Melatonin: A Molecule for Reducing Breast Cancer Risk
Abstract
:1. Introduction
1.1. Breast Cancer (BC) Risk
1.2. Melatonin
1.3. Objectives
2. Melatonin as a Possible Adjuvant Therapy When the Reduction of BC Risk is Based on Treatment with Antiestrogenic Drugs
2.1. The Role of Estrogens in BC
2.2. Antiestrogenic Drugs
2.3. Antiestrogenic Properties of Melatonin
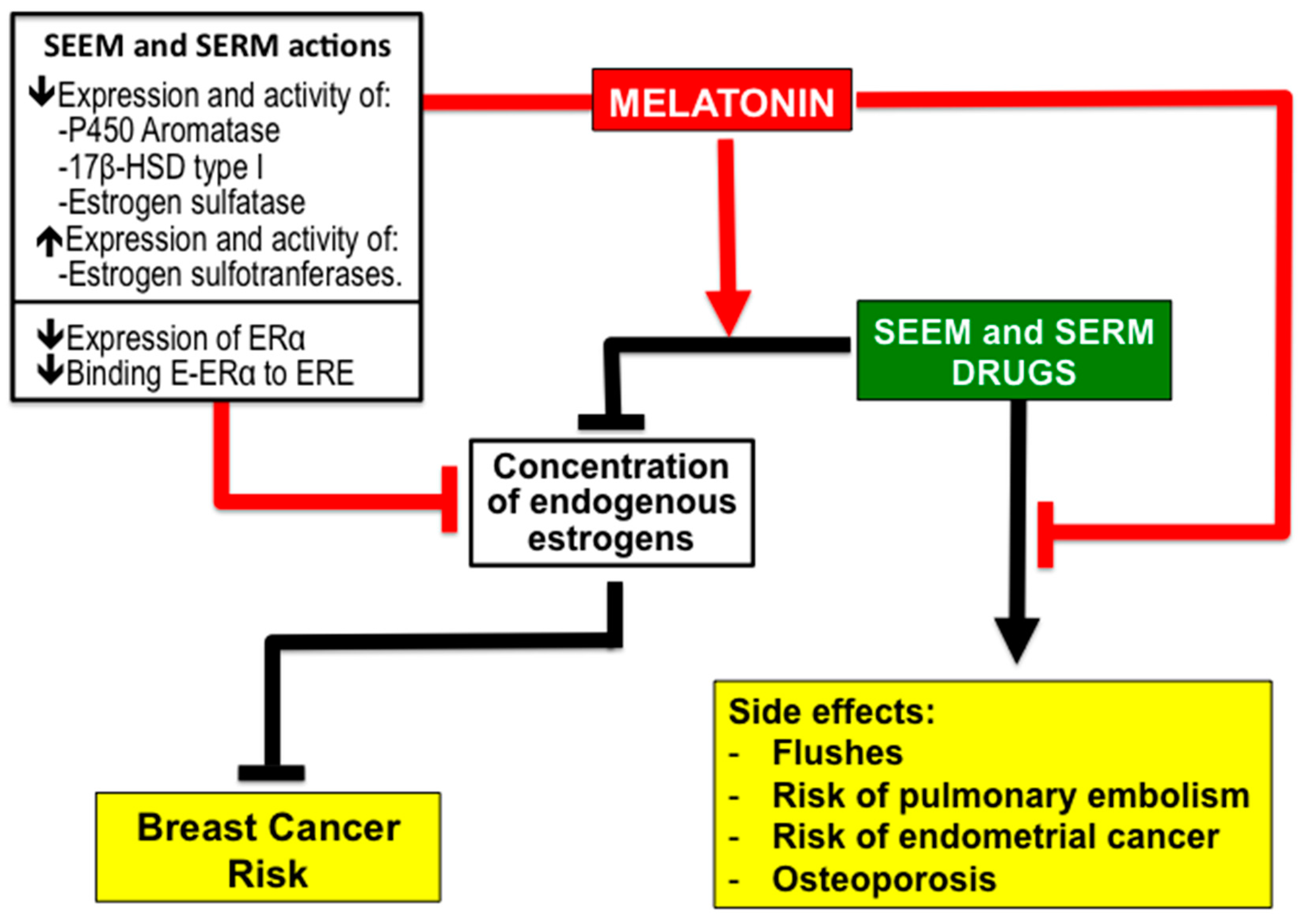
