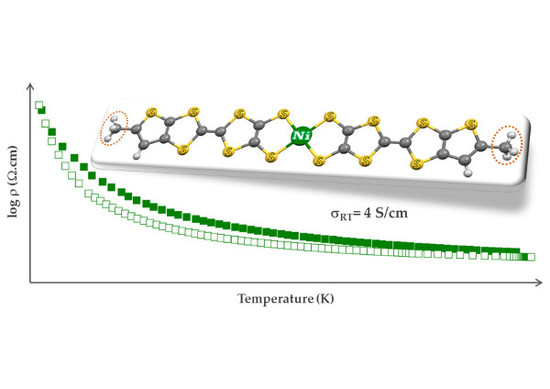
Gold and Nickel Extended Thiophenic-TTF Bisdithiolene Complexes
Abstract
:1. Introduction
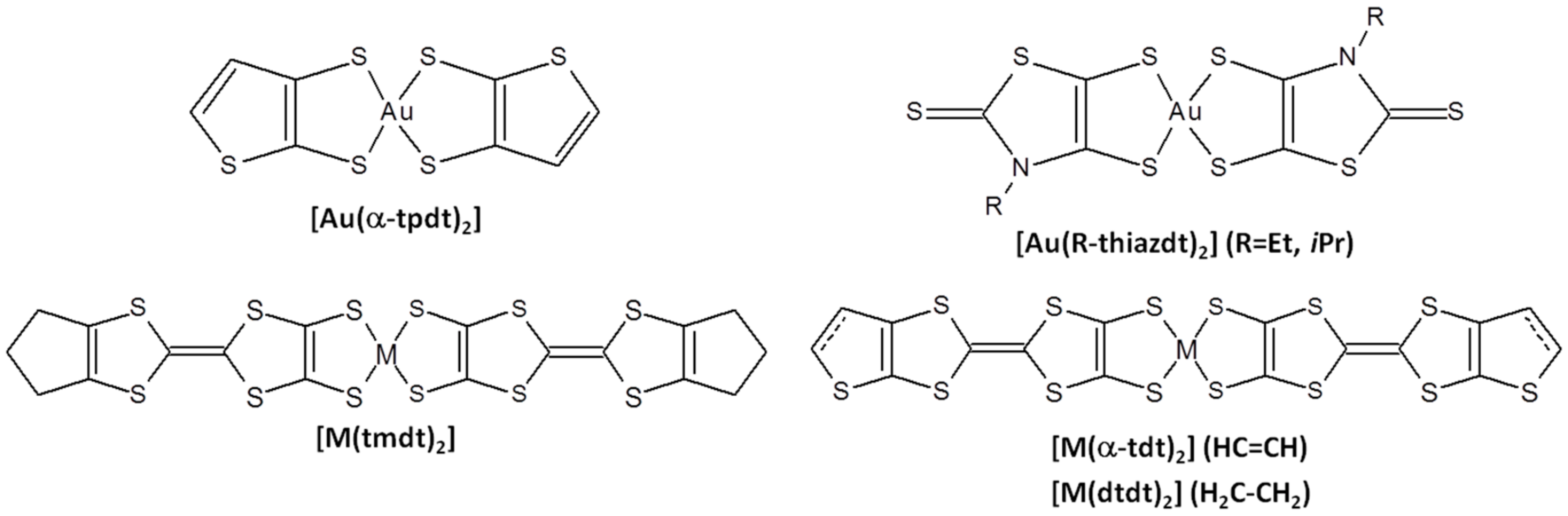
2. Results and Discussion
2.1. Synthesis
2.2. Electrochemical Studies
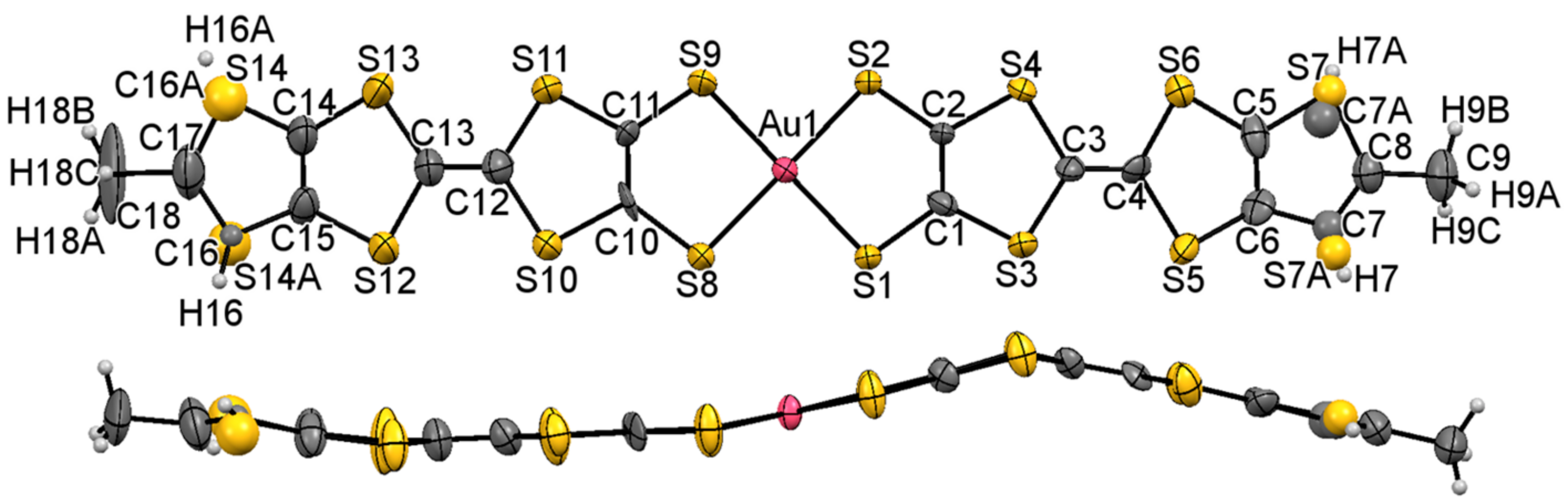
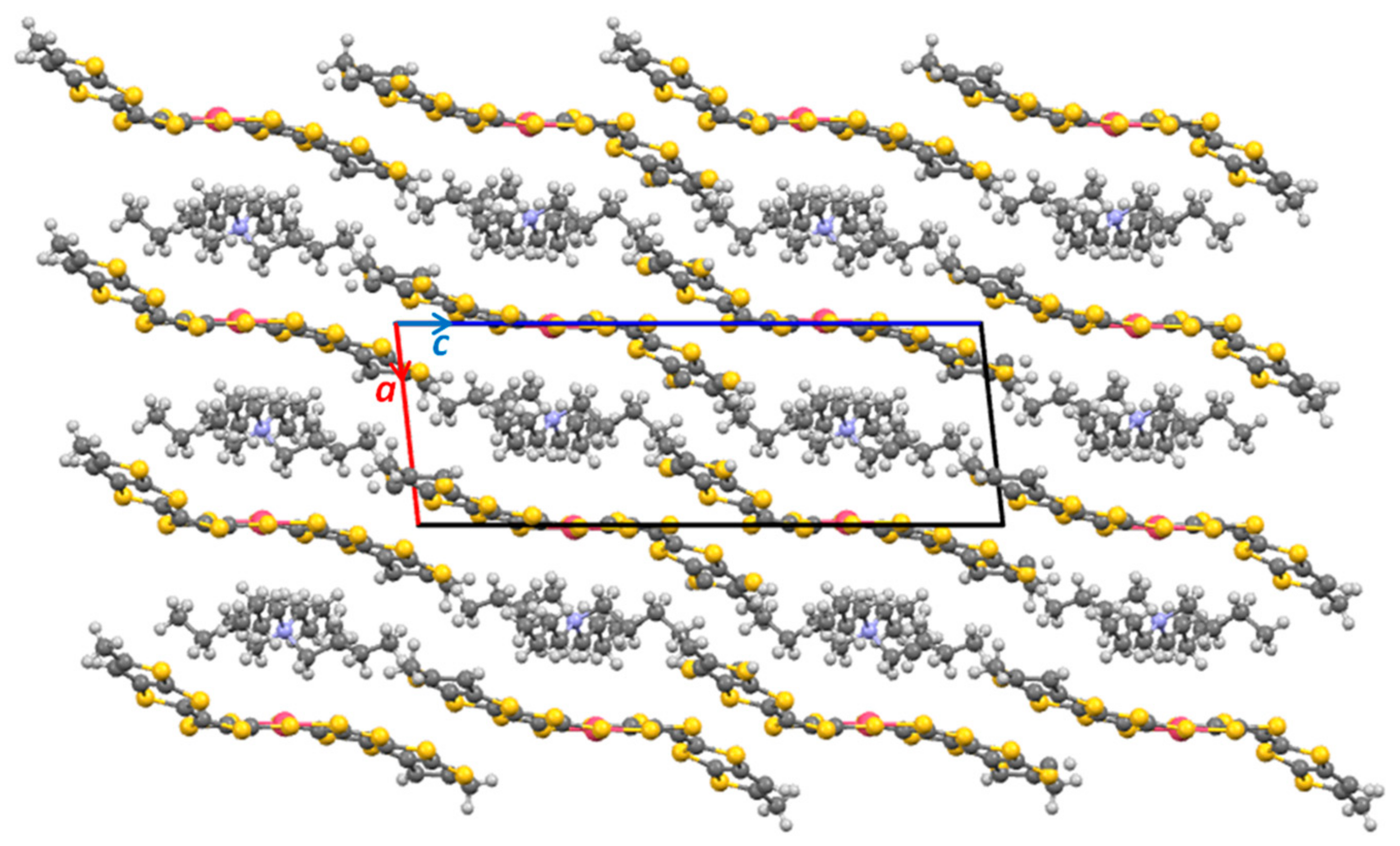
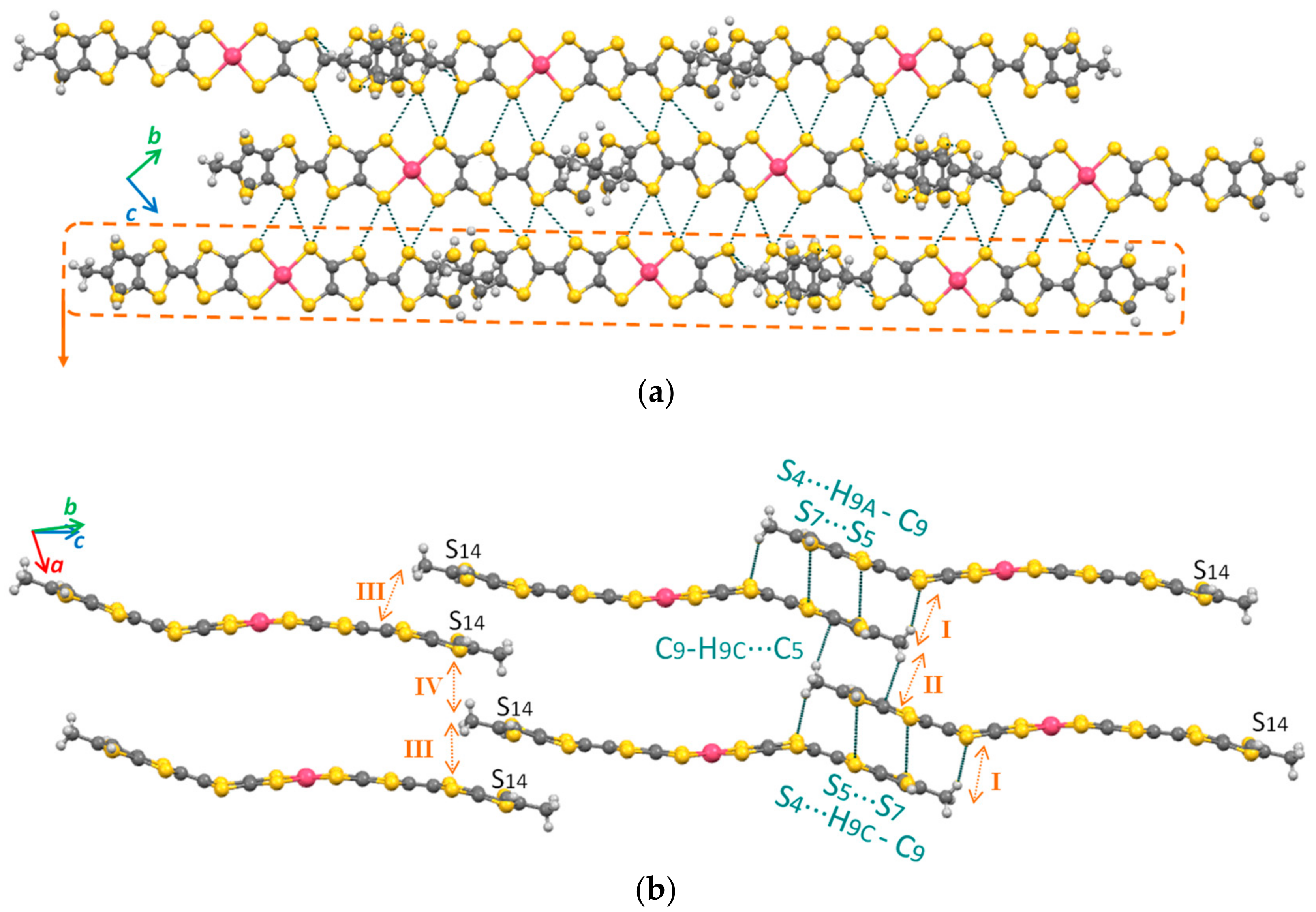
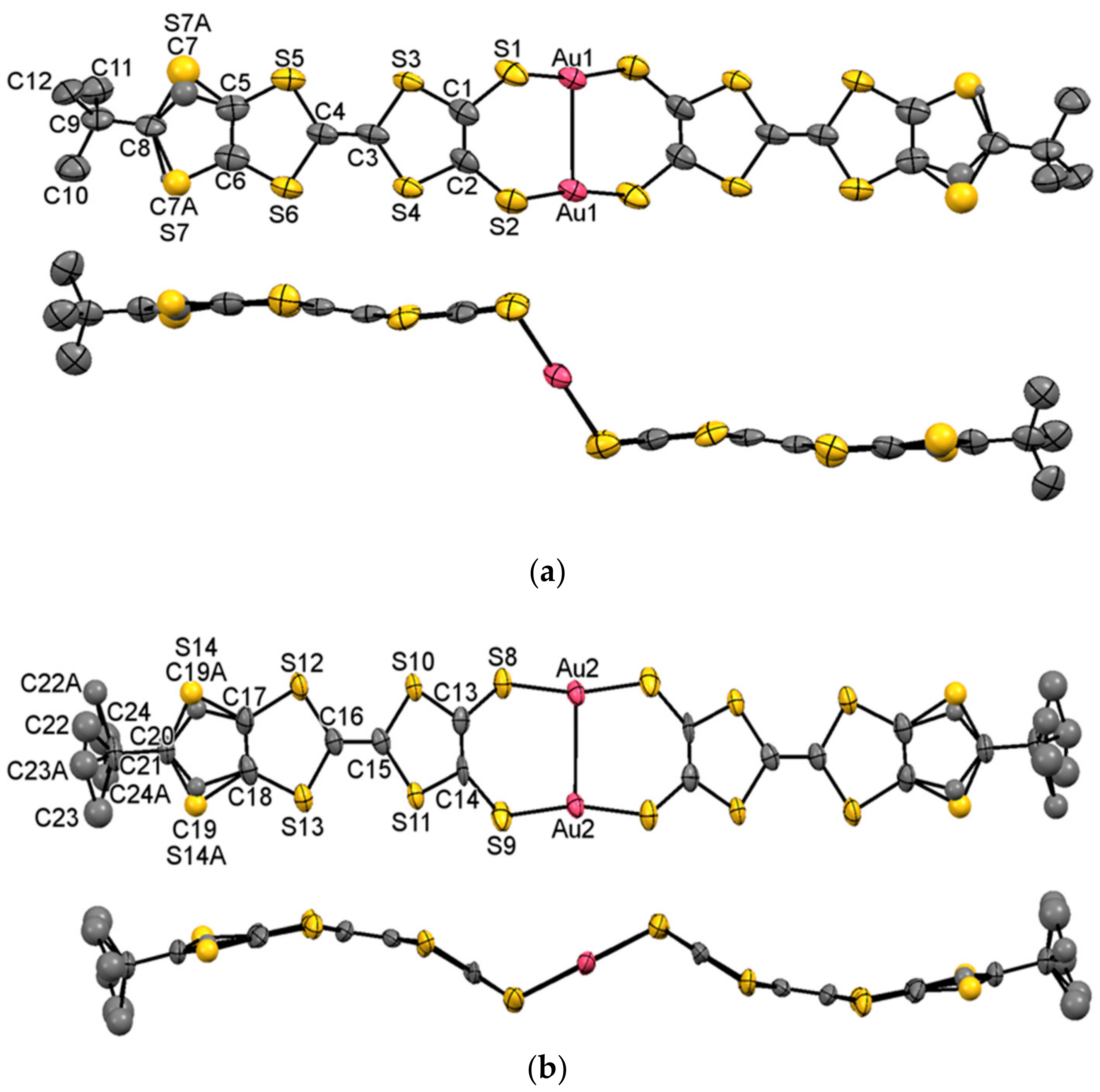
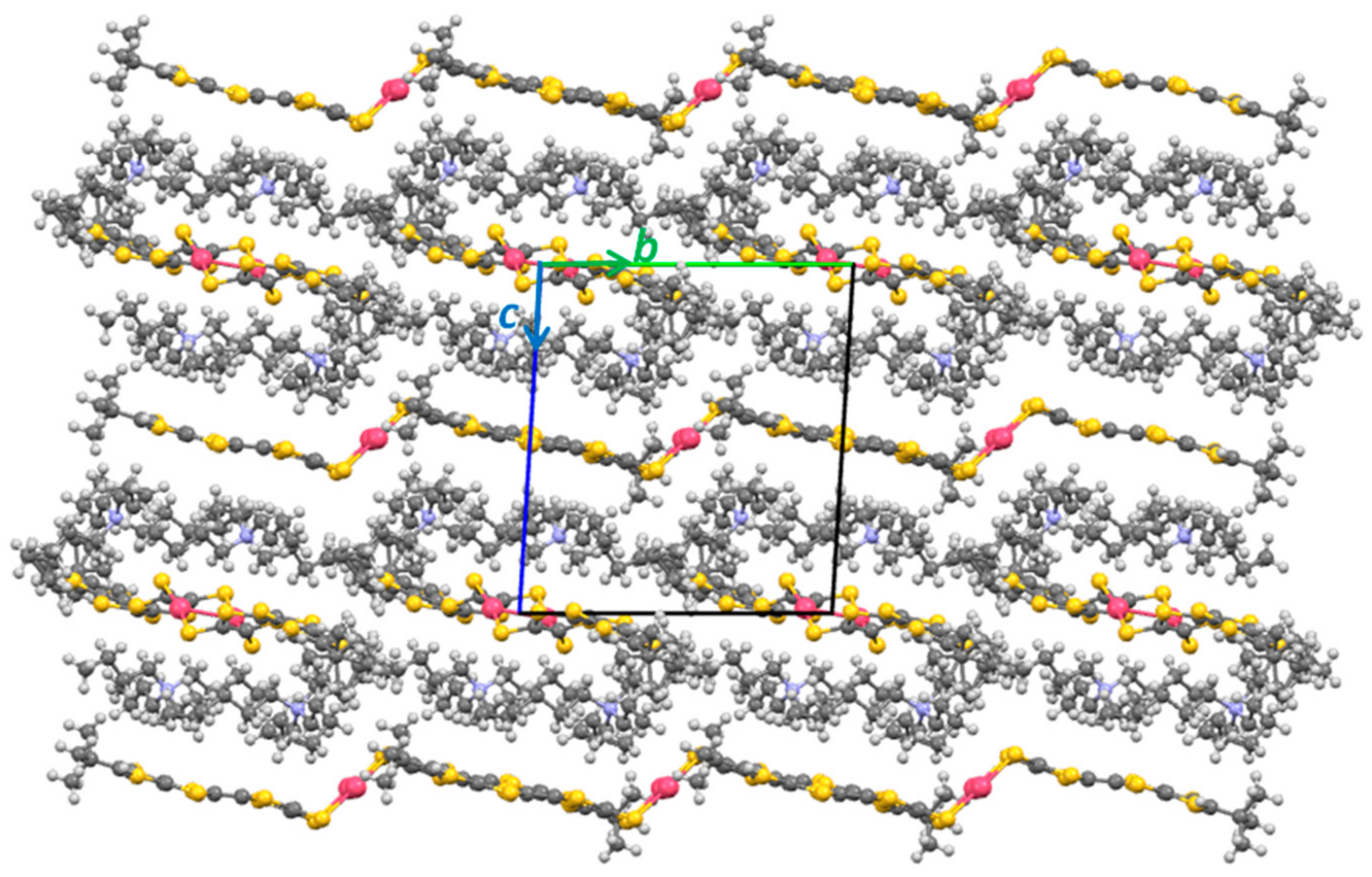
2.3. X-ray Structural Analysis
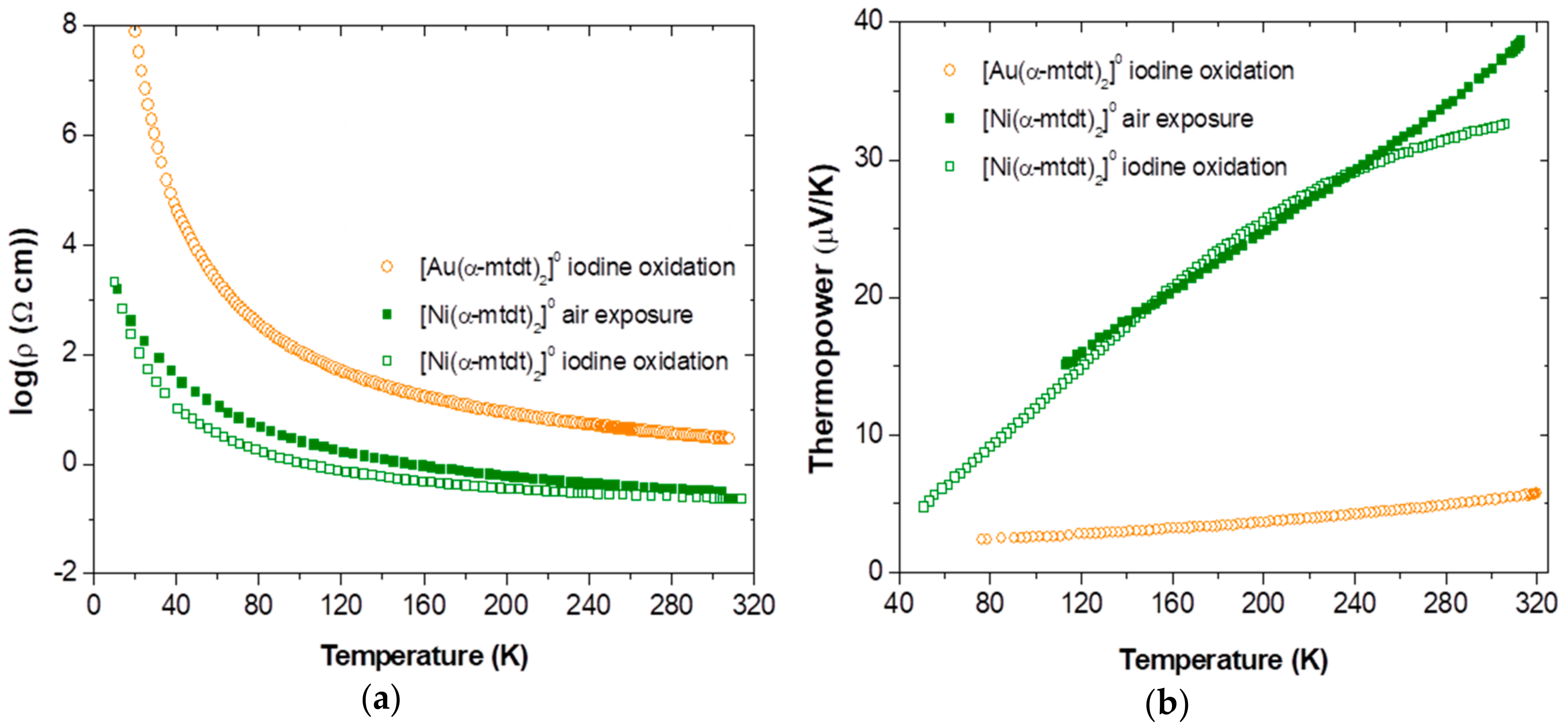
2.4. Electric Transport Properties
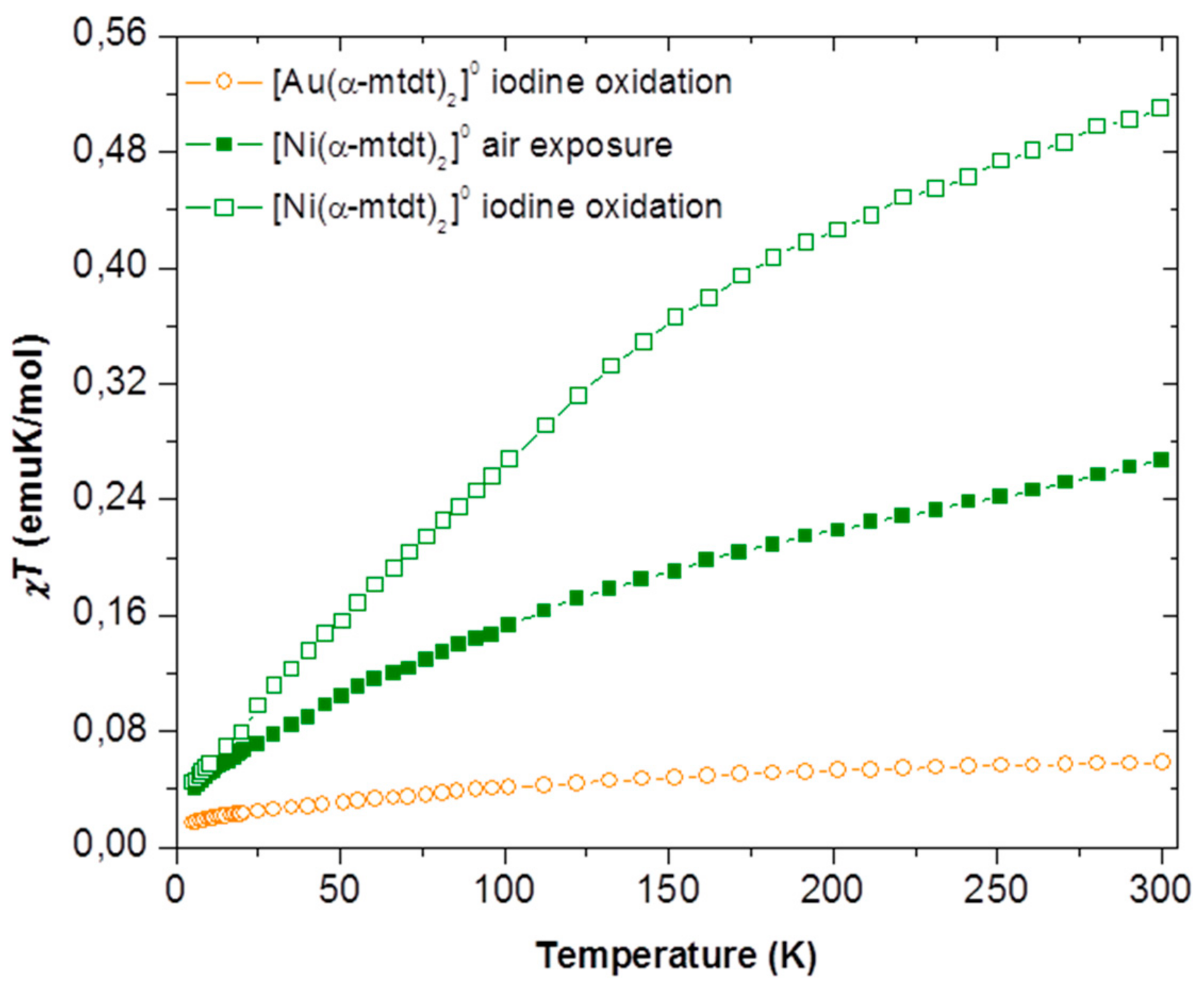
2.5. Magnetic Properties
3. Materials and Methods
3.1. General Information
3.2. Synthesis
3.3. Cyclic Voltammetry
3.4. X-ray Crystallography
3.5. Electric Transport Properties
3.6. Magnetic Susceptibility
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Belo, D.; Alves, H.; Lopes, E.B.; Duarte, M.T.; Gama, V.; Henriques, R.T.; Almeida, M.; Pérez-Benítez, A.; Rovira, C.; Veciana, J. Gold complexes with dithiothiophene ligands: A metal based on a neutral molecule. Chemistry 2001, 7, 511–519. [Google Scholar] [CrossRef]
