Novel Conjugated Polymers Prepared by Direct (Hetero) arylation: An Eco-Friendly Tool for Organic Electronics
Abstract
1. Introduction
2. Results and Discussion
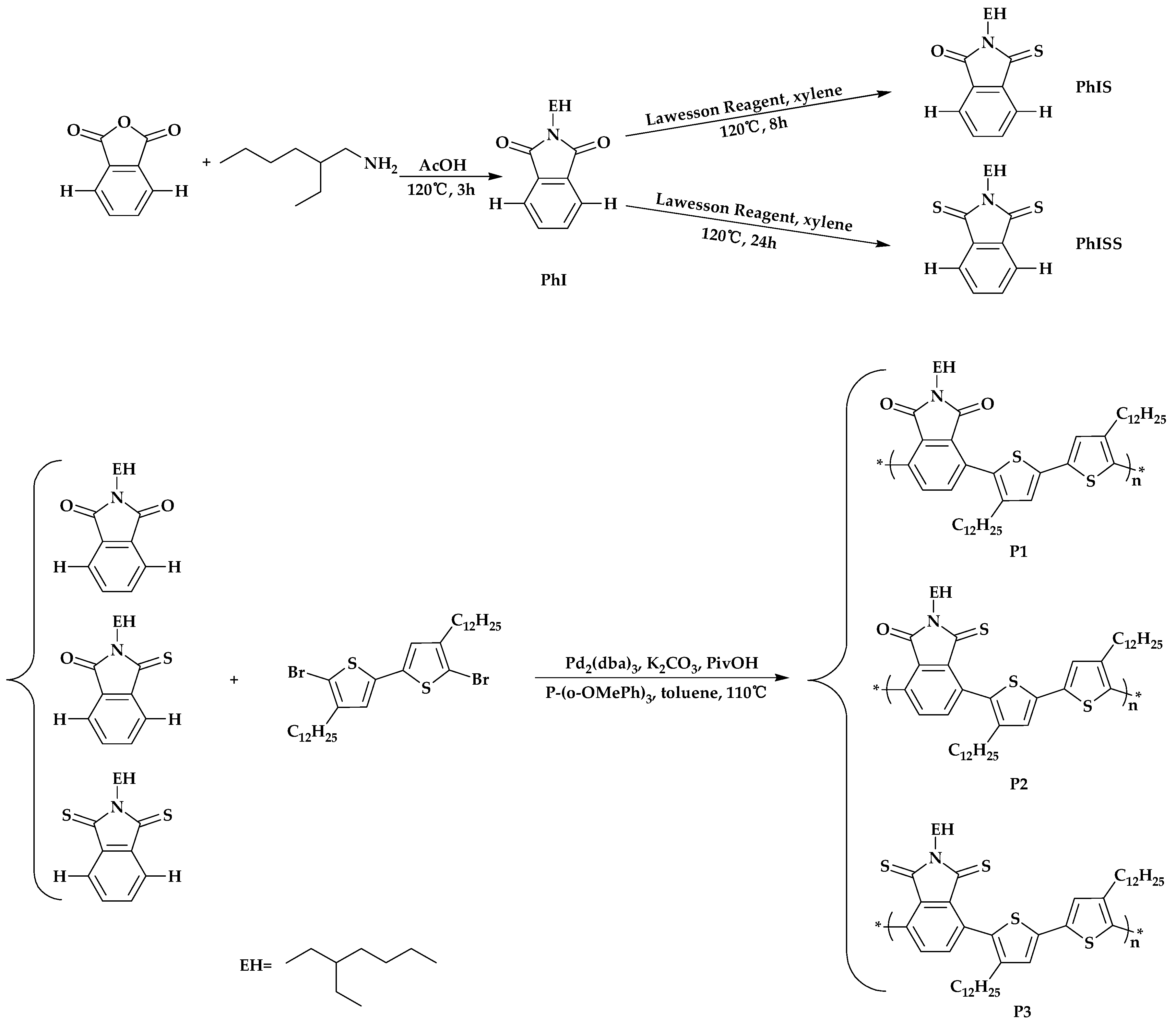
2.1. Synthesis of the Polymers
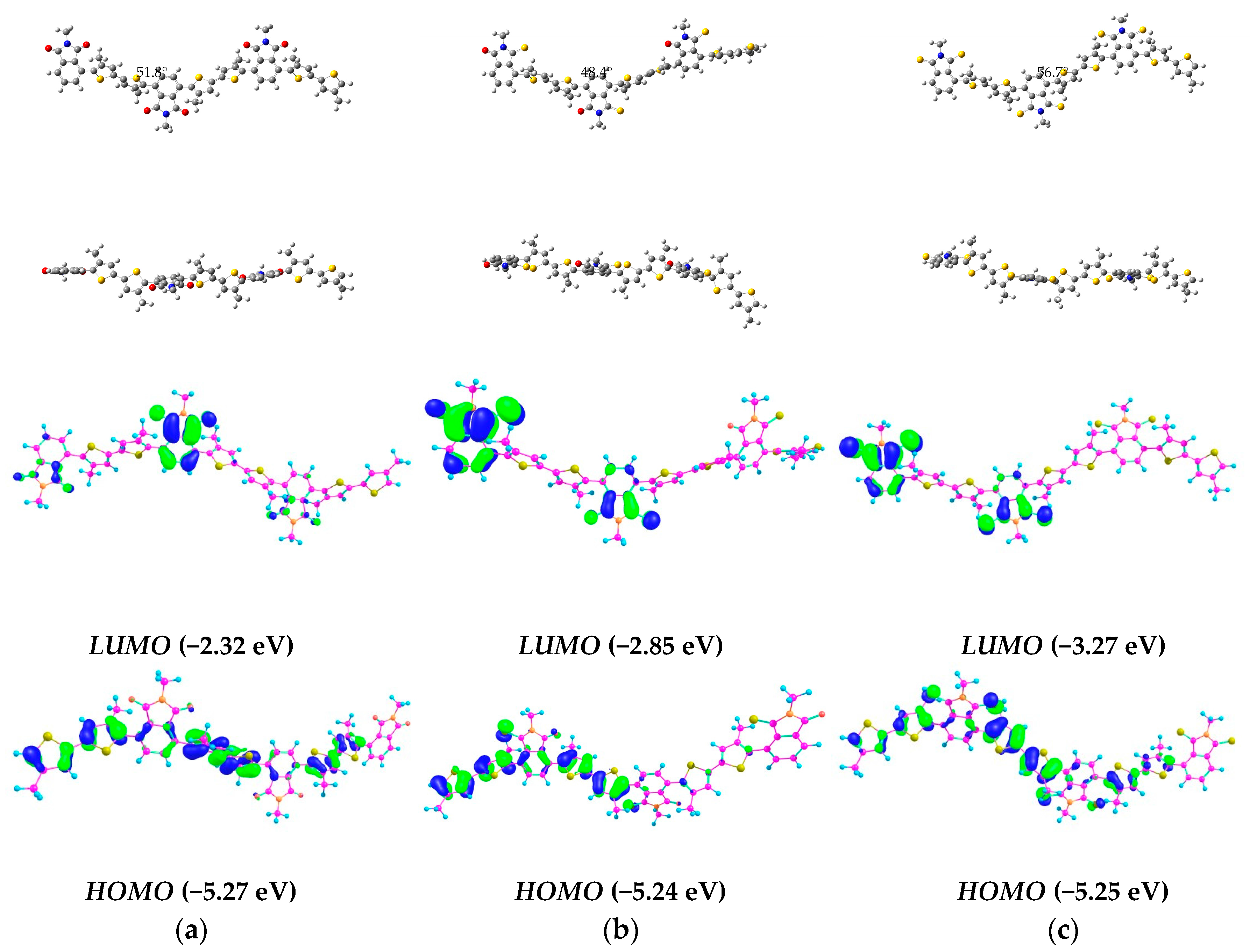
2.2. Computational Analysis
2.3. Optical Properties of the Polymers
2.4. Electrochemical Properties of the Polymers
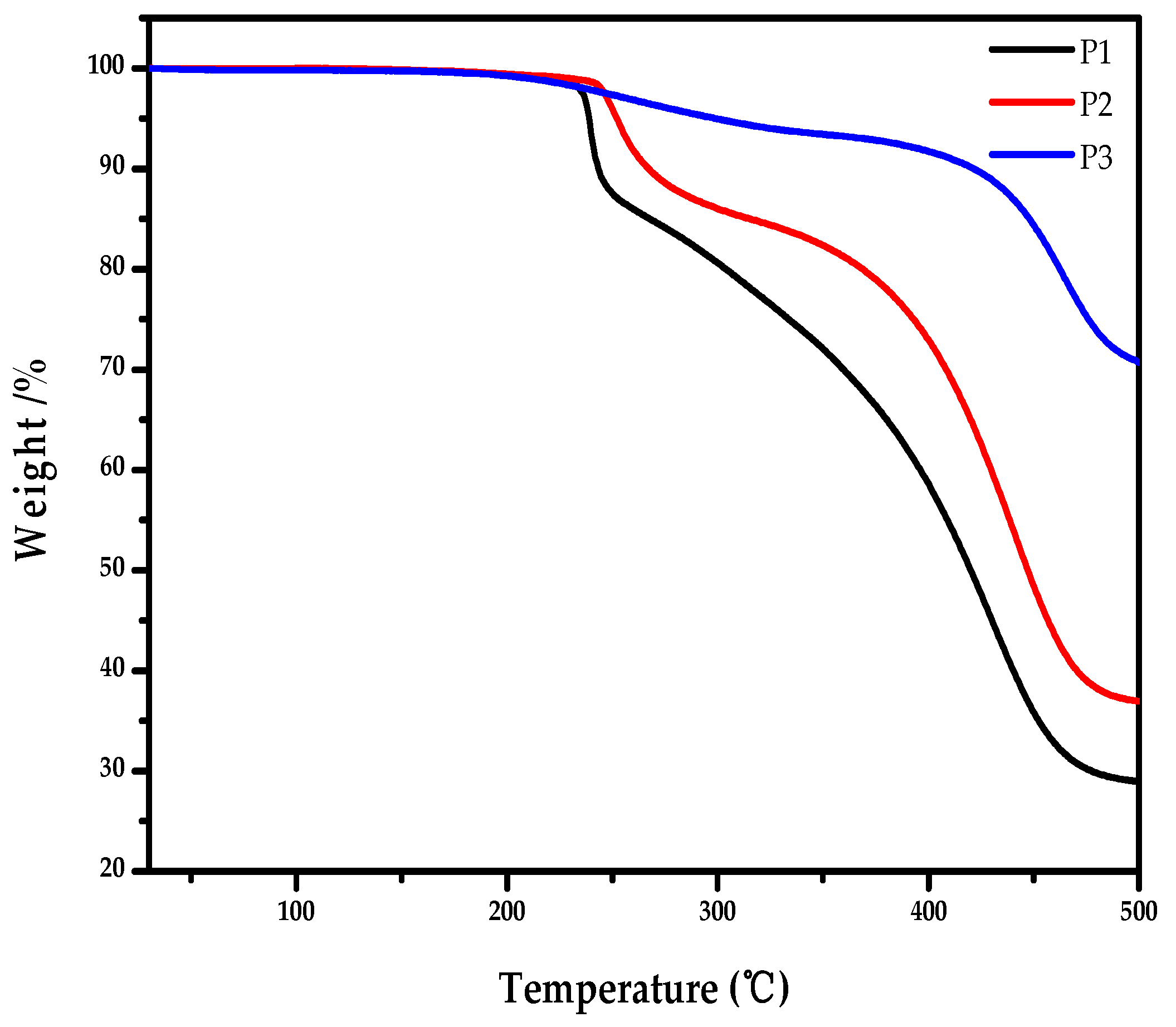
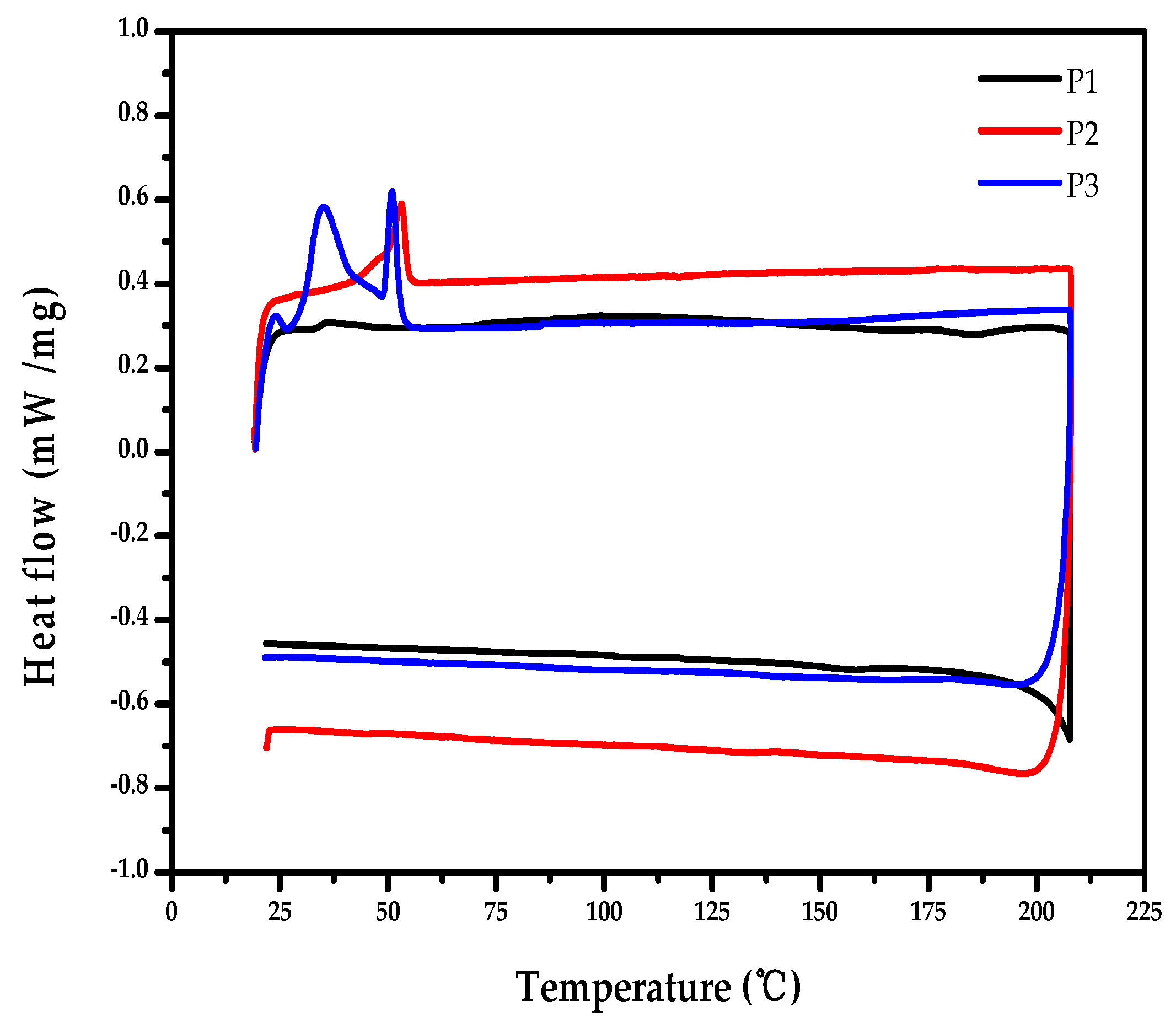
2.5. Thermal Properties of the Polymers
3. Experimental Section
3.1. Materials and Methods
3.2. General Characterization Methods
3.3. Synthesis of the Monomers
3.3.1. N-(2-Ethylhexyl) Phthalimide (PhI)
3.3.2. N-(2-Ethylhexyl)-thione Phthalimide (PhIS)
3.3.3. N-(2-Ethylhexyl)-dithione Phthalimide (PhISS)
3.4. Optimization of Reaction Conditions for DHAP
3.4.1. DHAP of PhI and Dibromobithiophene (P1)
3.4.2. DHAP of PhIS and Dibromobithiophene (P2)
3.4.3. DHAP of PhISS and Dibromobithiophene (P3)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zampetti, A.; Minotto, A.; Squeo, B.M.; Gregoriou, V.G.; Allard, S.; Scherf, U.; Chochos, C.L.; Cacialli, F. Highly Efficient Solid-State Near-infrared Organic Light-Emitting Diodes incorporating A-D-A Dyes based on α,β-unsubstituted “BODIPY” Moieties. Sci. Rep. 2017, 7, 1611. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, M.; Zhang, X.; Lei, Z.; Zhang, X.; Hao, L.; Fan, Q.; Lai, W.; Huang, W. Multilayered phosphorescent polymer light-emitting diodes using a solution-processed n-doped electron transport layer. J. Lumin. 2017, 186, 87–92. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, Z.; Chen, H.; Zheng, L.; Li, X.; Chen, J.; Sun, Y.; Liu, F.; Guo, Y.; Liu, Y. Regioregular Bis-Pyridal[2,1,3]thiadiazole-Based Semiconducting Polymer for High-Performance Ambipolar Transistors. J. Am. Chem. Soc. 2017, 139, 17735–17738. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yin, Z.; Chen, H.; Zheng, L.; Zhu, C.; Zhang, L.; Tan, S.; Wang, H.; Guo, Y.; Tang, Q.; et al. High-Performance, Air-Stable Field-Effect Transistors Based on Heteroatom-Substituted Naphthalenediimide-Benzothiadiazole Copolymers Exhibiting Ultrahigh Electron Mobility up to 8.5 cm V−1 s−1. Adv. Mater. 