2.4. Melatonin Potentiates the Efficiency of SEEM and SERM Drugs and Reduce Their Side Effects
3. Melatonin for Reduction of BC Risk due to Environmental Factors
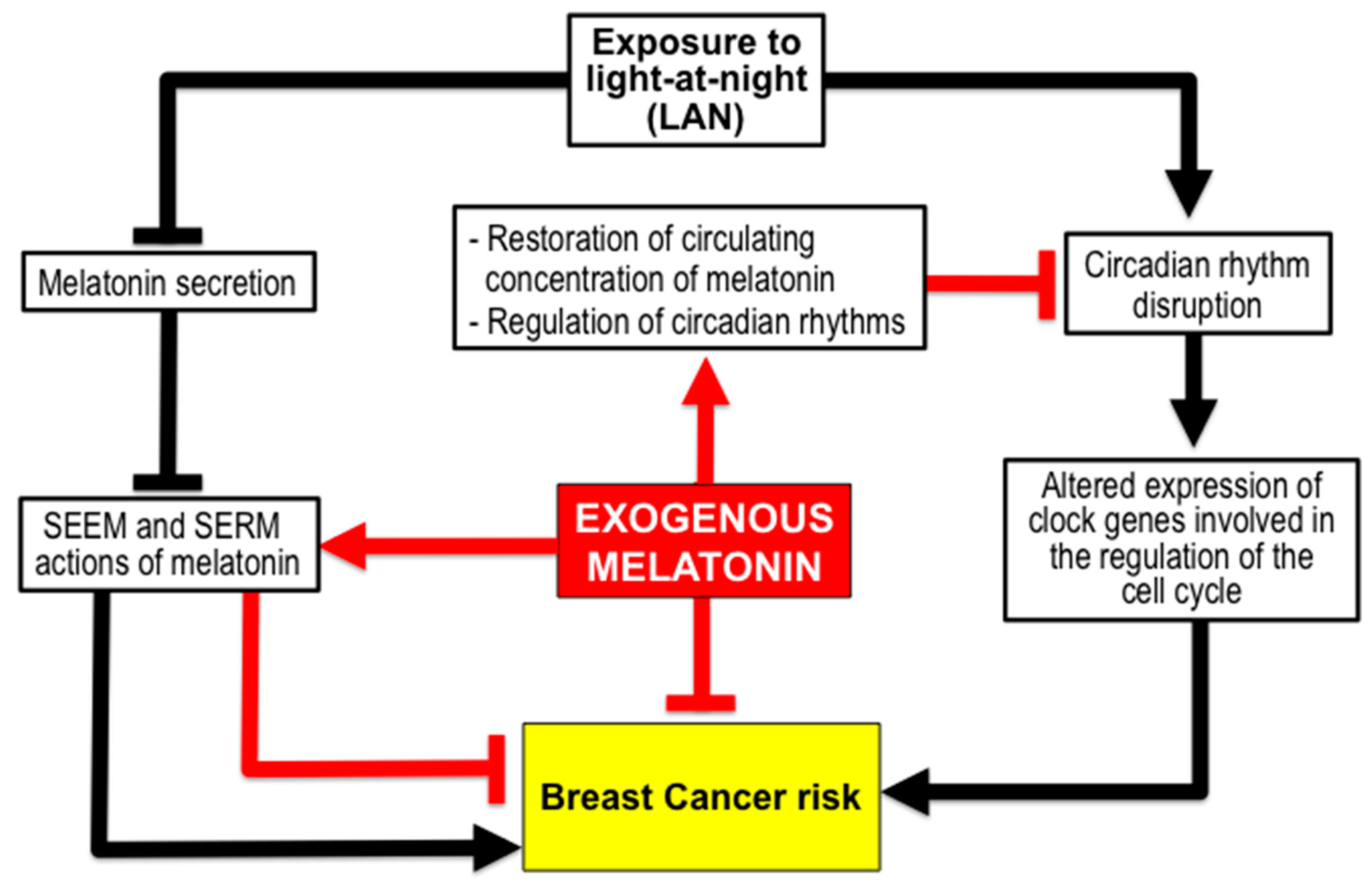
3.1. Melatonin Reduces BC Risk from Exposure to Light-at-Night (LAN) Producing Chronodisruption. The Shift Work
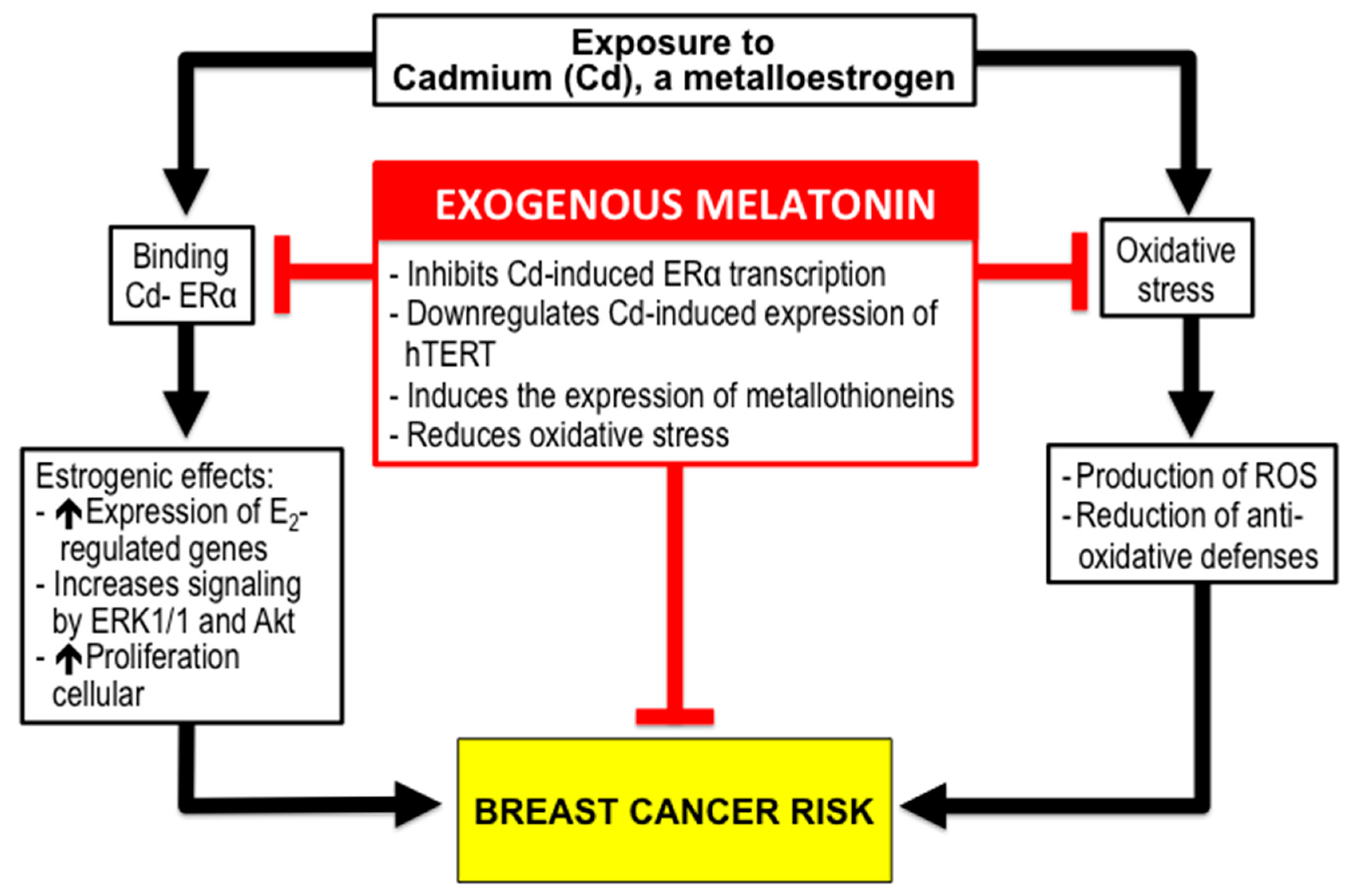
3.2. Melatonin and BC Risk due to Exposure to Xenoestrogens like Cadmium (Cd).