- Tanaka, H.; Okano, Y.; Kobayashi, H.; Suzuki, W.; Kobayashi, A. A three-dimensional synthetic metallic crystal composed of single-component molecules. Science 2001, 291, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Belo, D.; Alves, H.; Lopes, E.B.; Gama, V.; Henriques, R.T.; Duarte, M.; Almeida, M.; Pérez-Benítez, A.; Rovira, C.; Veciana, J. New dithiothiophene complexes for conducting and magnetic materials. Synth. Met. 2001, 120, 699–702. [Google Scholar] [CrossRef]
- Kobayashi, A.; Tanaka, H.; Kobayashi, H. Molecular design and development of single-component molecular metals. J. Mater. Chem. 2001, 11, 2078–2088. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kobayashi, H. Single-component molecular conductors. Mol. Cryst. Liq. Cryst. 2006, 455, 47–56. [Google Scholar] [CrossRef]
- Kobayashi, A.; Okano, Y.; Kobayashi, H. Molecular design and physical properties of single-component molecular metals. J. Phys. Soc. Jpn. 2006, 75, 051002-1-12. [Google Scholar] [CrossRef]
- Kobayashi, A.; Fujiwara, E.; Kobayashi, H. Single-component molecular metals with extended-TTF dithiolate ligands. Chem. Rev. 2004, 104, 5243–5264. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Sasa, M.; Suzuki, W.; Fujiwara, E.; Tanaka, H.; Tokumoto, M.; Okano, Y.; Fujiwara, H.; Kobayashi, H. Infrared electronic absorption in a single-component molecular metal. J. Am. Chem. Soc. 2004, 126, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, A.; Osuka, A. Fully conjugated porphyrin tapes with electronic absorption bands that reach into infrared. Science 2001, 293, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Belo, D.; Almeida, M. Transition metal complexes based on thiophene-dithiolene ligands. Coordin. Chem. Rev. 2010, 254, 1479–1492. [Google Scholar] [CrossRef]
- Kanno, M.; Bando, Y.; Shirahata, T.; Inoue, J.; Wada, H.; Mori, T. Stabilization of organic field-effect transistors in hexamethylenetetrathiafulvalene derivatives substituted by bulky alkyl groups. J. Mater. Chem. 2009, 19, 6548–6555. [Google Scholar] [CrossRef]
- Higashino, T.; Akiyama, Y.; Kojima, H.; Kawamoto, T.; Mori, T. Organic semiconductors and conductors with tert-butyl substituents. Crystals 2012, 2, 1222–1238. [Google Scholar] [CrossRef]