2017, 29, 1602410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Xiao, C.; Wu, Y.; Li, C.; Ji, Y.; Li, L.; Hu, W.; Wang, Z.; Ma, W.; Li, W. Effect of Fluorination on Molecular Orientation of Conjugated Polymers in High Performance Field-Effect Transistors. Macromolecules 2016, 49, 6431–6438. [Google Scholar] [CrossRef]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Y.; Yang, G.; Jiang, K.; Lin, H.; Ade, H.; Ma, W.; Yan, H. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 2016, 1, 15027. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Zhang, Z.G.; Bai, H.; Li, Y.; Zhu, D.; Zhan, X. An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Kozma, E.; Catellani, M. Perylene diimides based materials for organic solar cells. Dyes Pigment. 2013, 98, 160–179. [Google Scholar] [CrossRef]
- Wang, Y.; Nakano, M.; Michinobu, T.; Kiyota, Y.; Mori, T.; Takimiya, K. Naphthodithiophenediimide–Benzobisthiadiazole-Based Polymers: Versatile n-Type Materials for Field-Effect Transistors and Thermoelectric Devices. Macromolecules 2017, 50, 857–864. [Google Scholar] [CrossRef]
- Subramaniyan, S.; Earmme, T.; Murari, N.M.; Jenekhe, S.A. Naphthobisthiazole diimide-based n-type polymer semiconductors: Synthesis, π-stacking, field-effect charge transport, and all-polymer solar cells. Polym. Chem. 2014, 5, 5707–5715. [Google Scholar] [CrossRef]
- Lai, W.; Li, C.; Zhang, J.; Yang, F.; Colberts, F.J.M.; Guo, B.; Wang, Q.M.; Li, M.; Zhang, A.; Janssen, R.A.J.; et al. Diketopyrrolopyrrole-Based Conjugated Polymers with Perylene Bisimide Side Chains for Single-Component Organic Solar Cells. Chem. Mater. 2017, 29, 7073–7077. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Y.; Zhang, A.; Li, C.; Tang, Z.; Ma, W.; Wu, Y.; Li, W. Diketopyrrolopyrrole Polymers with Thienyl and Thiazolyl Linkers for Application in Field-Effect Transistors and Polymer Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 30328–30335. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ortiz, R.P.; Zheng, Y.; Kim, M.G.; Zhang, S.; Hu, Y.; Lu, G.; Facchetti, A.; Marks, T.J. Thieno[3,4-c]pyrrole-4,6-dione-based polymer semiconductors: Toward high-performance, air-stable organic thin-film transistors. J. Am. Chem. Soc. 2011, 133, 13685–13697. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Najari, A.; Berrouard, P.; Beaupre, S.; Aïch, B.R.; Tao, Y.; Leclerc, M. A Thieno[3,4-c]pyrrole-4,6-dione-Based Copolymer for Efficient Solar Cells. J. Am. Chem. Soc. 2010, 132, 5330–5331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wen, Y.; Zhou, W.; Guo, Y.; Ma, L.; Zhao, X.; Zhao, Z.; Barlow, S.; Marder, S.R.; Liu, Y.; et al. Perylene diimide copolymers with dithienothiophene and dithienopyrrole: Use in n-channel and ambipolar field-effect transistors. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1550–1558. [Google Scholar] [CrossRef]
