A Hexahomotrioxacalix[3]arene-Based Ditopic Receptor for Alkylammonium Ions Controlled by Ag+ Ions
Abstract
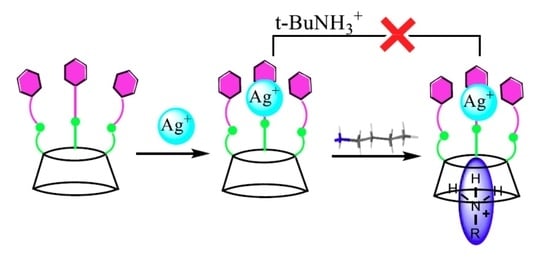
:1. Introduction
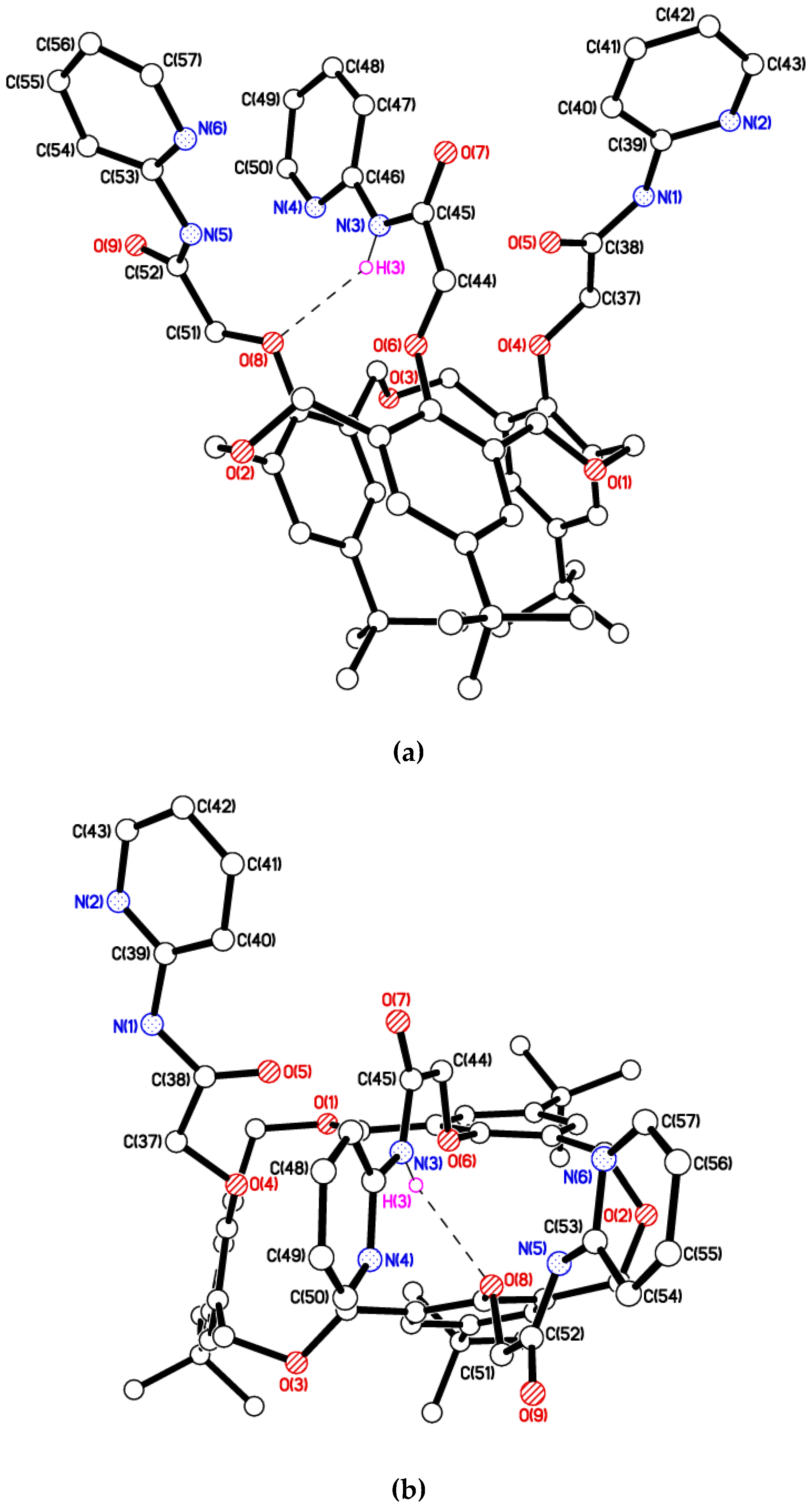
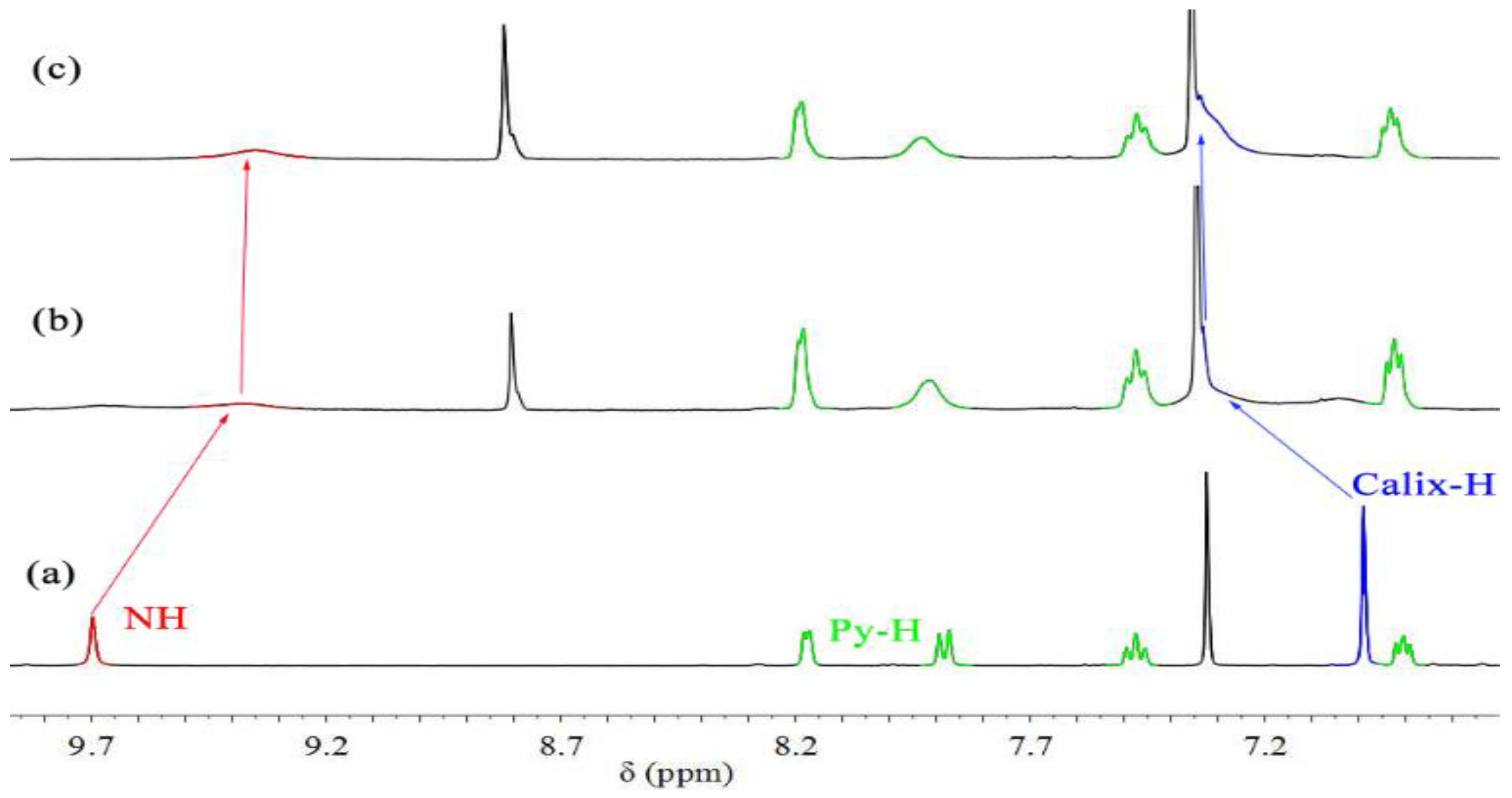
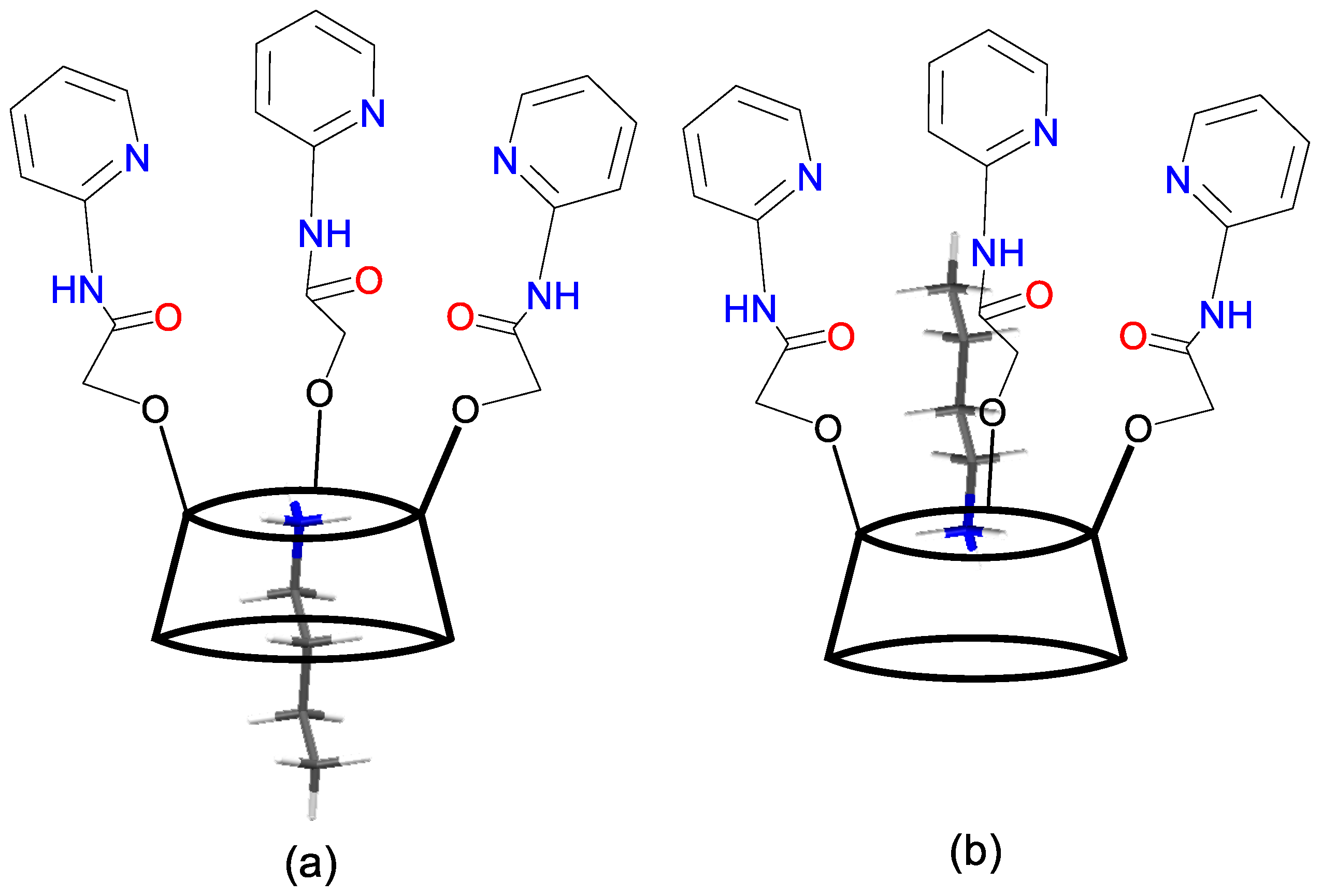
2. Results and Discussion
3. Experimental Section
3.1. General
3.2. Materials
3.3. Preparation of cone-4
3.4. Preparation of cone-1
3.5. 1H-NMR Spectroscopic Complexation Experiments
3.6. Single-Crystal X-Ray Diffraction Measurements for cone-1
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schmidtchen, F.P.; Berger, M. Artificial Organic Host Molecules for Anions. Chem. Rev. 1997, 97, 1609–1646. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Shinkai, S. Novel Cavity Design Using Calix[n]arene Skeletons: Toward Molecular Recognition and Metal Binding. Chem. Rev. 1997, 97, 1713–1734. [Google Scholar] [CrossRef] [PubMed]
- Morohashi, N.; Narumi, F.; Iki, N.; Hattori, T.; Miyano, S. Thiacalixarenes. Chem. Rev. 2006, 106, 5291–5316. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Quang, D.T. Calixarene-Derived Fluorescent Probes. Chem. Rev. 2007, 107, 3780–3799. [Google Scholar] [CrossRef] [PubMed]
- Marcos, P.M. Functionalization and Properties of Homooxacalixarenes. In Calixarenes and Beyond; Neri, P., Sessler, J. L., Wang, M.-X., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Joseph, R.; Rao, C.P. Ion and Molecular Recognition by Lower Rim 1,3-Di-conjugates of Calix[4]arene as Receptors. Chem. Rev. 2011, 111, 4658–4702. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ikejiri, Y.; Zeng, X.; Elsegood, M.R.J.; Redshaw, C.; Yamato, T. Synthesis of Mono-O-alkylated Homooxacalix[3]arene and a Protection–Deprotection Strategy for Homooxacalix[3]arene. Org. Lett. 2017, 19, 66–69. [Google Scholar]
- Kumar, M.; Kumar, R.; Bhalla, V. Optical Chemosensor for Ag+, Fe3+, and Cysteine: Information Processing at Molecular Level. Org. Lett. 2011, 13, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Kwon, P.S.; Lee, J. W.; Kim, J.I.; Hong, C.S.; Kim, J.; Yan, W.S.; Lee, J.Y.; Lee, J.H.; Joo, T.; et al. Coumarin-derived Cu(2+)-selective fluorescence sensor: synthesis, mechanisms, and applications in living cells. J. Am. Chem. Soc. 2009, 131, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Gutsche, C.D. Shaping the Baskets: Conformations of Calixarenes. In Calixarenes. An Introduction; Stoddart, J.F., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2008. [Google Scholar]
- Cottet, K.; Marcos, P.M.; Cragg, P.J. Fifty years of oxacalix[3]arenes: A review. Beilstein J. Org. Chem. 2012, 8, 201–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, X.-L.; Takimoto, M.; Zeng, X.; Yamato, T. Synthesis, structure and inclusion properties of cone-tris{[(5’-methyl-2,2’-bipyridyl)-5-yl]oxycarbonylmethoxy}hexahomotrioxacalix[3]arene. J. Incl. Phenom. Macrocycl. Chem. 2011, 71, 231–237. [Google Scholar] [CrossRef]