- Neves, A.I.S.; Santos, I.C.; Coutinho, J.T.; Pereira, L.C.J.; Henriques, R.T.; Lopes, E.B.; Alves, H.; Almeida, M.; Belo, D. 5-methylthiophene-2,3-dithiolene transition metal complexes. Eur. J. Inorg. Chem. 2014, 2014, 3989–3999. [Google Scholar] [CrossRef]
- Andrade, M.A.; Silva, R.A.L.; Santos, I.C.; Lopes, E.B.; Rabaça, S.; Pereira, L.C.J.; Coutinho, J.T.; Telo, J.P.; Rovira, C.; Almeida, M.; et al. Gold and nickel alkyl substituted bis-thiophenedithiolene complexes: Anionic and neutral forms. Inorg. Chem. Front. 2017, 4, 270–280. [Google Scholar] [CrossRef]
- Tenn, N.; Bellec, N.; Jeannin, O.; Piekara-Sady, L.; Auban-Senzier, P.; Íñiguez, J.; Canadell, E.; Lorcy, D. A single-component molecular metal based on a thiazole dithiolate gold complex. J. Am. Chem. Soc. 2009, 131, 16961–16967. [Google Scholar] [CrossRef] [PubMed]
- Filatre-Furcate, A.; Bellec, N.; Jeannin, O.; Auban-Senzier, P.; Fourmigué, M.; Íñiguez, J.; Canadell, E.; Brière, B.; Phuoc, V.T.; Lorcy, D. Single-component conductors: A sturdy electronic structure generated by bulky substituents. Inorg. Chem. 2016, 55, 6036–6046. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.P.N.; Figueira, M.J.; Belo, D.; Santos, I.C.; Ribeiro, B.; Lopes, E.B.; Henriques, R.T.; Gancedo, J.V.; Veciana, J.; Rovira, C. Transition metal bisdithiolene complexes based on extended ligands with fused tetrathiafulvalene and thiophene moieties: New single-component molecular metals. Chem. Eur. J. 2007, 13, 9841–9849. [Google Scholar] [CrossRef] [PubMed]
- Alves, H.; Neves, A.I.S.; Gouveia, W.; Silva, R.A.L.; Belo, D. Conducting films based on single-component molecular metals. Chem. Commun. 2015, 51, 13117–13119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narvor, N.L.; Robertson, N.; Weyland, T.; Kilburn, J.D.; Underhill, A.E.; Webster, M.; Svenstrup, N.; Becher, J. Synthesis, structure and properties of nickel complexes of 4,5-tetrathiafulvalene dithiolates: High conductivity in neutral dithiolate complexes. Chem. Commun. 1996, 0, 1363–1364. [Google Scholar] [CrossRef]
- Silva, R.A.L.; Vieira, B.J.C.; Andrade, M.A.; Santos, I.C.; Rabaça, S.; Belo, D.; Almeida, M. TTFs nonsymmetrically fused with alkylthiophenic moieties. Beilstein J. Org. Chem. 2015, 11, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.I.; Wang, S.; Fackler, J.P., Jr. Synthesis