- Nakano, K.; Nakano, M.; Xiao, B.; Zhou, E.; Suzuki, K.; Osaka, I.; Takimiya, K.; Tajima, K. Naphthodithiophene Diimide-Based Copolymers: Ambipolar Semiconductors in Field-Effect Transistors and Electron Acceptors with Near-Infrared Response in Polymer Blend Solar Cells. Macromolecules 2016, 49, 1752–1760. [Google Scholar] [CrossRef]
- Chen, H.; Guo, Y.; Mao, Z.; Yu, G.; Huang, J.; Zhao, Y.; Liu, Y. Naphthalenediimide-Based Copolymers Incorporating Vinyl-Linkages for High-Performance Ambipolar Field-Effect Transistors and Complementary-Like Inverters under Air. Chem. Mater. 2013, 25, 3589–3596. [Google Scholar] [CrossRef]
- Yue, W.; Nikolka, M.; Xiao, M.; Sadhanala, A.; Nielsen, C.B.; White, A.J.P.; Chen, H.-Y.; Onwubiko, A.; Sirringhaus, H.; McCulloch, I. Azaisoindigo conjugated polymers for high performance n-type and ambipolar thin film transistor applications. J. Mater. Chem. C 2016, 4, 9704–9710. [Google Scholar] [CrossRef]
- Brandt, R.G.; Yue, W.; Andersen, T.R.; Larsen-Olsen, T.T.; Hinge, M.; Bundgaard, E.; Krebs, F.C.; Yu, D. An isoindigo containing donor–acceptor polymer: Synthesis and photovoltaic properties of all-solution-processed ITO- and vacuum-free large area roll-coated single junction and tandem solar cells. J. Mater. Chem. C 2015, 3, 1633–1639. [Google Scholar] [CrossRef]
- Chen, C.-M.; Sharma, S.; Li, Y.-L.; Lee, J.-J.; Chen, S.-A. Thienoisoindigo-based copolymer with fused thieno[3,2-b]thiophene as a donor in thin film transistor applications with high performance. J. Mater. Chem. C 2015, 3, 33–36. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, F.; Ji, Y.; Wu, Y.; Zhang, A.; Li, C.; Li, W. A perylene bisimide derivative with a LUMO level of −4.56 eV for non-fullerene solar cells. J. Mater. Chem. C 2016, 4, 4134–4137. [Google Scholar] [CrossRef]
- Kang, H.; An, S.Y.; Walker, B.; Song, S.; Kim, T.; Kim, J.Y.; Yang, C. Thienoisoindigo (TIIG)-based small molecules for the understanding of structure–property–device performance correlations. J. Mater. Chem. A 2015, 3, 9899–9908. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, K.; Zhou, W.; Li, Z.; Chen, Z.; Xu, J.; Yan, D.; Han, Y.; Wong, M.S.; Li, F.; et al. Naphthodithieno[3,2-b]thiophene-based donor-acceptor copolymers: Synthesis, characterization, and their photovoltaic and charge transport properties. Dyes Pigment. 2016, 131, 1–8. [Google Scholar] [CrossRef]