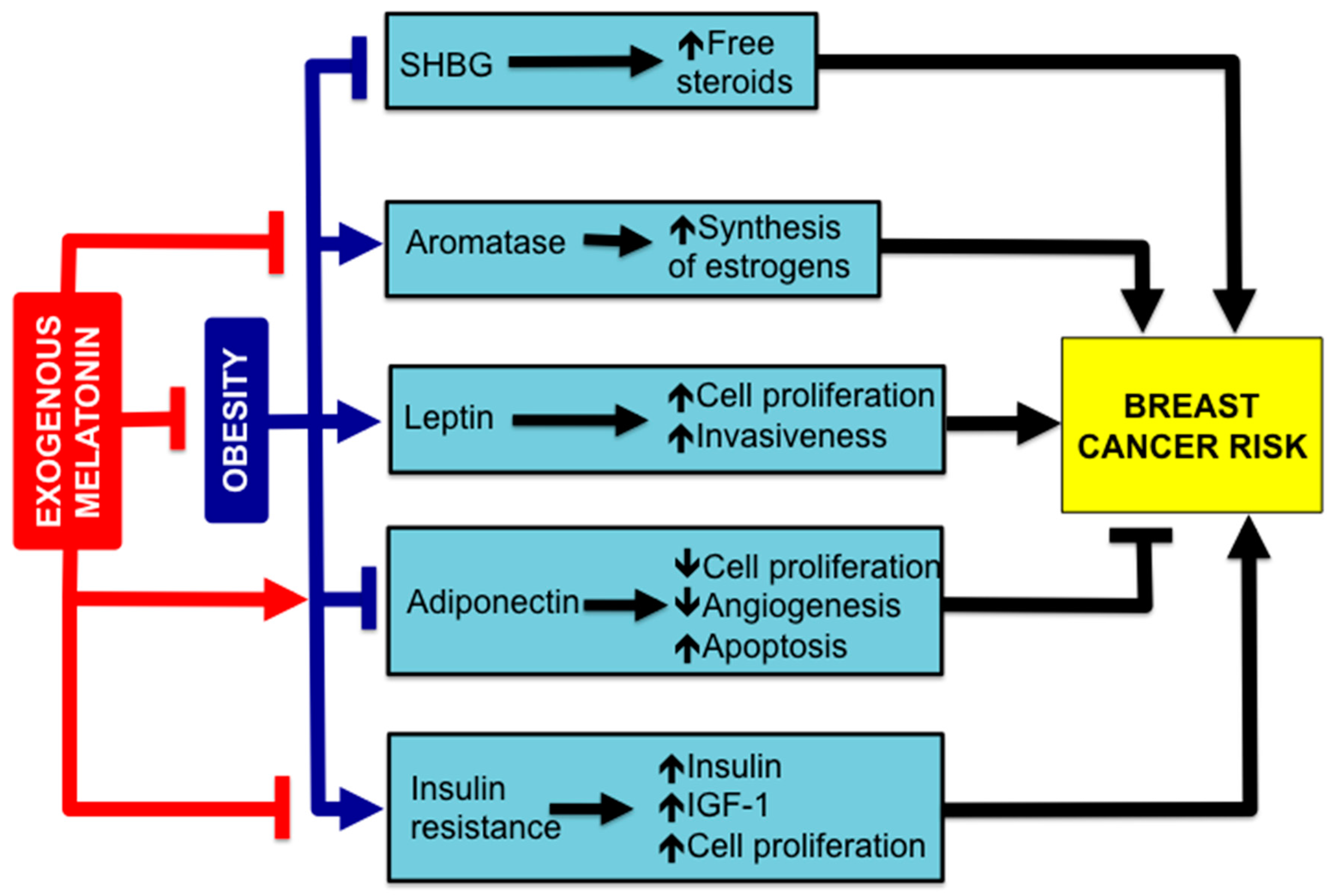
4. Melatonin, Obesity and BC Risk
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2017; American Cancer Society: Atlanta, GA, USA, 2017. [Google Scholar]
- PDQ® Screening and Prevention Editorial Board. PDQ Breast Cancer Prevention; National Cancer Institute: Bethesda, MD, USA, 2017. Available online: https.//www.cancer.gov/types/breast/hp/breast-prevention-pdq (accessed on 20 December 2017).
