Study of Hexane Adsorption on Activated Carbons with Differences in Their Surface Chemistry
Abstract
:1. Introduction



2. Results and Discussion
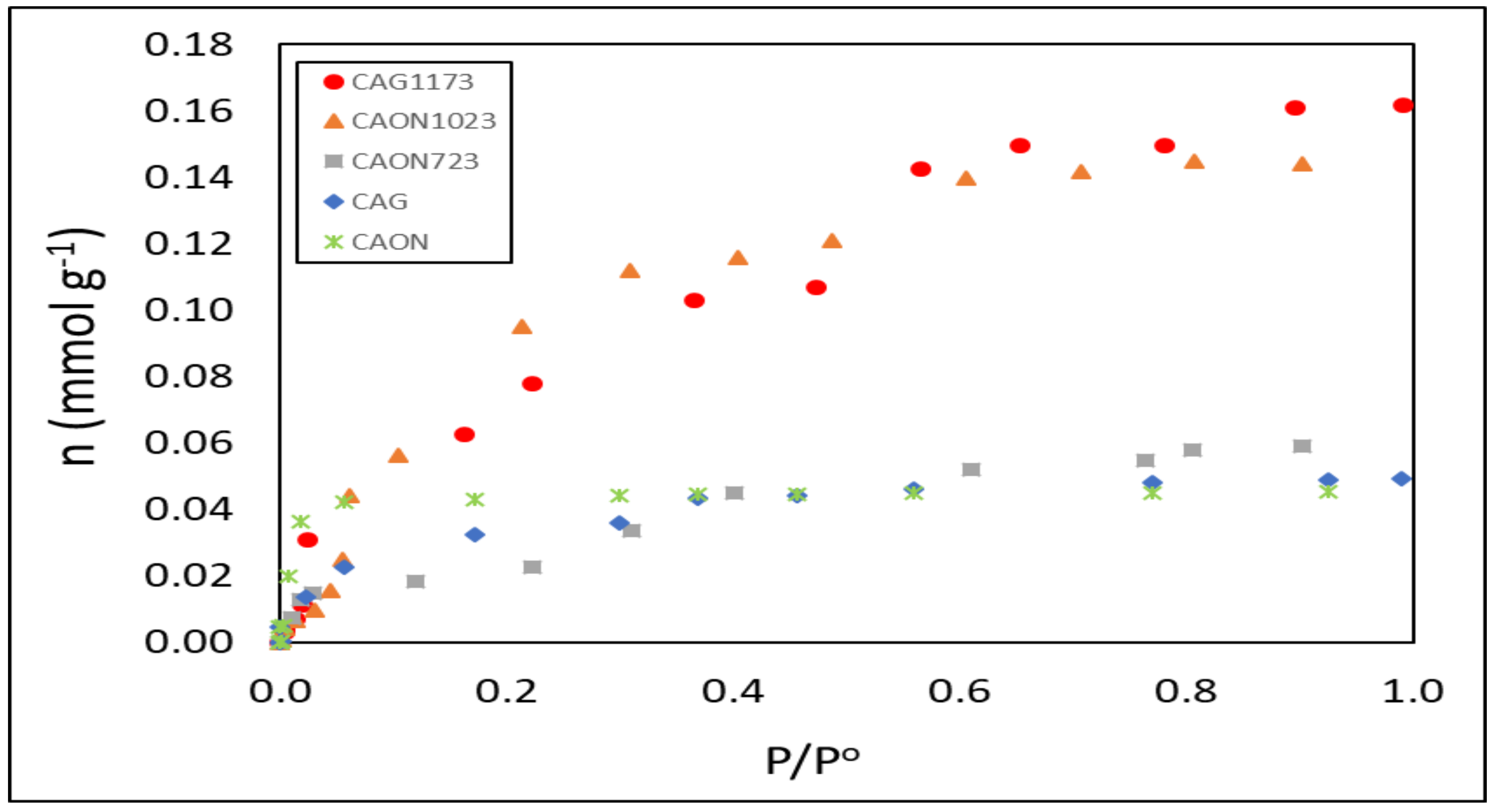
2.1. Relationship between the Physicochemical Characteristics of Activated Carbons and Gas Phase Adsorption Isotherms of Hexane
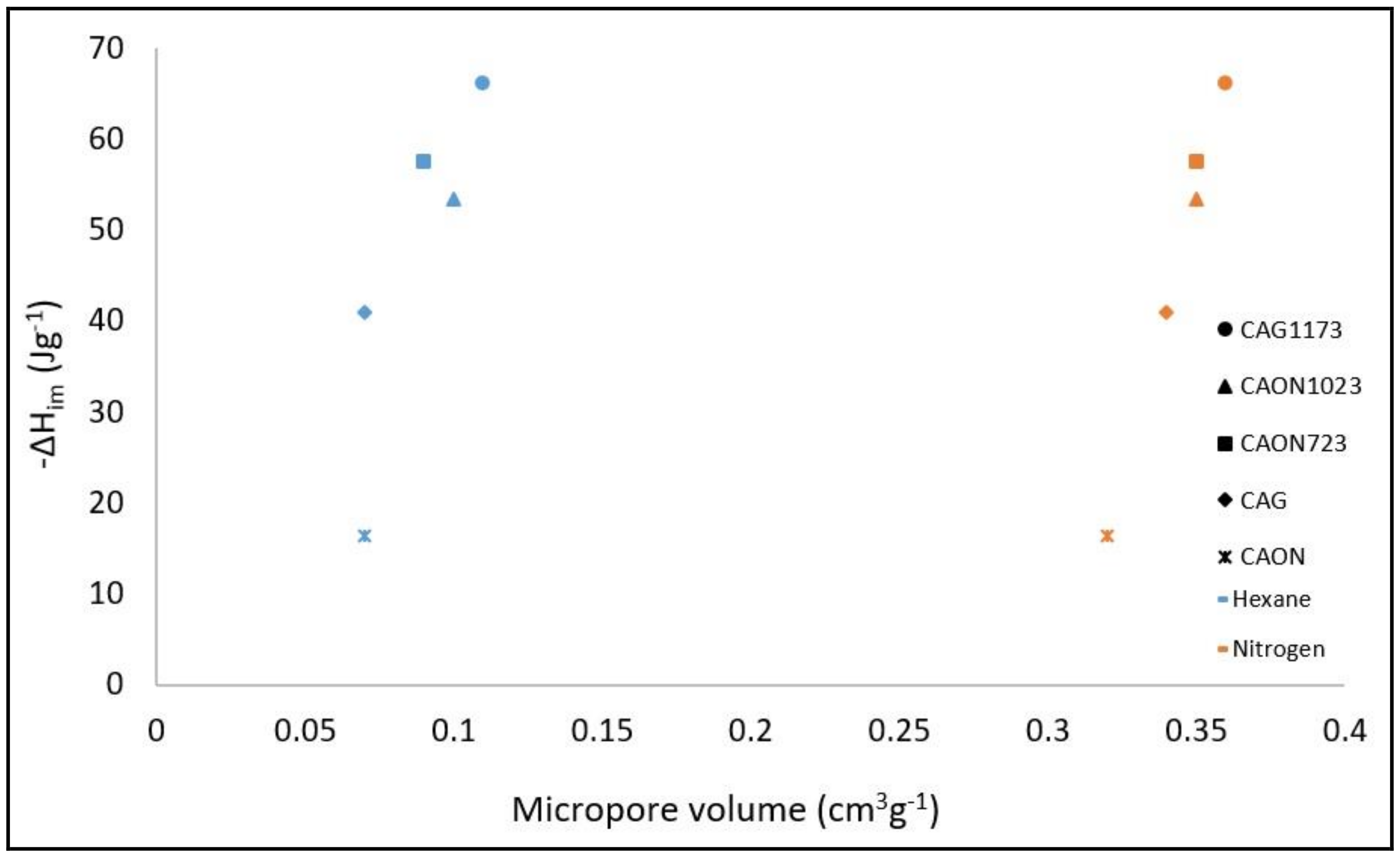
2.2. Relationship between the Physicochemical Characteristics of Activated Carbons and the Enthalpy of Immersion
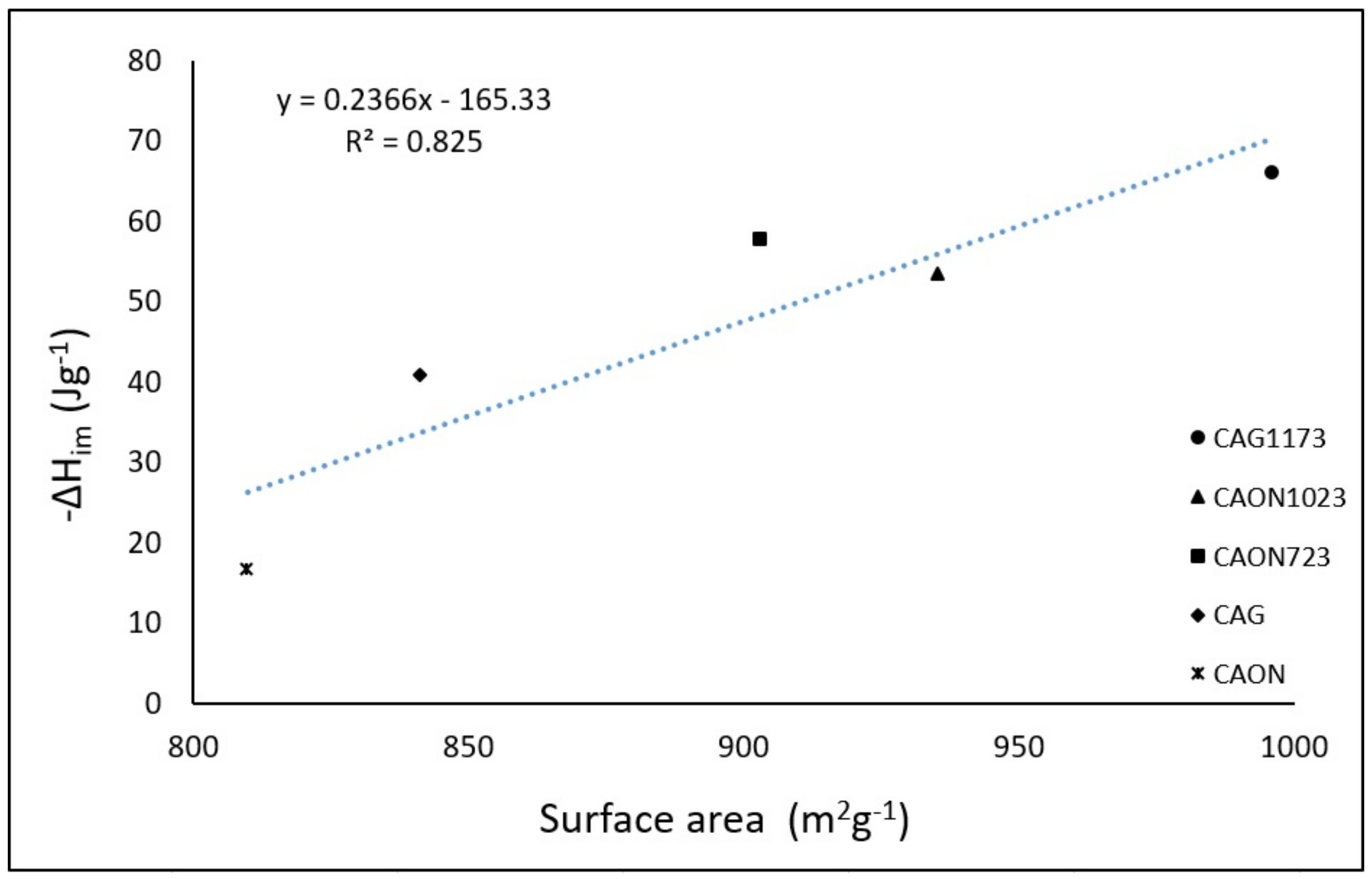
2.3. Relationship between the Energetic Characterization (Characteristic Energy and Enthalpy of Immersion) and Physicochemical Characteristics of Activated Carbons
3. Materials and Methods
3.1. Activated Carbon
3.2. Nitrogen Adsorption Isotherms
3.3. Boehm Titrations
3.4. Hexane Adsorption Isotherms
3.5. Enthalpy of Immersion of Activated Carbon in Solvents
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, L.; Liu, S.; Liu, J. Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J. Hazard. Mater. 2011, 192, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, S.C.; Peng, Y.; Shih, Y. Adsorption of volatile organic vapors by activated carbon derived from rice husk under various humidity conditions and its statistical evaluation by linear solvation energy relationships. Sep. Purif. Technol. 