- Wu, C.; Ikejiri, Y.; Zhao, J.-L.; Jiang, X.-K.; Ni, X.-L.; Zeng, X.; Redshaw, C.; Yamato, T. A pyrene-functionalized triazole-linked hexahomotrioxacalix[3]arene as a fluorescent chemosensor for Zn2+ ions. Sens. Actuators B. 2016, 228, 480–485. [Google Scholar] [CrossRef]
- Wu, C.; Wang, C.-Z.; Zhu, Q.; Zeng, X.; Redshawd, C.; Yamato, T. Click synthesis of a quinoline-functionalized hexahomotrioxacalix[3]arene: A turn-on fluorescence chemosensor for Fe3+. Sens. Actuators B. 2018, 254, 52–58. [Google Scholar] [CrossRef]
- Tsubaki, K.; Otsubo, T.; Morimoto, T.; Maruoka, H.; Furukawa, M.; Momose, Y.; Shang, M.H.; Fuji, K. Modification of the Upper Rim of Homooxacalix[3]arenes and Complexation between a Nitrohomooxacalix[3]arene Derivative and n-Hexylamine. J. Org. Chem. 2002, 67, 8151–8156. [Google Scholar] [CrossRef] [PubMed]
- Yamato, T.; Rahman, S.; Kitajima, F.; Zeng, X.; Gil, J.T. Synthesis and inclusion properties of O-tris(benzyloxy)hexahomotrioxacalix[3]arenes. J. Chem. Res. 2006, 8, 496–498. [Google Scholar] [CrossRef]
- Ikeda, A.; Hatano, T.; Shinkai, S.; Akiyama, T.; Yamada, S. Efficient Photocurrent Generation in Novel Self-Assembled Multilayers Comprised of [60]Fullerene−Cationic Homooxacalix[3]arene Inclusion Complex and Anionic Porphyrin Polymer. J. Am. Chem. Soc. 2001, 123, 4855–4856. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Yoshimura, M.; Udzu, H.; Fukuhara, C.; Shinkai, S. Inclusion of [60]Fullerene in a Homooxacalix[3]arene-Based Dimeric Capsule Cross-Linked by a PdII−Pyridine Interaction. J. Am. Chem. Soc. 1999, 121, 4296–4297. [Google Scholar] [CrossRef]