and structural characterization of the gold complex, [n-Bu4N]2[Au2(i-MNT)2] (i-MNT = 1,1-dicyanoethylene-2,2-dithiolate) and its oxidative-addition products [Ph4As]2[Au2(i-MNT)2Cl2], [n-Bu4N]2[Au2(i-MNT)2Br2], and [n-Bu4N][Au(i-MNT)2]. Spectral studies of the disproportionation of [n-Bu4N]2[Au2(i-MNT)2X2] (X = Cl−, Br−, I−) into [n-Bu4N][AuX2] and [n-Bu4N][Au(i-MNT)2]. Inorg. Chem. 1989, 28, 3579–3588. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. Aurophilic interactions as a subject of current research: An up-date. Chem. Soc. Rev. 2012, 41, 370–412. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.A.L. Thiophenic-TTF Derivatives and Thiophenic-Bisdithiolene Complexes for Magnetic and Conducting Material. Ph.D. Thesis, Degree in Chemistry-Instituto Superior Técnico, Lisbon, Portugal, 2017. [Google Scholar]
- Perrin, D.D.; Armarego, W.L.F. Purification of Laboratory Chemicals, 3rd ed.; Pergamon Press: Oxford, UK, 1988. [Google Scholar]
- Sheldrick, G.M. SADABS; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Bruker. SMART and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.; Giacovazzo, G.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL97, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for Windows—A version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Cryst. 1997, 30, 565. [Google Scholar] [CrossRef]
- Chaikin, P.M.; Kwak, J.F. Apparatus for thermopower measurements on organic conductors. Rev. Sci. Instrum. 1975, 46, 218–220. [Google Scholar] [CrossRef]
- Almeida, M.; Alcácer, L.; Oostra, S. Anisotropy of thermopower in N-methyl-N-ethylmorpholinium bistetracyanoquinodimethane, MEM(TCNQ)2, in the region of the high-temperature phase transitions. Phys. Rev. B 1984, 30, 2839–2844. [Google Scholar] [CrossRef]
- Lopes, E.B. INETI-Sacavém, Internal Report; Portugal, 1991.
- Huebner, R.P. Thermoelectric power of lattice vacancies in gold. Phys. Rev. 1964, 135, A1281–A1921. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–7 are available from the authors. |



| Complexes | Oxidation Process | σRT | Ea |
|---|---|---|---|
| (S/cm) | (meV) | ||
| (n-Bu4N)[Au(α-mtdt)2] [23] | − | 1.5 × 10−5 | 143 |
| [Au(α-mtdt)2] [23] | Iodine | 0.32 | 54 |
| [Ni(α-mtdt)2] [23] | Iodine | 4.2 | 20 |