2016, 170, 102–108. [Google Scholar] [CrossRef]
- Bansal, R.; Goyal, M. Activated Carbon and Its Surface Structure. In Activated Carbon Adsorption; Bansal, R., Ed.; CRC Press: Boca Ratón, FL, USA, 2010; pp. 1–65. [Google Scholar]
- Tham, Y.J.; Latif, P.A.; Abdullah, A.M.; Shamala-Devi, A.; Taufiq-Yap, Y.H. Performances of toluene removal by activated carbon derived from durian shell. Bioresour. Technol. 2011, 102, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Liu, J.; Gong, G.; Jiang, Y.; Xie, Q. Preparation and modification of activated carbon for benzene adsorption by steam activation in the presence of KOH. Int. J. Min. Sci. Technol. 2013, 23, 395–401. [Google Scholar] [CrossRef]
- Pak, S.; Jeon, M.; Jeon, Y. Study of sulfuric acid treatment of activated carbon used to enhance mixed VOC removal. Int. Biodeterior. Biodegrad. 2016, 113, 195–200. [Google Scholar] [CrossRef]
- Mazlan, M.; Uemura, Y.; Yusup, S.; Elhassan, F.; Uddin, A.; Hiwada, A.; Demiya, M. activated carbon from rubber wood sawdust by carbon dioxide activation. Procedia Eng. 2016, 148, 530–537. [Google Scholar] [CrossRef]