- Cuzick, J. Prevention therapy for cancer. Lancet Oncol. 2017, 18, e472–e482. [Google Scholar] [CrossRef]
- Costa, M.; Saldanha, P. Risk reduction strategies in breast cancer prevention. Eur. J. Breast Health 2017, 13, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Tamarkin, L.; Cohen, M.; Roselle, D.; Reichert, C.; Lippman, M.; Chabner, B. Melatonin inhibition and pinealectomy enhancement of 7,12-dimethylbenz(a)anthracene-induced mammary tumors in the rat. Cancer Res. 1981, 41, 4432–4436. [Google Scholar] [PubMed]
- Subramanian, A.; Kothari, L. Suppressive effect by melatonin on different phases of 9,10-dimethyl-1,2-benzanthracene(DMBA)-induced rat mammary gland carcinogenesis. Anticancer Drugs 1991, 2, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Cos, S.; Sánchez-Barceló, E.J. Melatonin, experimental basis for a possible application in breast cancer prevention and treatment. Histol. Histopathol. 2000, 15, 637–647. [Google Scholar] [PubMed]
- Cos, S.; Sánchez-Barceló, E.J. Melatonin and mammary pathological growth. Front. Neuroendocrinol. 2000, 21, 133–170. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.M.; Blask, D.E. Effects of the pineal hormone melatonin on the proliferation and morphological characteristics of human breast cancer cells (MCF-7) in culture. Cancer Res. 1988, 48, 6121–6126. [Google Scholar] [PubMed]
- Sánchez-Barceló, E.J.; Cos, S.; Fernández, R.; Mediavilla, M.D. Melatonin and mammary cancer: A short review. Endocr. Relat. Cancer 2003, 10, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Cos, S.; Fernández, R.; Güézmes, A.; Sánchez-Barceló, E.J. Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Res. 1998, 58, 4383–4390. [Google Scholar] [PubMed]
- Mediavilla, M.D.; Sanchez-Barcelo, E.J.; Tan, D.X.; Manchester, L.; Reiter, R.J. Basic mechanisms involved in the anti-cancer effects of melatonin. Curr. Med. Chem. 2010, 17, 4462–4481. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.A.; Tan, D.X.; Acuña-Castroviejo, D.; Quin, L.; Yang, S.F.; Xu, K. Melatonin, a Full Service Anti-Cancer Agent: Inhibition of Initiation, Progression and Metastasis. Int. J. Mol. Sci. 2017, 18, 843. [Google Scholar] [CrossRef] [PubMed]
- Innominato, P.F.; Lim, A.S.; Palesh, O.; Clemons, M.; Trudeau, M.; Eisen, A.; Wang, C.; Kiss, A.; Pritchard, K.I.; Bjarnason, G.A. The effect of melatonin on sleep and quality of life in patients with advanced breast cancer. Support. Care Cancer 2016, 24, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.V.; Andersen, L.T.; Madsen, M.T.; Hageman, I.; Rasmussen, L.S.; Bokmand, S.; Rosenberg, J.; Gögenur, I. Effect of melatonin on depressive symptoms and anxiety in patients undergoing breast cancersurgery: A randomized, double-blind, placebo-controlled trial. Breast Cancer Res. Treat. 2014, 145, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, M.A.; Elkayam, R.; Gelernter, I.; Pfeffer, R.M. Melatonin for Prevention of Breast Radiation Dermatitis: A Phase II, Prospective, Double-Blind Randomized Trial. Isr. Med. Assoc. J. 2016, 18, 188–192. [Google Scholar] [PubMed]
- Lissoni, P.; Barni, S.; Mandalà, M.; Ardizzoia, A.; Paolorossi, F.; Vaghi, M.; Longarini, R.; Malugani, F.; Tancini, G. Decreased toxicity and incre-ased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. Eur. J. Cancer 1999, 35, 1688–1692. [Google Scholar] [CrossRef]
- Turner, N.C.; Neven, P.; Loibl, S.; Andre, F. Advances in the treatment of advanced oestrogen-receptor-positive breast cancer. Lancet 2017, 389, 2403–2414. [Google Scholar] [CrossRef]
- Oualla, K.; El-Zawahry, H.M.; Arun, B.; Reuben, J.M.; Woodward, W.A.; Gamal El-Din, H.; Lim, B.; Mellas, N.; Ueno, N.T.; Fouad, T.M. Novel therapeutic strategies in the treatment of triple-negative breast cancer. Ther. Adv. Med. Oncol. 2017, 9, 493–511. [Google Scholar] [CrossRef] [PubMed]
- Endogenous Hormones and Breast Cancer Collaborative Group; Key, T.J.; Appleby, P.N.; Reeves, G.K.; Travis, R.C.; Alberg, A.J.; Barricarte, A.; Berrino, F.; Krogh, V.; Sieri, S.; et al. Sex hormones and risk of breast cancer in premenopausal women: A collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013, 14, 1009–1019. [Google Scholar] [PubMed]
- Pasqualini, J.R.; Chetrite, G.S. Recent insight on the control of enzymes involved in estrogen formation and transformation in human breast cancer. J. Steroid Biochem. Mol. Biol. 2005, 93, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Conley, A.; Hinshelwood, M. Mammalian aromatases. Reproduction 2001, 121, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Miki, Y.; Nakata, T.; Shiotsu, Y.; Akinaga, S.; Inoue, K.; Ishida, T.; Kimura, M.; Moriya, T.; Sasano, H. Steroid sulfatase and estrogen sulfotransferase in normal human tissue and breast carcinoma. J. Steroid Biochem. Mol. Biol. 2003, 86, 449–454. [Google Scholar] [CrossRef]
- Miettinen, M.M.; Mustonen, M.V.; Poutanen, M.H.; Isomaa, V.V.; Vihko, R.K. Human 17 beta-hydroxysteroid dehydrogenase type 1 and type 2 isoenzymes have opposite activities in cultured cells and characteristic cell- and tissue-specific expression. Biochem. J. 1996, 314, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Hilborn, E.; Stål, O.; Jansson, A. Estrogen and androgen-converting enzymes 17β-hydroxysteroid dehydrogenase and their involvement in cancer: With a special focus on 17β-hydroxysteroid dehydrogenase type 1, 2, and breast cancer. Oncotarget 2017, 8, 30552–30562. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Sestak, I.; Cawthorn, S.; Hamed, H.; Holli, K.; Howell, A.; Forbes, J.F.; IBIS-I, Investigators. Tamoxifen for prevention of breast cancer: Extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015, 16, 67–75. [Google Scholar] [CrossRef]