| Air | 3.2 | 33 | |
| [Au(α-tdt)2] [17] | Iodine | 5 | <20 |
| [Au(dtdt)2] [17] | Iodine | 8 | <20 |
| [Ni(α-tdt)2] [17] | Iodine | 24 | <20 |
| Air | 2 | <20 | |
| [Ni(dtdt)2] [17] | Iodine | 200 | <20 |
| Air | 2.5 | <20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.A.L.; Vieira, B.J.C.; Andrade, M.M.; Santos, I.C.; Rabaça, S.; Lopes, E.B.; Coutinho, J.T.; Pereira, L.C.J.; Almeida, M.; Belo, D. Gold and Nickel Extended Thiophenic-TTF Bisdithiolene Complexes. Molecules 2018, 23, 424. https://doi.org/10.3390/molecules23020424
Silva RAL, Vieira BJC, Andrade MM, Santos IC, Rabaça S, Lopes EB, Coutinho JT, Pereira LCJ, Almeida M, Belo D. Gold and Nickel Extended Thiophenic-TTF Bisdithiolene Complexes. Molecules. 2018; 23(2):424. https://doi.org/10.3390/molecules23020424
Chicago/Turabian StyleSilva, Rafaela A. L., Bruno J. C. Vieira, Marta M. Andrade, Isabel C. Santos, Sandra Rabaça, Elsa B. Lopes, Joana T. Coutinho, Laura C. J. Pereira, Manuel Almeida, and Dulce Belo. 2018. "Gold and Nickel Extended Thiophenic-TTF Bisdithiolene Complexes" Molecules 23, no. 2: 424. https://doi.org/10.3390/molecules23020424