- Schmidtchen, F.P.; Berger, M. Artificial Organic Host Molecules for Anions. Chem. Rev. 1997, 97, 1609. [Google Scholar] [CrossRef] [PubMed]
- Beer, P.D.; Gale, P.A. Anion Recognition and Sensing: The State of the Art and Future Perspectives. Angew. Chem. Int. Ed. 2001, 40, 486–516. [Google Scholar] [CrossRef]
- Sansone, F.; Baldini, L.; Casnati, A.; Ungaro, R. Calixarenes: from biomimetic receptors to multivalent ligands for biomolecular recognition. New J. Chem. 2010, 34, 2715–2728. [Google Scholar] [CrossRef]
- Casnati, A.; Pochini, A.; Ungaro, R.; Ugozzoli, F.; Arnaud, F.; Fanni, S.; Schwing, M.-J.; Egberink, R.J.; de Jong, F.; Reinhoudt, D.N. Synthesis, Complexation, and Membrane Transport Studies of 1,3-Alternate Calix[4]arene-crown-6 Conformers: A New Class of Cesium Selective Ionophores. J. Am. Chem. Soc. 1995, 117, 2767–2777. [Google Scholar] [CrossRef]
- Yamamoto, H.; Ueda, K.; Suenaga, H.; Sakaki, T.; Shinkai, S. Exploitation of Na+-Selective Electrodes for Protein Solutions from Crown-Bridged Calix[4]quinones. Chem. Lett. 1996, 25, 39–40. [Google Scholar] [CrossRef]
- Dozol, J.F.; Ludwig, R. Extraction of Radioactive by Caliarenes. Elements Ion Exch. Solvent Extr. 2010, 19, 195–310. [Google Scholar]
- Ludwig, R.; Dzung, N.T.K. Calixarene-Based Molecules for Cation Recognition. Sensors 2002, 2, 397–416. [Google Scholar] [CrossRef]