- Yang, G.; Nowsheen, S.; Aziz, K.; Georgakilas, A.G. Toxicity and adverse effects of Tamoxifen and other anti-estrogen drugs. Pharmacol. Ther. 2013, 139, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Vogel, V.G.; Costantino, J.P.; Wickerham, D.L.; Cronin, W.M.; Cecchini, R.S.; Atkins, J.N.; Bevers, T.B.; Fehrenbacher, L.; Pajon, E.R.; Wade, J.L.; et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006, 295, 2727–2741. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; Hannon, R. Long-term effects of aromatase inhibitors on bone. J. Steroid Biochem. Mol. Biol. 2005, 95, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Cos, S.; Martínez-Campa, C.; Mediavilla, M.D.; Sánchez-Barceló, E.J. Melatonin modulates aromatase activity in MCF-7 human breast cancer cells. J. Pineal Res. 2005, 38, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Cos, S.; González, A.; Martínez-Campa, C.; Mediavilla, M.D.; Alonso-González, C.; Sánchez-Barceló, E.J. Estrogen-signaling pathway: A link between breast cancer and melatonin oncostatic actions. Cancer Detect. Prev. 2006, 30, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Cos, S.; González, A.; Güezmes, A.; Mediavilla, M.D.; Martínez-Campa, C.; Alonso-González, C.; Sánchez-Barceló, E.J. Melatonin inhibits the growth of DMBA-induced mammary tumors by decreasing the local biosynthesis of estrogens through the modulation of aromatase activity. Int. J. Cancer 2006, 118, 274–278. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Martínez-Campa, C.; Mediavilla, M.D.; Alonso-González, C.; Sánchez-Mateos, S.; Hill, S.M.; Sánchez-Barceló, E.J.; Cos, S. Effects of MT1 melatonin receptor overexpression on the aromatase-suppressive effect of melatonin in MCF-7 human breast cancer cells. Oncol. Rep. 2007, 17, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Cos, S.; Martinez-Campa, C.; Alonso-Gonzalez, C.; Sanchez-Mateos, S.; Mediavilla, M.D.; Sanchez-Barcelo, E.J. Selective estrogen enzyme modulator actions of melatonin in human breast cancer cells. J. Pineal Res. 2008, 45, 86–92. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Alvarez-García, V.; Martínez-Campa, C.; Mediavilla, M.D.; Alonso-González, C.; Sánchez-Barceló, E.J.; Cos, S. In vivo inhibition of the estrogen sulfatase enzyme and growth of DMBA-induced mammary tumors by melatonin. Curr. Cancer Drug Targets 2010, 10, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Campa, C.; González, A.; Mediavilla, M.D.; Alonso-González, C.; Alvarez-García, V.; Sánchez-Barceló, E.J.; Cos, S. Melatonin inhibits aromatase promoter expression by regulating cyclooxygenases expression and activity in breast cancer cells. Br. J. Cancer 2009, 101, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Barceló, E.J.; Cos, S.; Mediavilla, D.; Martínez-Campa, C.; González, A.; Alonso-González, C. Melatonin-estrogen interactions in breast cancer. J. Pineal Res. 2005, 38, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Barcelo, E.J.; Mediavilla, M.D.; Alonso-Gonzalez, C.; Rueda, N. Breast cancer therapy based on melatonin. Recent. Pat. Endocr. Metab. Immune Drug Discov. 2012, 6, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Ram, P.T.; Kiefer, T.; Silverman, M.; Song, Y.; Brown, G.M.; Hill, S.M. Estrogen receptor transactivation in MCF-7 breast cancer cells by melatonin and growth factors. Mol. Cell. Endocrinol. 1998, 141, 53–64. [Google Scholar] [CrossRef]
- Ram, P.T.; Dai, J.; Yuan, L.; Dong, C.; Kiefer, T.L.; Lai, L.; Hill, S.M. Involvement of the mt1 melatonin receptor in human breast cancer. Cancer Lett. 2002, 179, 141–150. [Google Scholar] [CrossRef]
- Yuan, L.; Collins, A.R.; Dai, J.; Dubocovich, M.L.; Hill, S.M. MT(1) melatonin receptor overexpression enhances the growth suppressive effects of melatonin in human breast cancer cells. Mol. Cell. Endocrinol. 2002, 192, 147–156. [Google Scholar] [CrossRef]
- Molis, T.M.; Spriggs, L.L.; Hill, S.M. Modulation of estrogen receptor mRNA expression by melatonin in MCF-7 human breast cancer cells. Mol. Endocrinol. 1994, 8, 1681–1690. [Google Scholar] [PubMed]
- Rato, A.G.; Pedrero, J.G.; Martinez, M.A.; del Rio, B.; Lazo, P.S.; Ramos, S. Melatonin blocks the inactivation of estrogen receptor for DNA binding. FASEB J. 1999, 13, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.T.; Blask, D.E.; Lemus-Wilson, A.M. Melatonin augments the sensitivity of MCF-7 human breast cancer cells to tamoxifen in vitro. J. Clin. Endocrinol. Metab. 1992, 75, 669–670. [Google Scholar] [PubMed]
- Martínez-Campa, C.; González, A.; Mediavilla, M.D.; Alonso-González, C.; Sánchez-Barceló, E.J.; Cos, S. Melatonin enhances the inhibitory effect of aminoglutethimide on aromatase activity in MCF-7 human breast cancer cells. Breast Cancer Res. Treat. 2005, 94, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Aydin, M.; Oktar, S.; Ozkan, O.V.; Alçin, E.; Oztürk, O.H.; Nacar, A. Letrozole induces hepatotoxicity without causing oxidative stress: The protective effect of melatonin. Gynecol. Endocrinol. 2011, 27, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Ladizesky, M.G.; Boggio, V.; Cutrera, R.A.; Mautalen, C. Melatonin effects on bone: Experimental facts and clinical perspectives. J. Pineal Res. 2003, 34, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Barceló, E.J.; Mediavilla, M.D.; Tan, D.X.; Reiter, R.J. Scientific basis for the potential use of melatonin in bone diseases: Osteoporosis and adolescent idiopathic scoliosis. J. Osteoporos. 2010. [Google Scholar] [CrossRef] [PubMed]