- Liu, H.; Yang, B.; Xue, N. Enhanced adsorption of benzene vapor on granular activated carbon under humid conditions due to shifts in hydrophobicity and total micropore volume. J. Hazard. Mater. 2016, 318, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Lopes, A.; De Carvalho, S.; Do Socorro Barros, D.; De Alcântara Mendes, R.; Lima, M. Surface modification of commercial activated carbon (CAG) for the adsorption of benzene and toluene. Am. J. Anal. Chem. 2015, 6, 528–538. [Google Scholar] [CrossRef]
- Yang, X.; Yi, H.; Tang, X.; Zhao, S.; Yang, Z.; Ma, Y.; Feng, T.; Cui, X. Behaviors and kinetics of toluene adsorption -desorption on activated carbons with varying pore structure. J. Environ. Sci. 2017, (in press). [Google Scholar] [CrossRef]
- Martínez de Yuso, A.; Izquierdo, M.; Valenciano, R.; Rubio, B. Toluene and n-hexane adsorption and recovery behavior on activated carbons derived from almond shell wastes. Fuel Process. Technol. 2013, 110, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pei, J.; Zhang, J. Determination of adsorption isotherm and diffusion coefficient of toluene on activated carbon at low concentrations. Build. Sci. 2012, 48, 66–76. [Google Scholar] [CrossRef]
- Tham, Y.; Latif, P.; Abdullah, A.; Shamala-Devi, A.; Taufiq-Yap, Y. Performances of toluene removal by activated carbon derived from durian shell. Bioresour. Technol. 2011, 102, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Tazibet, S.; Boucheffa, Y.; Lodewyckx, P. Heat treatment effect on the textural, hydrophobic and adsorptive properties of activated carbons obtained from olive waste. Microporous Mesoporous Mater. 2013, 170, 293–298. [Google Scholar] [CrossRef]
- Betancur-Sánchez, A.; Vásquez-Trespalacios, E.; Sardi-Correa, C. Impaired colour vision in workers exposed to organic solvents: A systematic review. Arch. Soc. Esp. Oftalmol. (Engl. Ed.) 2017, 92, 12–18. [Google Scholar] [CrossRef]
- Park, H.; Park, H.; Jang, J. Exposure characteristics of construction painters to organic solvents. Saf. Health Work 2016, 7, 63–71. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services. Toxicological profile for n-hexane. Available online: http://www.atsdr.cdc.gov/toxprofiles/tp113.pdf/ (accessed on 21 January 2018).