- Estrada, L.A.; Deininger, J.J.; Kamenov, G.D.; Reynolds, J.R. Direct (Hetero)arylation Polymerization: An Effective Route to 3,4-Propylenedioxythiophene-Based Polymers with Low Residual Metal Content. ACS Macro Lett. 2013, 2, 869–873. [Google Scholar] [CrossRef]
- Shao, J.; Wang, G.; Wang, K.; Yang, C.; Wang, M. Direct arylation polycondensation for efficient synthesis of narrow-bandgap alternating D–A copolymers consisting of naphthalene diimide as an acceptor. Polym. Chem. 2015, 6, 6836–6844. [Google Scholar] [CrossRef]
- Kuwabara, J.; Nohara, Y.; Choi, S.J.; Fujinami, Y.; Lu, W.; Yoshimura, K.; Oguma, J.; Suenobu, K.; Kanbara, T. Direct arylation polycondensation for the synthesis of bithiophene-based alternating copolymers. Polym. Chem. 2013, 4, 947–953. [Google Scholar] [CrossRef]
- Kuramochi, M.; Kuwabara, J.; Lu, W.; Kanbara, T. Direct Arylation Polycondensation of Bithiazole Derivatives with Various Acceptors. Macromolecules 2014, 47, 7378–7385. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Fukuzawa, H.; Fujita, K.; Mori, H. Direct arylation synthesis of thienoisoindigo-based low-band-gap polymer from asymmetric donor-acceptor monomer. J. Polym. Sci. Part A Polym. Chem. 2017. [Google Scholar] [CrossRef]
- Pouliot, J.R.; Grenier, F.; Blaskovits, J.T.; Beaupre, S.; Leclerc, M. Direct (Hetero)arylation Polymerization: Simplicity for Conjugated Polymer Synthesis. Chem. Rev. 2016, 116, 14225–14274. [Google Scholar] [CrossRef] [PubMed]
- Bura, T.; Blaskovits, J.T.; Leclerc, M. Direct (Hetero)arylation Polymerization: Trends and Perspectives. J. Am. Chem. Soc. 2016, 138, 10056–10071. [Google Scholar] [CrossRef] [PubMed]
- Bohra, H.; Wang, M. Direct C–H arylation: A “Greener” approach towards facile synthesis of organic semiconducting molecules and polymers. J. Mater. Chem. A 2017, 5, 11550–11571. [Google Scholar] [CrossRef]
- Yu, S.; Liu, F.; Yu, J.; Zhang, S.; Cabanetos, C.; Gao, Y.; Huang, W. Eco-friendly direct (hetero)-arylation polymerization: Scope and limitation. J. Mater. Chem. C 2017, 5, 29–40. [Google Scholar] [CrossRef]
