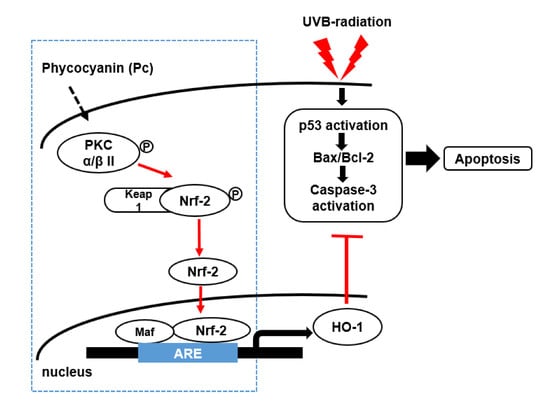
Phycocyanin Protects Against UVB-induced Apoptosis Through the PKC α/βII-Nrf-2/HO-1 Dependent Pathway in Human Primary Skin Cells
Abstract
:1. Introduction
2. Results
2.1. Pc-Induces HO-1 mRNA and Protein Expression without Cytotoxicity in Human Primary Skin Cells
2.2. Pc-Induced HO-1 Expression Is Mediated by Nrf-2
2.3. Pc Protects against UVB-Induced Apoptotic Cell Death in Primary Skin Cells
2.4. Induction of HO-1 and Activation of Nrf-2 by Pc via Phosphorylation of PKC α/β II
3. Discussion
4. Material and Methods
4.1. Reagents
4.2. Primary Cell Culture
4.3. UVB Radiation
4.4. RNA Interference
4.5. DNA Fragmentation Assay
4.6. Quantitative Reverse Transcription PCR (RT-qPCR)
4.7. Preparation of Cytosolic and Nuclear Fractions
4.8. Western Blotting
4.9. ARE-Luciferase Assay
4.10. Evaluation of Apoptotic Cell Death
4.11. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, Z.P.; Liu, L.N.; Chen, X.L.; Wang, J.X.; Chen, M.; Zhang, Y.Z.; Zhou, B.C. Factors that affect antioxidant activity of C-phycocyanins from Spirulina platensis. J. Food. Biochem. 2005, 29, 313–322. [Google Scholar] [CrossRef]
- Cherng, S.C.; Cheng, S.N.; Tarn, A.; Chou, T.C. Anti-inflammatory activity of c-phycocyanin in lipopolysaccharide-stimulated RAW 264.7 macrophages. Life Sci. 2007, 81, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Manconia, M.; Pendás, J.; Ledón, N.; Moreira, T.; Sinico, C.; Saso, L.; Fadda, A.M. Phycocyanin liposomes for topical anti-inflammatory activity: In vitro in vivo studies. J. Pharm. Pharmacol. 2009, 61, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Xu, G.; Chen, T.; Wong, Y.S.; Zhao, H.L.; Fan, R.R.; Gu, X.M.; Tong, P.C.; Chan, J.C. Phycocyanin protects INS-1E pancreatic beta cells against human islet amyloid polypeptide-induced apoptosis through attenuating oxidative stress and modulating JNK and p38 mitogen-activated protein kinase pathways. J. Biochem. Cell Biol. 2009, 41, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Madhyastha, H.K.; Radha, K.S.; Nakajima, Y.; Omura, S.; Maruyama, M. uPA dependent and independent mechanisms of wound healing by C-phycocyanin. J. Cell. Mol. Med. 2008, 12, 2691–2703. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.F.; Yang, S.P.; Kuo, Y.L.; Lai, Y.S.; Chou, T.C. Mechanisms involved in the antiplatelet effect of C-phycocyanin. Br. J. Nutr. 2006, 95, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, G.; Chou, P.H.; Shen, M.Y.; Chou, D.S.; Lin, C.H.; Sheu, J.R. C-phycocyanin, a very potent and novel platelet aggregation inhibitor from Spirulina platensis. J. Agric. Food. Chem. 2005, 53, 7734–7740. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chu, X.; Gao, M.; Li, W. Apoptotic mechanism of MCF-7 breast cells in vivo and in vitro induced by photodynamic therapy with C-phycocyanin. Acta Biochim. Biophys. Sin. (Shanghai) 2010, 42, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, C.; Li, B.; Liu, C.; He, P. Photodynamic effect of two kinds of phycobiliproteins on human liver cancer cell line SMMC-7721 in vitro. Sheng Wu Gong Cheng Xue Bao 2009, 25, 1417–1423. [Google Scholar] [PubMed]
- Meunier, L. Ultraviolet light and dendritic cells. Eur. J. Dermtol. 1999, 9, 269–275. [Google Scholar]