- Environmental Protection Agency. Hexane. Available online: https://www.epa.gov/sites/production/files/2016-9/documents/hexane. pdf/ (accessed on 21 January 2018).
- Bates, M.; Reed, B.; Liu, E.; Eisen, M.; Hammond, S. Solvent exposure and cognitive function in automotive technicians. NeuroToxicology 2016, 57, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, P.; Giraldo, L.; Moreno-Piraján, J.C. Modified surface chemistry of activated carbons. Correlation with immersion enthalpy. J. Therm. Anal. Calorim. 2013, 114, 245–251. [Google Scholar] [CrossRef]
- Stoeckli, F.; Centeno, T.A. On the characterization of microporous carbons by immersion calorimetry alone. Carbon. 1997, 35, 1097–1100. [Google Scholar] [CrossRef]
- Moreno-Piraján, J.C.; Giraldo, L. .; García-Cuello, V., Vargas-Delgadillo, D., Rodríguez-Estupiñán, P., Murillo-Acevedo, Y., Cantillo, M. Thermodynamic of the interactions between gas-solid and solid-liquid on carbonaceous materials. In Thermodynamics/Book 1, Moreno-Piraján, J.C., Eds.; INTECH: Rijeka, Croatia, 2011; pp. 164–195. [Google Scholar]
- Stoeckli, H.F.; Kraehenbuehl, F.; Ballerini, L.; De Bernardini, S. Recent developments in the Dubinin equation. Carbon 1989, 27, 125–128. [Google Scholar] [CrossRef]
- Moreno, J.C.; Giraldo, L. Determination of the immersion enthalpy of activated carbon by microcalorimetry of the heat conduction. Inst. Sci. Technol. 2000, 28, 171–178. [Google Scholar]
- Li, M.; Wu, S.; Peng, Y.; Shih, Y. Adsorption of volatile organic vapors by activated carbon derived from rice husk under various humidity conditions and its statistical evaluation by linear solvation energy relationships. Sep. Purif. Technol. 2016, 170, 102–108. [Google Scholar] [CrossRef]
- Shim, W.G.; Lee, J.W.; Moon, H. Equilibrium and fixed-bed adsorption of n-hexane on activated carbon. Sep. Sci. Technol. 2003, 38, 3905–3926. [Google Scholar] [CrossRef]
- Singh, K.; Mohan, D.; Tandon, G.S.; Gupta, G.S.D. Vapor-phase adsorption of hexane and benzene on activated carbon fabric cloth: Equilibria and rate studies. Ind. Eng. Chem. Res. 2002, 41, 2480–2486. [Google Scholar] [CrossRef]
- Parvulescu, O.; Ion, V.; Dobre, T.; Niu, S.G. The effect of process factors on n-hexane adsorption onto copper impregnated activated carbon. UPB Sci. Bull. Series B 2016, 78, 121–130. [Google Scholar]
- Izquierdo, M.; Martínez de Yuso, A.; Valenciano, R.; Rubio, B.; Pino, M. Influence of activated carbon characteristics on toluene and hexane adsorption: Application of surface response methodology. Appl. Surf. Sci. 2013, 264, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Ramos, M.E.; Bonelli, P.R.; Cukierman, A.L.; Ribeiro Carrott, M.M.L.; Carrott, P.J.M. Adsorption of volatile organic compounds onto activated carbon cloths derived from a novel regenerated cellulosic precursor. J. Hazard. Mater. 2010, 177, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Shim, W.G.; Moon, H. Adsorption equilibrium of solvent vapors on activated carbons. Korean J. Chem. Eng. 2001, 18, 518–524. [Google Scholar] [CrossRef]
- Ryu, Y.; Kim, K.; Lee, C. Adsorption and desorption of n-hexane, methyl ethyl ketone, and toluene on an activated carbon fiber from supercritical carbon dioxide. Ind. Eng. Chem. Res. 2000, 39, 2510–2518. [Google Scholar] [CrossRef]