- Maria, S.; Witt-Enderby, P.A. Melatonin effects on bone: Potential use for the prevention and treatment for osteopenia, osteoporosis, periodontal disease and for use in bone-grafting procedures. J. Pineal Res. 2014, 56, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Amstrup, A.K.; Sikjaer, T.; Mosekilde, L.; Rejnmark, L. Melatonin and the skeleton. Osteoporos. Int. 2013, 24, 2919–2927. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.H.E.; Helfrich, M.H.; Wallace, H.M.; Ralston, S.H. Hydrogen peroxide, but not superoxide, stimulates bone resorption in mouse calvariae. Bone 1996, 19, 223–226. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, X.; Yan, J.; Li, M.; Liu, T.; Zhu, C.; Pan, G.; Guo, Q.; Yang, H.; Pei, M.; et al. Melatonin at pharmacological concentrations suppresses osteoclastogenesis via the attenuation of intracellular ROS. Osteoporos. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kotlarczyk, M.P.; Lassila, H.C.; O’Neil, C.K.; D’Amico, F.; Enderby, L.T.; Witt-Enderby, P.A.; Balk, J.L. Melatonin osteoporosis prevention study (MOPS): A randomized, double-blind, placebo-controlled study examining the effects of melatonin on bone health and quality of life in perimenopausal women. J. Pineal Res. 2012, 52, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Amstrup, A.K.; Sikjaer, T.; Heickendorff, L.; Mosekilde, L.; Rejnmark, L. Melatonin improves bone mineral density at the femoral neck in postmenopausal women with osteopenia: A randomized controlled trial. J. Pineal Res. 2015, 59, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Feskanich, D.; Hankinson, S.E.; Schernhammer, E.S. Nightshift work and fracture risk. The Nurses’ Health Study. Osteoporos. Int. 2009, 20, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Karasek, M. Melatonin, human aging, and age-related diseases. Exp. Gerontol. 2004, 39, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: Collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet 1997, 350, 1047–1059. [Google Scholar]
- Gray, J.M.; Rasanayagam, S.; Engel, C.; Rizz, J. State of the evidence 2017: An update on the connection between breast cancer and the environment. Environ. Health 2017. [Google Scholar] [CrossRef] [PubMed]
- Papagiannakopoulos, T.; Bauer, M.R.; Davidson, S.M.; Heimann, M.; Subbaraj, L.; Bhutkar, A.; Bartlebaugh, J.; Vander Heiden, M.G.; Jacks, T. Circadian Rhythm Disruption Promotes Lung Tumorigenesis. Cell Metab. 2016, 24, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Ercolani, L.; Ferrari, A.; De Mei, C.; Parodi, C.; Wade, M.; Grimaldi, B. Circadian clock: Time for novel anticancer strategies? Pharmacol. Res. 2015, 100, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Cos, S.; Mediavilla, M.D.; Martínez-Campa, C.; González, A.; Alonso-González, C.; Sánchez-Barceló, E.J. Exposure to light-at-night increases the growth of DMBA-induced mammary adenocarcinomas in rats. Cancer Lett. 2006, 235, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Dauchy, R.T.; Tirrell, P.C.; Wu, S.S.; Lynch, D.T.; Jitawatanarat, P.; Burrington, C.M.; Dauchy, E.M.; Blask, D.E.; Greene, M.W. Light at night activates IGF-1R/PDK1 signaling and accelerates tumor growth in human breast cancer xenografts. Cancer Res. 2011, 71, 2622–2631. [Google Scholar] [CrossRef] [PubMed]
- Van Dycke, K.C.; Rodenburg, W.; van Oostrom, C.T.; van Kerkhof, L.W.; Pennings, J.L.; Roenneberg, T.; van Steeg, H.; van der Horst, G.T. Chronically alternating light cycles increase breast cancer risk in mice. Curr. Biol. 2015, 25, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- Kettner, N.M.; Katchy, C.A.; Fu, L. Circadian gene variants in cancer. Ann. Med. 2014, 46, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Sahar, S.; Sassone-Corsi, P. Metabolism and cancer: The circadian clock connection. Nat. Rev. Cancer 2009, 9, 886–896. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Anand, S.T.; Ebell, M.H.; Vena, J.E.; Robb, S.W. Circadian disrupting exposures and breast cancer risk: A meta-analysis. Int. Arch. Occup. Environ. Health 2015, 88, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Purdue, M.P.; Hutchings, S.J.; Rushton, L.; Silverman, D.T. The proportion of cancer attributable to occupational exposures. Ann. Epidemiol. 2015, 25, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Straif, K.; Baan, R.; Grosse, Y.; Secretan, B.; Ghissassi, F.E.; Bouvard, V.; Altieri, A.; Benbrahim-Tallaa, L.; Cogliano, V.; WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007, 8, 1065–1066. [Google Scholar] [CrossRef]
- Wise, J. Danish night shift workers with breast cancer awarded compensation. BMJ 2009, 338. [Google Scholar] [CrossRef] [PubMed]
- Travis, R.C.; Balkwill, A.; Fensom, G.K.; Appleby, P.N.; Reeves, G.K.; Wang, X.S.; Roddam, A.W.; Gathani, T.; Peto, R.; Green, J.; et al. Night Shift Work and Breast Cancer Incidence: Three Prospective Studies and Meta-analysis of Published Studies. J. Natl. Cancer Inst. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.L.; Smit, A.N.; Mistlberger, R.E.; Landry, G.J.; Koehoorn, M. Organisational characteristics associated with shift work practices and potential opportunities for intervention: Findings from a Canadian study. Occup. Environ. Med. 2017, 74, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J. Night shift work and risk of breast cancer. Curr. Environ. Health Rep. 2017, 4, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Blask, D.E.; Brainard, G.C.; Dauchy, R.T.; Hanifin, J.P.; Davidson, L.K.; Krause, J.A.; Sauer, L.A.; Rivera-Bermudez, M.A.; Dubocovich, M.L.; Jasser, S.A.; et al. Melatonin-depletedblood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 2005, 65, 11174–11184. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.M.; Figueiro, M.G. Measuring light at night and melatonin levels in shift workers: A review of the literature. Biol. Res. Nurs. 2017, 19, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Blask, D.E.; Hill, S.M.; Dauchy, R.T.; Xiang, S.; Yuan, L.; Duplessis, T.; Mao, L.; Dauchy, E.; Sauer, L.A. Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night. J. Pineal Res. 2011, 51, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Langley, A.R.; Graham, C.H.; Grundy, A.L.; Tranmer, J.E.; Richardson, H.; Aronson, K.J. A cross-sectional study of breast cancer biomarkers among shift working nurses. BMJ Open 2012. [Google Scholar] [CrossRef] [PubMed]