- Ni, X.-L.; Wang, S.; Zeng, X.; Tao, Z.; Yamato, T. Pyrene-Linked Triazole-Modified Homooxacalix[3]arene: A Unique C3 Symmetry Ratiometric Fluorescent Chemosensor for Pb2+. Org. Lett. 2011, 13, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.-L.; Zeng, X.; Redshaw, C.; Yamato, T. Synthesis and evaluation of a novel pyrenyl-appended triazole-based thiacalix[4]arene as a fluorescent sensor for Ag+ ion. Tetrahedron 2011, 67, 3248–3253. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.K.; Ikejiri, Y.; Jin, C.C.; Wu, C.; Zhao, J.L.; Ni, X.-L.; Zeng, X.; Redshaw, C.; Yamato, T. Synthesis and evaluation of a novel fluorescent sensor based on hexahomotrioxacalix[3]arene for Zn2+ and Cd2+. Tetrahedron 2016, 72, 4854–4858. [Google Scholar] [CrossRef]
- Yamato, T.; Kitajima, F.; Gil, J.T. Alkyl Ammonium Ion Selectivity of Hexahomotrioxacalix[3]arene Triamide Derivative having the Intramolecular Hydrogen-bonding Group. J. Incl. Phenom. Macrocycl. Chem. 2005, 53, 257–262. [Google Scholar] [CrossRef]
- Yamato, T.; Zhang, F.; Tsuzuki, H.; Miura, Y. Synthesis and and lnclusion Properties of C3-Symetrically Capped Hexaholmotrioxacalix[3]arenes with Ester Groups on the Lower Rim. Eur. J. Org. Chem. 2001, 1069–1075. [Google Scholar] [CrossRef]
- Yamato, T.; Zhang, F. Synthesis, conformations and inclusion properties of hexahomotrioxacalix[3]arene triamide derivatives having hydrogen-bonding groups. J. Incl. Phenom. Macrocycl. Chem. 2001, 39, 55–64. [Google Scholar] [CrossRef]
- Takeshita, M.; Shinkai, S. Novel fluorometric sensing of ammonium ions by pyrene functionalized homotrioxacalix[3]arenes. Chem. Lett. 1994, 32, 125–128. [Google Scholar] [CrossRef]