- Murillo, Y.S.; Giraldo, L.; Moreno-Piraján, J.C. Contribution enthalpic in the interaction of activated carbon with polar and apolar solvents. Arab. J. Chem. 2013, 6, 347–351. [Google Scholar] [CrossRef]
- Almazán-Almazán, M.C.; Domingo-García, M.; Fernández-Morales, I.; López-Garzón, F.J.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A.; Martínez-Alonso, A. Adsorption and microcalorimetric measurements on activated carbons prepared from Polyethylene Terephtalate. Stud. Surf. Sci. Catal. 2007, 160, 185–192. [Google Scholar]
- Hernández-Monje, D.; Giraldo, L.; Moreno-Piraján, J.C. Estudio entálpico de la inmersión de carbones activados granulares modificados en benceno, hexano y ciclohexano. Afinidad Revista de Química Teórica y Aplicada 2016, 74, 291–298. [Google Scholar]
- Li, K.; Jiang, Y.; Wang, X.; Bai, D.; Li, H.; Zheng, Z. Effect of nitric acid modification on the lead(II) adsorption of mesoporous biochars with different mesopore size distributions. Clean Techn. Environ. Policy 2016, 18, 797–805. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Òrfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Jaramillo, J.; Álvarez, P.; Gómez-Serrano, V. Preparation and ozone-surface modification of activated carbon. Thermal stability of oxygen surface groups. Appl. Surf. Sci. 2010, 256, 5232–5236. [Google Scholar] [CrossRef]
- Jaramillo, J.; Modesto, P.; Gómez, V. Oxidation activated carbon by dry and wet methods. Surface chemistry and textural modifications. Fuel Process. Technol. 2010, 91, 1768–1775. [Google Scholar] [CrossRef]
- Foo, K.; Hameed, B. Insights into the modeling of adsorption isotherm systems. Chem Eng J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Chen, X. Modeling of experimental adsorption isotherm data. Information 2015, 6, 14–22. [Google Scholar] [CrossRef]
- Marsh, H.; Rodrìguez-Reinoso, F. Applicability of Activated Carbon. In Activated Carbon; Rodrìguez-Reinoso, F., Ed.; Taylor & Francis Group: San Diego, CA, USA, 2016; pp. 383–453. [Google Scholar]
- Echeverría, J.; Juncal, E.; Barbería, V.; Musgo, J.; Garrido, J. Synthesis and characterization of ultramicroporous silica xerogels. J. Non-Cryst. Solids 2010, 356, 378–382. [Google Scholar] [CrossRef]
- Villar-Rodil, S.; Navarrete, R.; Denoyel, R.; Albiniak, A.; Paredes, J.; Martínez-Alonso, A.; Tascón, J. Carbon molecular sieve cloths prepared by chemical vapour deposition of methane for separation of gas mixtures. Micropor. Mesopor. Mat. 2005, 77, 109–118. [Google Scholar] [CrossRef]
- Stoeckli, F.; Slasli, A.; Hugi-Cleary, D.; Guillot, A. The characterization of microporosity in carbons with molecular sieve effects. Micropor. Mesopor. Mat. 2002, 51, 197–202. [Google Scholar] [CrossRef]
- Gómez, F.; Giraldo, L.; Moreno-Piraján, J.C. Granular activated carbons characterization by CO2 adsorption isotherms and immersion enthalpy. J. Therm. Anal. Calorim. 2015, 120, 1657–1664. [Google Scholar] [CrossRef]
- Vargas, D.P.; Giraldo, L.; Moreno-Piraján, J.C. Accessible area and hydrophobicity of activated carbons obtained from the enthalpy characterization. Adsorption 2016, 22, 3–11. [Google Scholar] [CrossRef]