- Menegaux, F.; Truong, T.; Anger, A.; Cordina-Duverger, E.; Lamkarkach, F.; Arveux, P.; Kerbrat, P.; Févotte, J.; Guénel, P. Night work and breast cancer: A population-based case-control study in France (the CECILE study). Int. J. Cancer 2013, 132, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Fonken, L.K.; Nelson, R. The effects of light at night on circadian clocks and metabolism. Endocr. Rev. 2014, 35, 648–670. [Google Scholar] [CrossRef] [PubMed]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Tamarkin, L.; Danforth, D.; Lichter, A.; DeMoss, E.; Cohen, M.; Chabner, B.; Lippman, M. Decreased nocturnal plasma melatonin peak in patients with estrogen receptor positive breast cancer. Science 1982, 216, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Nagao, Y.; Yamamoto, S.; Shibuya, C.; Kashiki, Y.; Shimizu, H. Light exposure at night, urinary 6-sulfatoxymelatonin, and serum estrogens and androgens in postmenopausal Japanese women. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Acebo, I.; Dierssen-Sotos, T.; Papantoniou, K.; García-Unzueta, M.T.; Santos-Benito, M.F.; Llorca, J. Association between exposure to rotating night shift versus day shift using levels of 6-sulfatoxymelatonin and cortisol and other sex hormones in women. Chronobiol. Int. 2015, 32, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Papantoniou, K.; Castaño-Vinyals, G.; Espinosa, A.; Aragonés, N.; Pérez-Gómez, B.; Ardanaz, E.; Altzibar, J.M.; Sanchez, V.M.; Gómez-Acebo, I.; Llorca, J.; et al. Breast cancer risk and night shift work in a case-control study in a Spanish population. Eur. J. Epidemiol. 2016, 31, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Unsal-Kaçmaz, K.; Mullen, T.E.; Kaufmann, W.K.; Sancar, A. Coupling of human circadian and cell cycles by the timeless protein. Mol. Cell. Biol. 2005, 25, 3109–3116. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, F.C.; Rao, A.; Maguire, A. Circadian molecular clocks and cancer. Cancer Lett. 2014, 342, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, G.A.; Jordan, R. Circadian variation of cell proliferation and cell cycle protein expression in man: Clinical implications. Prog. Cell Cycle Res. 2000, 4, 193–206. [Google Scholar] [PubMed]
- Borgs, L.; Beukelaers, P.; Vandenbosch, R.; Belachew, S.; Nguyen, L.; Malgrange, B. Cell “circadian” cycle: New role for mammalian core clock genes. Cell Cycle 2009, 8, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Dauchy, R.T.; Xiang, S.; Mao, L.; Brimer, S.; Wren, M.A.; Yuan, L.; Anbalagan, M.; Hauch, A.; Frasch, T.; Rowan, B.G.; et al. Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res. 2014, 74, 4099–4110. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Dauchy, R.T.; Hauch, A.; Mao, L.; Yuan, L.; Wren, M.A.; Belancio, V.P.; Mondal, D.; Frasch, T.; Blask, D.E.; et al. Doxorubicin resistance in breast cancer is driven by light at night-induced disruption of the circadian melatonin signal. J. Pineal Res. 2015, 59, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Rabstein, S.; Harth, V.; Justenhoven, C.; Pesch, B.; Plöttner, S.; Heinze, E.; Heinze, E.; Lotz, A.; Baisch, C.; Schiffermann, M.; et al. Polymorphisms in circadian genes, night work and breast cancer: Results from the GENICA study. Chronobiol. Int. 2014, 31, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Grundy, A.; Schuetz, J.M.; Lai, A.S.; Janoo-Gilani, R.; Leach, S.; Burstyn, I.; Burstyn, I.; Richardson, H.; Brooks-Wilson, A.; Spinelli, J.J.; Aronson, K.J. Shift work, circadian gene variants and risk of breast cancer. Cancer Epidemiol. 2013, 37, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Reszka, E.; Przybek, M.; Muurlink, O.; Pepłonska, B. Circadian gene variants and breast cancer. Cancer Lett. 2017, 390, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Liira, J.; Verbeek, J.; Ruotsalainen, J. Pharmacological interventions for sleepiness and sleep disturbances caused by shift work. JAMA 2015, 313, 961–962. [Google Scholar] [CrossRef] [PubMed]
- Sadeghniiat-Haghighi, K.; Bahrami, H.; Aminian, O.; Meysami, A.; Khajeh-Mehrizi, A. Melatonin therapy in shift workers with difficulty falling asleep: A randomized, double-blind, placebo-controlled crossover field study. Work 2016, 55, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, M.; Boudreau, P.; Cermakian, N.; Boivin, D.B. Rapid resetting of human peripheral clocks by phototherapy during simulated night shift work. Sci. Rep. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Barriuso, R.; Fernández, M.F.; Castaño-Vinyals, G.; Whelan, D.; Pérez-Gómez, B.; Llorca, J.; Villanueva, C.M.; Guevara, M.; Molina-Molina, J.M.; Artacho-Cordón, F.; et al. Total effective xenoestrogen burdenn in serum samples and risk for breast cancer in a population-based multicase-control study in Spain. Environ. Health Perspect. 2016, 124, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Brody, J.G.; Rudel, R.A. Environmental pollutants and breast cancer. Environ. Health Perspect. 2003, 111, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D. Metalloestrogens: An emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. J. Appl. Toxicol. 2006, 26, 191–197. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Cancer Research (IARC). Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. In IARC Monographs on the Evaluation of Carcinogenic Risk to Humans; IARC Scientific Publications: Lyon, France, 1993; Volume 28, pp. 119–237. ISBN 92-832-1258-4. [Google Scholar]