- Punzi, A.; Nicoletta, F.; Marzano, G.; Fortuna, C.G.; Dagar, J.; Brown, T.M.; Farinola, G.M. Synthetic Routes to TEG-Substituted Diketopyrrolopyrrole-Based Low Band-Gap Polymers. Eur. J. Org. Chem. 2016, 19, 3233–3242. [Google Scholar] [CrossRef]
- Punzi, A.; Coppi, D.I.; Matera, S.; Capozzi, M.A.M.; Operamolla, A.; Ragni, R.; Babudri, F.; Farinola, G.M. Pd-Catalyzed Thiophene-Aryl Coupling Reaction via C–H Bond Activation in Deep Eutectic Solvents. Org. Lett. 2017, 19, 4754–4757. [Google Scholar] [CrossRef] [PubMed]
- Marzano, G.; Ciasca, C.V.; Babudri, F.; Bianchi, G.; Pellegrino, A.; Po, R.; Farinola, G.M. Organometallic Approaches to Conjugated Polymers for Plastic Solar Cells: From Laboratory Synthesis to Industrial Production. Eur. J. Org. Chem. 2014, 30, 6583–6614. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Huang, J.; Gao, D.; Wei, C.; Lin, Z.; Wang, L.; Yu, G. Fluorinated Dithienylethene–Naphthalenediimide Copolymers for High-Mobility n-Channel Field-Effect Transistors. Macromolecules 2017, 50, 6098–6107. [Google Scholar] [CrossRef]
- Jung, J.W.; Jo, J.W.; Chueh, C.C.; Liu, F.; Jo, W.H.; Russell, T.P.; Jen, A.K. Fluoro-Substituted n-Type Conjugated Polymers for Additive-Free All-Polymer Bulk Heterojunction Solar Cells with High Power Conversion Efficiency of 6.71. Adv. Mater. 2015, 27, 3310–3317. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, S.; Gendron, D.; Bérubé, N.; Grenier, F.; Leclerc, M.; Côté, M. Thiocarbonyl Substitution in 1,4-Dithioketopyrrolopyrrole and Thienopyrroledithione Derivatives: An Experimental and Theoretical Study. J. Phys. Chem. C 2014, 118, 3953–3959. [Google Scholar] [CrossRef]
- Ie, Y.; Jinnai, S.; Nitani, M.; Aso, Y. Arenedithiocarboxyimide-containing extended π-conjugated systems with high electron affinity. J. Mater. Chem. C 2013, 1, 5373–5380. [Google Scholar] [CrossRef]
- Li, Z.; Tang, X.; Jiang, Y.; Zuo, M.; Wang, Y.; Chen, W.; Zeng, X.; Sun, Y.; Lin, L. Tandem thionation of biomass derived levulinic acid with Lawesson’s reagent. Green Chem. 2016, 18, 2971–2975. [Google Scholar] [CrossRef]
- Pollum, M.; Jockusch, S.; Crespo-Hernandez, C.E. Increase in the photoreactivity of uracil derivatives by doubling thionation. Phys. Chem. Chem. Phys. 2015, 17, 27851–27861. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Nakano, M.; Takimiya, K.; Zhang, Q. Selective thionation of naphtho[2,3-b]thiophene diimide: Tuning of the optoelectronic properties and packing structure. Org. Chem. Front. 2017, 4, 704–710. [Google Scholar] [CrossRef]