- Kolgen, W.; Both, H.; Van Weelden, H.; Guikers, K.L.; Brujinzeel-Koomen, C.A.; Knol, E.F.; Van Vloten, W.A.; De Gruijl, F.R. Epidermal langerhans cell depletion after artificial ultraviolet B irradiation of human skin in vivo: Apoptosis versus migration. J. Investig. Dermatol. 2002, 118, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Ohtani, T.; Mizuashi, M.; Mollah, Z.U.; Ito, Y.; Tagami, H.; Aiba, S. p38 Mitogen-activated protein kinase mediates dual role of ultraviolet B radiation in induction of maturation and apoptosis of monocyte-derived dendritic cells. J. Investig. Dermatol. 2004, 123, 361–370. [Google Scholar] [CrossRef] [PubMed]
- McGowan, B.S.; Ciccimaro, E.F.; Chan, T.O.; Feldman, A.M. The balance between pro-apoptotic and anti-apoptotic pathways in the failing myocardium. Cardivasc. Toxicol. 2003, 123, 361–370. [Google Scholar] [CrossRef]
- Maines, M.D. Heme oxygenase: Function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988, 2, 2557–2568. [Google Scholar] [CrossRef] [PubMed]
- Wiesel, P.; Patel, A.P.; DiFonzo, N.; Marria, P.B.; Sim, C.U.; Pellacani, A.; Maemura, K.; LeBlanc, W.; Marino, K.; Doerschuk, C.M.; et al. Endotoxin-induced mortality is related to increased oxidative stress and end-organ dysfunction, not refractory hypotension, in heme oxygenase-1-deficient mice. Circulation 2000, 102, 3015–3022. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.F.; Kuo, C.H.; Juang, J.H. Effects of heme oxygenase-1 transgenic islets in transplantation. Transplant. Proc. 2005, 37, 3463–3467. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Llu, C.; Wan, G.; Wang, X.; Cheng, X.; Ou, Y. Phycocyanin prevents methylglyoxal-induced mitochondrial-dependent apoptosis in INS-1 cells by Nrf2. Food Funct. 2016, 7, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Chen, P.Y.; Lin, J.C.; Kirkby, N.S.; Ou, C.H.; Chang, T.C. Melaleuca alternifolia Induces Heme Oxygenase-1 Expression in Murine RAW264.7 Cells through Activation of the Nrf2-ARE Pathway. Am. J. Chin. Med. 2017, 45, 1631–1648. [Google Scholar] [CrossRef] [PubMed]
- De Vries, H.E.; Witte, M.; Hondius, D.; Rozermuller, A.J.; Drukarch, B.; Hoozemans, J.; Van Horssen, J. Nrf-2-induced antioxidant protection: A promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic. Biol. Med. 2008, 45, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Bae, C.J.; Choi, Y.J.; Kim, S.I.; Kwon, Y.S.; Lee, H.J.; Kim, S.S.; Chun, W. 3,4,5-trihydroxycinnamic acid inhibits lipopolysaccharide (LPS)-induced inflammation by Nrf2 activation in vitro and improves survival of mice in LPS-induced endotoxemia model in vivo. Mol. Cell. Biochem. 2014, 390, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Jang, H.D. Nrf-2-mediated HO-1 induction coupled with the ERK signaling pathway contribute to indirect antioxidant capacity of caffeic acid phenethyl ester in HepG2 cell. Int. J. Mol. Sci. 2014, 15, 12149–12165. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Shen, S.C.; Lee, W.R.; Lin, H.Y.; Ko, C.H.; Lee, T.J. Nitric oxide and prostaglandin E2 participate in lipopolysaccharide/interferon-gamma-induced heme oxygenase and prevents RAW264.7 macrophages from UV-irradiation-induced cell death. J. Cell. Biochem. 