- Gabba, F.P.; Schier, A.; Riede, J.; Hynes, M.J. Recognition of 1,2-diazines by a bidentate Lewis acid. Chem. Commun. 1998, 8, 897–898. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Bifulco, G.; Riccio, R.; Gaeta, C.; Neri, P. Quantum Mechanical Calculations of Conformationally Relevant 1H and 13C-NMR Chemical Shifts of N-, O-, and S-Substituted Calixarene Systems. Chem. Eur. J. 2007, 13, 7185–7194. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.; Hashimoto, N.; Otsuka, H.; Shinkai, S. Synthesis and ion selectivity of conformers derived from hexahomotrioxacalix[3]arene. J. Org. Chem. 1993, 58, 5958–5963. [Google Scholar] [CrossRef]
- Raymo, F.M.; Stoddart, J.F. Interlocked Macromolecules. Chem. Rev. 1999, 99, 1643–1664. [Google Scholar] [CrossRef] [PubMed]
- Cafeo, G.; Gargiulli, C.; Gattuso, G.; Kohnke, F.H.; Notti, A.; Occhipinti, S.; Pappalardo, S.; Parisi, M.F. Recognition and binding of paraquat dichloride by cyclodextrin/calix[6]pyrrole binary host systems. Tetrahedron Lett. 2002, 43, 8103–8106. [Google Scholar] [CrossRef]
- Ikeda, A.; Udzu, H.; Zhong, Z.; Shinkai, S.; Sakamoto, S.; Yamaguchi, K. A Self-Assembled Homooxacalix[3]arene-based Dimeric Capsule Constructed by a PdII−Pyridine Interaction Which Shows a Novel Chiral Twisting Motion in Response to Guest Inclusion. J. Am. Chem. Soc. 2001, 123, 3872–3877. [Google Scholar] [CrossRef] [PubMed]
- Prins, L.J.; Huskens, J.; de Jong, F.; Timmerman, P.; Reinhoudt, D.N. Complete asymmetric induction of supramolecular chirality in a hydrogen-bonded assembly. Nature 1999, 398, 498–502. [Google Scholar] [CrossRef]