- Radovic, L.R.; Moreno-Castilla, C.; Rivera-Utrilla, J. Carbon Materials as Adsorbents in Aqueous Solutions. In Chemistry and Physics of Carbon. A Serie of Advances; Walker, P., Ed.; Marcel Dekker: New York, NY, USA, 2000; pp. 293–297. [Google Scholar]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Kim, Y.; Park, C. Titration Method for the Identification of Surface Functional Groups. In Materials Science and Engineering of Carbon Characterization; Inagaki, M., Kang, F., Eds.; Tsinghua University Press Limited: Hamamatsu, Japan, 2016; pp. 273–286. [Google Scholar]
- Vargas, D.P.; Giraldo, L.; Moreno-Piraján, J.C. Enthalpic characterisation of activated carbon monoliths obtained from lignocellulosic materials. J. Therm. Anal. Cal. 2013, 111, 1067–1072. [Google Scholar] [CrossRef]
Sample Availability: Samples are available from the authors. |




| Activated Carbon | BET Surface Area (m2g−1) | Vo(N2) (cm3g−1) | Average Pore Size (N2-QSDFT) (nm) | Oxygenated Group Content (µmolg−1) |
|---|---|---|---|---|
| CAG | 840 | 0.34 | 0.785 | 0.33 |
| CAON | 816 | 0.32 | 0.753 | 0.54 |
| CAON723 | 903 | 0.35 | 0.785 | 0.43 |
| CAON1023 | 935 | 0.35 | 0.785 | 0.34 |
| CAG1173 | 996 | 0.36 | 0.785 | 0.30 |
| Activated Carbon | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| b (mbar−1) | nm (mmolg−1) | R2 | Kf (mmolg−1mbar−1) | 1/n | R2 | |
| CAG | 0.087 | 0.052 | 0.986 | 0.053 | 0.309 | 0.974 |
| CAON | 0.010 | 0.046 | 0.972 | 0.052 | 0.194 | 0.843 |
| CAON723 | 0.463 | 0.090 | 0.948 | 0.064 | 0.503 | 0.973 |
| CAON1023 | 0.261 | 0.193 | 0.989 | 0.171 | 0.520 | 0.959 |
| CAG1173 | 0.627 | 0.269 | 0.983 | 0.172 | 0.589 | 0.984 |
| Activated Carbon | −∆Him(C6H14) (Jg−1) | −∆Him(H2O) (Jg−1) | Hydrophobic Factor |
|---|---|---|---|
| CAG | 40.9 | 49.6 | 0.07 |
| CAON | 16.4 | 66.6 | 0.07 |
| CAON723 | 57.6 | 53.3 | 0.09 |
| CAON1023 | 53.4 | 37.4 | 0.10 |
| CAG1173 | 66.1 | 32.4 | 0.11 |
| Activated Carbon | Area Accessible to Hexane (m2g−1) | Eo(C6H14) (Jmol-1) | Vo(C6H14) (cm3g−1) |
|---|---|---|---|
| CAG | 379 | 6881 | 0.07 |
| CAON | 152 | 6922 | 0.07 |
| CAON723 | 533 | 6149 | 0.09 |
| CAON1023 | 494 | 5792 | 0.10 |
| CAG1173 | 612 | 5656 | 0.11 |
| Activated Carbon | Treatment |
|---|---|
| CAG | Starting carbon |
| CAON | CAG exposed to oxidation with a solution of HNO3 |
| CAON723 | CAON exposed to heat treatment at 723 K for 2 h under N2 atmosphere |
| CAON1023 | CAON exposed to heat treatment at 1023 K for 2 h under N2 atmosphere |
| CAG1173 | CAG exposed to heat treatment at 1173 K for 2 h under N2 atmosphere |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Monje, D.; Giraldo, L.; Moreno-Piraján, J.C. Study of Hexane Adsorption on Activated Carbons with Differences in Their Surface Chemistry. Molecules 2018, 23, 476. https://doi.org/10.3390/molecules23020476
Hernández-Monje D, Giraldo L, Moreno-Piraján JC. Study of Hexane Adsorption on Activated Carbons with Differences in Their Surface Chemistry. Molecules. 2018; 23(2):476. https://doi.org/10.3390/molecules23020476
Chicago/Turabian StyleHernández-Monje, Diana, Liliana Giraldo, and Juan Carlos Moreno-Piraján. 2018. "Study of Hexane Adsorption on Activated Carbons with Differences in Their Surface Chemistry" Molecules 23, no. 2: 476. https://doi.org/10.3390/molecules23020476