2002, 86, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, G.; Zheng, Y.; Lu, L.; Wu, C.; Zhang, Y.; Lin, Q.; Cao, X. TLR4 signaling induces functional nerve growth factor receptor p75NTR on mouse dendritic cells via p38 MAPK and NF-κB pathways. Mol. Immunol. 2008, 45, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.K.; Lin, C.K.; Chang, H.W.; Wu, Y.H.; Yen, F.L.; Chang, F.R.; Chen, W.C.; Yeh, C.C.; Lee, J.C. Aqueous extract of Gracilaria. tenuistipitata suppresses LPS-induced NF-κB and MAPK activation in RAW 264.7 and rat peritoneal macrophages and exerts hepatoprotective effects on carbon tetrachloride-treated rat. PLoS ONE 2014, 9, e86557. [Google Scholar] [CrossRef]
- Ibuki, Y.; Allanson, M.; Dixon, K.D.; Reeve, V.E. Radiation sources providing increased UVA/UVB ratios attenuate the apoptotic effects of the UVB waveband UVA-dose-dependently in hairless mice. J. Investig. Dermatol. 2008, 127, 2236–2244. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.Y.; Pang, X.W.; Zhang, G.Q.; Guo, J.Y. Salidroside’s Protection Against UVB-Mediated Oxidative Damage and Apoptosis Is Associated with the Upregulation of Nrf2 Expression. Photomed. Laser Surg. 2017, 1, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Reddy, S.P.; Kleeberger, S.R. Nrf2 defends the lung from oxidative stress. Antioxid. Redox Signal. 2006, 8, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Romay, C.H.; Gonzalez, R.; Ledon, N.; Remiez, D.; Rimbau, V. C-phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, N.T. Production of phycocyanin—A pigment with applications in biology, biotechnology, foods and medicine. Appl. Microbiol. Biotechnol. 2008, 80, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Zheng, S.; Lin, L.; Jiang, Q.; Yang, X. Protective effect of C-phycocyanin against carbon tetrachloride-induced hepatocytes damage in vitro and in vivo. Chem. Biol. Interact. 2010, 185, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Chaiklahan, R.; Chirasuwan, N.; Loha, V.; Tia, S.; Bunnag, B. Separation and purification of phycocyanin from Spirulina sp. using a membrane process. Bioresour. Technol. 2011, 102, 7159–7164. [Google Scholar] [CrossRef] [PubMed]
- Pentón-Rol, G.; Marín-Prida, J.; Pardo-Andreu, G.; Martínez-Sánchez, G.; Acosta-Medina, E.F.; Valdivia-Acosta, A.; Lagumersindez-Denis, N.; Rodríguez-Jiménez, E.; Llópiz-Arzuaga, A.; López-Saura, P.A.; et al. C-phycocyanin is neuroprotective against global cerebral ischemia/reperfusion injury in gerbils. Brain Res. Bull. 2011, 86, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Halliday, G.M.; Surjana, D.; Damian, D.L. Nicotinamide prevents ultraviolet radiation-induced cellular energy loss. Photochem. Photobiol. 2010, 86, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Damian, D.L. Photoprotective effects of nicotinamide. Photochem. Photobiol. Sci. 2010, 9, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Sander, C.S.; Chang, H.; Hamm, F.; Elsner, P.; Thiele, J.J. Role of oxidative stress and antioxidant network in cutaneous carcinogenesis. Int. J. Dermatol. 2004, 43, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Budden, T.; Bowden, N.A. The role of altered nucleotide excision repair and UVB-induced DNA damage in melanomagenesis. Int. J. Mol. Sci. 2013, 14, 1132–1151. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Wu, S.B.; Hong, C.H.; Yu, H.S.; Wei, Y.H. Molecular mechanisms of UV-induced apoptosis and its effect on skin residential cells; The implication in UV-based phototherapy. Int. J. Mol. Sci. 2013, 14, 6414–6435. [Google Scholar] [CrossRef] [PubMed]