- Prins, L.J.; Jolliffe, K.A.; Hulst, R.; Timmerman, P.; Reinhoudt, D.N. Control of Structural Isomerism in Noncovalent Hydrogen-Bonded Assemblies Using Peripheral Chiral Information. J. Am. Chem. Soc. 2000, 122, 3617–3627. [Google Scholar] [CrossRef]
- Takimoto, M.; Ni, X.-L.; Rahman, S.; Zeng, X.; Yamato, T. Heteroditopic receptors tris(2-pyridylamide) derivatives derived from hexahomotrioxacalix[3]arene triacetic acid. J. Incl. Phenom. Macrocycl. Chem. 2011, 70, 69–80. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284. [Google Scholar]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299. [Google Scholar] [CrossRef]
- Spartan’10, version 1.1.0; Wavefunction Inc.: Irvine, CA, USA.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- SAINT and APEX, version 2; Software for CCD Diffractometers; Bruker AXS Inc.: Madison, WI, USA, 2012.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Van der Sluis, P.; Spek, A.L. BYPASS: an effective method for the refinement of cristal structures containing diosordered solvent regions. Acta Crystallogr. 1990, A46, 194–201. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound are not available from the authors. |
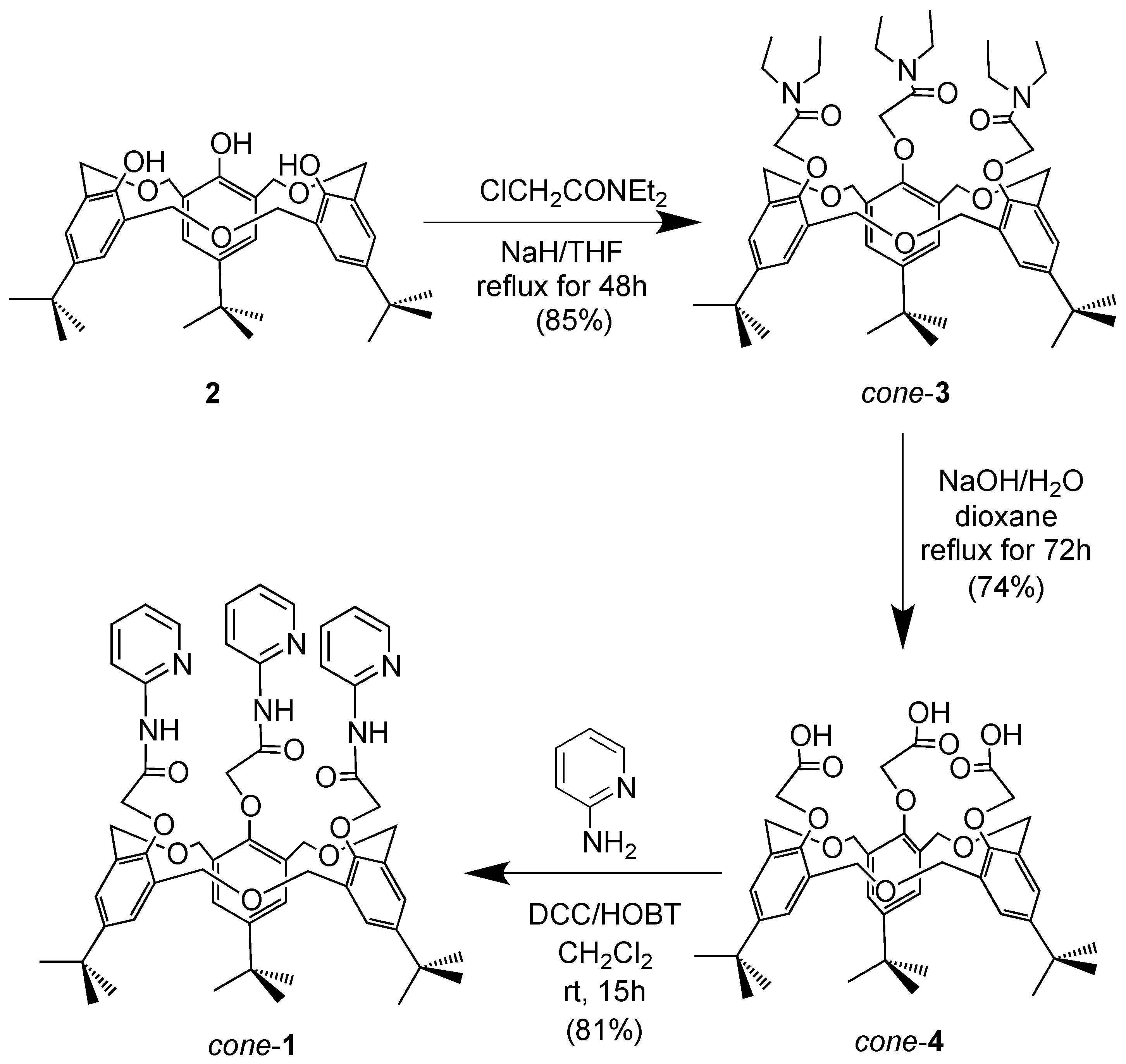



| Chemical Shift of Proton | |||||
|---|---|---|---|---|---|
| CH3 | CH3CH2 | CH3CH2CH2 | CH2 | ||
| Free | 0.94 | 1.40 | 1.67 | 3.02 | |
| n-BuNH3+ | Complex | 0.88 | 0.30 | −0.33 | 0.30 |
| Δδ | −0.06 | −1.10 | −2.00 | −2.72 | |