- Llewellyn, B.A.; Davies, E.S.; Pfeiffer, C.R.; Cooper, M.; Lewis, W.; Champness, N.R. Thionated perylene diimides with intense absorbance in the near-IR. Chem. Commun. 2016, 52, 2099–2102. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.F.; Huang, S.H.; Chiu, Y.P.; Chen, B.H.; Shih, Y.W.; Chang, Y.C.; Yao, J.Y.; Lee, Y.J.; Kuo, M.Y. Pyromellitic dithioimides: Thionation improves air-stability and electron mobility of N-type organic field-effect transistors. Chem. Commun. 2015, 51, 13772–13775. [Google Scholar] [CrossRef] [PubMed]
- Tilley, A.J.; Guo, C.; Miltenburg, M.B.; Schon, T.B.; Yan, H.; Li, Y.; Seferos, D.S. Thionation Enhances the Electron Mobility of Perylene Diimide for High Performance n-Channel Organic Field Effect Transistors. Adv. Funct. Mater. 2015, 25, 3321–3329. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Wang, Y.; Qin, X.; Wu, J.; Hou, J. Fluorinated and non-fluorinated conjugated polymers showing different photovoltaic properties in polymer solar cells with PFNBr interlayers. Org. Electron. 2016, 28, 178–183. [Google Scholar] [CrossRef]
- Wang, M.; Ford, M.; Phan, H.; Coughlin, J.; Nguyen, T.-Q.; Bazan, G.C. Fluorine Substitution Influence on Benzo[2,1,3]thiadiazole Based Polymers for Field-Effect Transistor Applications. Chem. Commun. 2016, 52, 3207–3210. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shi, K.; Cai, K.; Guo, Y.; Yang, X.; Wang, J.-Y.; Pei, J.; Zhao, D. Syntheses of polycyclic aromatic diimides via intramolecular cyclization of maleic acid derivatives. New J. Chem. 2016, 40, 113–121. [Google Scholar] [CrossRef]
- Psutka, K.M.; Maly, K.E. Synthesis and characterization of novel dibenz[a,c]anthracenedicarboxythioimides: The effect of thionation on self-assembly. RSC Adv. 2016, 6, 78784–78790. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J.; Long, G.; Liu, Y.; Zhang, Q. From Non-Detectable to Decent: Replacement of Oxygen with Sulfur in Naphthalene Diimide Boosts Electron Transport in Organic Thin-Film Transistors (OTFT). J. Mater. Chem. C 2015, 3, 8219–8224. [Google Scholar] [CrossRef]
- Kozycz, L.M.; Guo, C.; Manion, J.G.; Tilley, A.J.; Lough, A.J.; Li, Y.; Seferos, D.S. Enhanced electron mobility in crystalline thionated naphthalene diimides. J. Mater. Chem. C 2015, 3, 11505–11515. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Ding, G.; Guo, J.; Lu, H.; Qiu, L.; Ma, W. Synthesis and photovoltaic application of low-bandgap conjugated polymers by incorporating highly electron-deficient pyrrolo[3,4-d]pyridazine-5,7-dione units. Polymer 2016, 93, 213–220. [Google Scholar] [CrossRef]