- Bowden, G.T. prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signaling. Nat. Rev. Cancer 2004, 4, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Balogan, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterini, R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Hu, B.; Cederbaum, A.I. Diallyl sulfide induces heme oxygenase-1 through MAPK pathway. Arch. Biochem. Biophys. 2004, 432, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Hebbar, V.; Nair, S.; Xu, C.; Li, W.; Lin, W.; Keum, Y.S.; Han, J.; Gallo, M.A.; Kong, A.N. Regulation of Nrf2 transactivation domain activity. The differential effects of mitogen-activated protein kinase cascades and synergistic stimulatory effect of Raf and CREB-binding protein. J. Biol. Chem. 2004, 279, 23052–23060. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Nguyen, T.; Pickett, C.B. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation NF-E2-related factor 2. Proc. Natl. Acad. Sci. USA 2000, 97, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Nguyen, T.; Pickett, C.B. Phosphorylation of Nrf2 at Ser40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002, 277, 42769–42774. [Google Scholar] [CrossRef] [PubMed]
- Zipper, L.M.; Mulcahy, R.T. Erk activation is required for Nrf2 nuclear localization during pyrroidine dithiocarbamate induction of glutamate cysteine ligase modulatory gene expression in HepG2 cells. Toxicol. Sci. 2003, 73, 124–134. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Chanas, S.A.; Henderson, C.J.; McLellan, L.I.; Wolf, C.R.; Cavin, C.; Hayes, J.D. The Cap ‘n’ Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001, 61, 3299–3307. [Google Scholar] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1-Nrf-2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef] [PubMed]
- Im, R.A.; Kim, Y.H.; Uddin, M.R.; Lee, H.W.; Chae, S.W.; Kim, Y.W.; Jung, W.S.; Kang, B.J.; Mun, C.S.; Lee, M.Y. Scutellaria baicalensis extracts and flavonoids protect Rat L6 Cells from antimycin A-induced mitochondrial dysfunction. Evid. Based Complement. Alternat. Med. 2012, 2012, 517965–517973. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: not available. |





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.M.; Lee, J.Y.; Im, A.-R.; Chae, S. Phycocyanin Protects Against UVB-induced Apoptosis Through the PKC α/βII-Nrf-2/HO-1 Dependent Pathway in Human Primary Skin Cells. Molecules 2018, 23, 478. https://doi.org/10.3390/molecules23020478
Kim KM, Lee JY, Im A-R, Chae S. Phycocyanin Protects Against UVB-induced Apoptosis Through the PKC α/βII-Nrf-2/HO-1 Dependent Pathway in Human Primary Skin Cells. Molecules. 2018; 23(2):478. https://doi.org/10.3390/molecules23020478
Chicago/Turabian StyleKim, Ki Mo, Joo Young Lee, A-Rang Im, and Sungwook Chae. 2018. "Phycocyanin Protects Against UVB-induced Apoptosis Through the PKC α/βII-Nrf-2/HO-1 Dependent Pathway in Human Primary Skin Cells" Molecules 23, no. 2: 478. https://doi.org/10.3390/molecules23020478