| Free | 1.40 | ||||
| t-BuNH3+ | Complex | −0.34 | |||
| Δδ | −1.74 | ||||
| Ka (M−1) | |
|---|---|
| n-BuNH3+ | 2680 ± 155 |
| n-BuNH3+ ⊂ [cone-1 ⊃ Ag+] | 3270 ± 190 |
| t-BuNH3+ | 360 ± 20 |
| t-BuNH3+ ⊂ [cone-1 ⊃ Ag+] | No complexation |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.-K.; Ikejiri, Y.; Wu, C.; Rahman, S.; Georghiou, P.E.; Zeng, X.; Elsegood, M.R.J.; Redshaw, C.; Teat, S.J.; Yamato, T. A Hexahomotrioxacalix[3]arene-Based Ditopic Receptor for Alkylammonium Ions Controlled by Ag+ Ions. Molecules 2018, 23, 467. https://doi.org/10.3390/molecules23020467
Jiang X-K, Ikejiri Y, Wu C, Rahman S, Georghiou PE, Zeng X, Elsegood MRJ, Redshaw C, Teat SJ, Yamato T. A Hexahomotrioxacalix[3]arene-Based Ditopic Receptor for Alkylammonium Ions Controlled by Ag+ Ions. Molecules. 2018; 23(2):467. https://doi.org/10.3390/molecules23020467
Chicago/Turabian StyleJiang, Xue-Kai, Yusuke Ikejiri, Chong Wu, Shofiur Rahman, Paris E. Georghiou, Xi Zeng, Mark R. J. Elsegood, Carl Redshaw, Simon J. Teat, and Takehiko Yamato. 2018. "A Hexahomotrioxacalix[3]arene-Based Ditopic Receptor for Alkylammonium Ions Controlled by Ag+ Ions" Molecules 23, no. 2: 467. https://doi.org/10.3390/molecules23020467
APA StyleJiang, X.-K., Ikejiri, Y., Wu, C., Rahman, S., Georghiou, P. E., Zeng, X., Elsegood, M. R. J., Redshaw, C., Teat, S. J., & Yamato, T. (2018). A Hexahomotrioxacalix[3]arene-Based Ditopic Receptor for Alkylammonium Ions Controlled by Ag+ Ions. Molecules, 23(2), 467. https://doi.org/10.3390/molecules23020467