- Kim, J.; Yeo, J.-S.; Jeong, H.-G.; Yun, J.-M.; Kim, Y.-A.; Kim, D.-Y. A thienylenevinylene-phthalimide copolymer based polymer solar cell with high open circuit voltage: Effect of additive concentration on the open circuit voltage. Sol. Energy Mater. Sol. Cells 2014, 125, 253–260. [Google Scholar] [CrossRef]
- Makhseed, S.; Ibrahim, F.; Samuel, J. Phthalimide based polymers of intrinsic microporosity. Polymer 2012, 53, 2964–2972. [Google Scholar] [CrossRef]
- Guo, X.; Kim, F.S.; Jenekhe, S.A.; Watson, M.D. Phthalimide-Based Polymers for High Performance Organic Thin-Film Transistors. J. Am. Chem. Soc. 2009, 131, 7206–7207. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Guo, X.; Kim, F.S.; Ren, G.; Watson, M.D.; Jenekhe, S.A. Efficient solar cells based on a new phthalimide-based donor–acceptor copolymer semiconductor: Morphology, charge-transport, and photovoltaic properties. J. Mater. Chem. 2009, 19, 5303–5310. [Google Scholar] [CrossRef]
- He, M.; Soulé, J.-F.; Doucet, H. Synthesis of (Poly)fluorobiphenyls through Metal-catalyzed C–H Bond Activation/Arylation of (Poly)fluorobenzene Derivatives. ChemCatChem 2014, 6, 1824–1859. [Google Scholar] [CrossRef]
- Asaumi, T.; Matsuo, T.; Fukuyama, T.; Ie, Y.; Kakiuchi, F.; Chatani, N. Ruthenium- and Rhodium-Catalyzed Direct Carbonylation of the Ortho C–H Bond in the Benzene Ring of N-Arylpyrazoles. J. Org. Chem. 2004, 69, 4433–4440. [Google Scholar] [CrossRef] [PubMed]
- Ie, Y.; Chatani, N.; Ogo, T.; Marshall, D.R.; Fukuyama, T.; Kakiuchi, F.; Murai, S. Direct Carbonylation at a C–H Bond in the Benzene Ring of 2-Phenyloxazolines Catalyzed by Ru3(CO)12. Scope, Limitations, and Mechanistic Aspects. J. Org. Chem. 2000, 65, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Zhang, M.; Zhao, G. The promotion effects of thionation and isomerization on charge carrier mobility in naphthalene diimide crystals. Phys. Chem. Chem. Phys. 2017, 19, 28175–28181. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Yokoshima, S.; Fukuyama, T. Synthesis of Cardiopetaline via a Wagner-Meerwein Rearrangement without Preactivation of the Pivotal Hydroxy Group. Org. Lett. 2017, 19, 5833–5835. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Hernandez, J.G.; Bolm, C. Mechanochemical Ruthenium-Catalyzed Hydroarylations of Alkynes under Ball-Milling Conditions. Org. Lett. 2017, 19, 6284–6287. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.T.; Ha, H.; Kang, G.; Kim, O.S.; Ryu, H.; Biswas, A.K.; Lim, S.M.; Baik, M.H.; Joo, J.M. Ligand-controlled Regiodivergent C–H Alkenylation of Pyrazoles and its Application to the Synthesis of Indazoles. Angew. Chem. Int. Ed. 2017, 56, 16262–16266. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| Polymer | Mn (kDa) | PDI | Td (°C) | λmax (nm) | λonset (nm) | (eV) | EHOMO (eV) | ELUMO (eV) |
|---|---|---|---|---|---|---|---|---|
| P1 | 12.2 | 1.66 | 239 | 322 | 459 | 2.70 | −5.68 | −2.98 |
| P2 | 9.4 | 2.87 | 256 | 328 | 468 | 2.65 | −5.93 | −3.28 |
| P3 | 11.5 | 2.01 | 298 | 336 | 484 | 2.56 | −5.73 | −3.17 |
| Entry | Pd Catalyst | Base | Ligand | Mn b (kDa) | PDI b | Yield c (%) |
|---|---|---|---|---|---|---|
| 1 | Pd(PPh3)4 | Cs2CO3 | P(o-OMePh)3 | 7.8 | 3.89 | 27.6 |
| 2 | Pd(OAc)2 | Cs2CO3 | P(o-OMePh)3 | 5.7 | 3.64 | 19.7 |
| 3 | Pd2(dba)3 | Cs2CO3 | P(o-OMePh)3 | 11.0 | 2.09 | 49.6 |
| 4 | Pd(PPh3)2Cl2 | Cs2CO3 | P(o-OMePh)3 | 8.9 | 2.47 | 22.2 |
| 5 | Pd2(dba)3 | K2CO3 | P(o-OMePh)3 | 12.2 | 1.66 | 64.1 |
| 6 | Pd2(dba)3 | K2CO3 | P(o-tol)3 | 10.1 | 1.92 | 52.4 |
| 7 | Pd2(dba)3 | K2CO3 | PCy3·HBF4 | 8.6 | 3.49 | 26.1 |
| 8 | Pd2(dba)3 | KOAc | P(o-OMePh)3 | 9.1 | 2.38 | 39.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Zhang, Y.; Wang, H.; Zhang, S. Novel Conjugated Polymers Prepared by Direct (Hetero) arylation: An Eco-Friendly Tool for Organic Electronics. Molecules 2018, 23, 408. https://doi.org/10.3390/molecules23020408
Liu F, Zhang Y, Wang H, Zhang S. Novel Conjugated Polymers Prepared by Direct (Hetero) arylation: An Eco-Friendly Tool for Organic Electronics. Molecules. 2018; 23(2):408. https://doi.org/10.3390/molecules23020408
Chicago/Turabian StyleLiu, Fuchuan, Yangqian Zhang, Hang Wang, and Shiming Zhang. 2018. "Novel Conjugated Polymers Prepared by Direct (Hetero) arylation: An Eco-Friendly Tool for Organic Electronics" Molecules 23, no. 2: 408. https://doi.org/10.3390/molecules23020408
APA StyleLiu, F., Zhang, Y., Wang, H., & Zhang, S. (2018). Novel Conjugated Polymers Prepared by Direct (Hetero) arylation: An Eco-Friendly Tool for Organic Electronics. Molecules, 23(2), 408. https://doi.org/10.3390/molecules23020408