- Lappano, R.; Malaguarnera, R.; Belfiore, A.; Maggiolini, M. Recent advances on the stimulatory effects of metals in breast cancer. Mol. Cell. Endocrinol. 2017, 457, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Health Risk Assessment of Dietary Cadmium Intake: Do Current Guidelines Indicate How Much is Safe? Environ. Health Perspect. 2017, 125, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Ramos, E.; de Los Ríos, C.; Egea, J.; Del Pino, J.; Reiter, R.J. A review of metal-catalyzed molecular damage: Protection bymelatonin. J. Pineal Res. 2014, 56, 343–370. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Lu, J.; Nordberg, M. Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology 1998, 19, 529–535. [Google Scholar] [PubMed]
- Riederer, A.M.; Belova, A.; George, B.J.; Anastas, P.T. Urinary cadmium in the 1999–2008 U.S. National Health and Nutrition Examination Survey (NHANES). Environ. Sci. Technol. 2013, 47, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Cantor, K.P.; Stewart, P.A.; Brinton, L.A.; Dosemeci, M. Occupational exposures and female breast cancer mortality in the United States. J. Occup. Med. 1994, 37, 336–348. [Google Scholar] [CrossRef]
- McElroy, J.A.; Shafer, M.M.; Trentham-Dietz, A.; Hampton, J.M.; Newcomb, P.A. Cadmium exposure and breast cancer risk. J. Natl. Cancer Inst. 2006, 98, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, C.M.; Chen, J.J.; Kovach, J.S. Environmental cadmium and breast cancer risk. Aging 2010, 2, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Julin, B.; Wolk, A.; Bergkvist, L.; Bottai, M.; Akesson, A. Dietary cadmiumexposure and risk of postmenopausal breast cancer: A population-based prospective cohort study. Cancer Res. 2012, 72, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Huang, Y.; Zhang, J.; Peng, Y.; Lin, X.; Wu, K.; Huo, X. Cadmium exposure and the risk of breast cancer in Chaoshan population of southeast China. Environ. Sci. Pollut. Res. Int. 2015, 22, 19870–19878. [Google Scholar] [CrossRef] [PubMed]
- Strumylaite, L.; Bogusevicius, A.; Abdrachmanovas, O.; Baranauskiene, D.; Kregzdyte, R.; Pranys, D.; Poskiene, L. Cadmium concentration in biological media of breast cancer patients. Breast Cancer Res. Treat. 2011, 125, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Nagao, Y.; Nakamura, K.; Wada, K.; Tamai, Y.; Tsuji, M.; Yamamoto, S.; Kashiki, Y. Cadmium exposure and the risk of breast cancer in Japanese women. Breast Cancer Res. Treat. 2013, 138, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, F.; Lei, Y. Dietary intake and urinary level of cadmium and breast cancer risk: A meta-analysis. Cancer Epidemiol. 2016, 42, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Orsini, N.; Wolk, A. Urinary cadmium concentration and risk of breast cancer: A systematic review and dose-response meta-analysis. Am. J. Epidemiol. 2015, 182, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, E.; Socha, K.; Reszka, E.; Wieczorek, E.; Skokowski, J.; Kalinowski, L.; Fendler, W.; Seroczynska, B.; Wozniak, M.; Borawska, M.H.; et al. Cadmium, arsenic, selenium and iron- Implications for tumor progression in breast cancer. Environ. Toxicol. Pharmacol. 2017, 53, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Iwasaki, M.; Sawada, N.; Takachi, R.; Kasuga, Y.; Yokoyama, S.; Onuma, H.; Nishimura, H.; Kusama, R.; Yokoyama, K.; et al. Dietary cadmium intake and breast cancer risk in Japanese women: A case-control study. Int. J. Hyg. Environ. Health 2014, 217, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Parodi, D.A.; Greenfield, M.; Evans, C.; Chichura, A.; Alpaugh, A.; Williams, J.; Cyrus, K.C.; Martin, M.B. Alteration of Mammary Gland Development and Gene Expression by In Utero Exposure to Cadmium. Int. J. Mol. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, K.T.; McElroy, J.A.; Harrington, J.M.; Levine, K.E.; Pedersen, C.; Sørensen, M.; Tjønneland, A.; Meliker, J.R.; Raaschou-Nielsen, O. Urinary Cadmium and breast cancer: A prospective Danish Cohort Study. J. Natl. Cancer Inst. 2016. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.V.; Shafer, M.M.; Bonner, M.R.; LaCroix, A.Z.; Manson, J.E.; Meliker, J.R.; Neuhouser, M.L.; Newcomb, P.A. Urinary Cadmium and risk of invasive breast cancer in the Women’s Health Initiative. Am. J. Epidemiol. 2016, 183, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Stoica, A.; Katzenellenbogen, B.S.; Martin, M.B. Activation of estrogen receptor-alpha by the heavy metal cadmium. Mol. Endocrinol. 2000, 14, 545–553. [Google Scholar] [PubMed]
- Byrne, C.; Divekar, S.D.; Storchan, G.B.; Parodi, D.A.; Martin, M.B. Metals and breast cancer. J. Mammary Gland Biol. Neoplasia 2013, 18, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Kenney, N.; Stoica, A.; Hilakivi-Clarke, L.; Singh, B.; Chepko, G.; Clarke, R.; Sholler, P.F.; Lirio, A.A.; Foss, C.; et al. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat. Med. 2003, 9, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Sandbichler, A.M.; Höckner, M. Cadmium Protection Strategies—A hidden trade-off? Int. J. Mol. Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Campa, C.; Alonso-González, C.; Mediavilla, M.D.; Cos, S.; González, A.; Ramos, S.; Sánchez-Barceló, E.J. Melatonin inhibits both ER alpha activation and breast cancer cell proliferation induced by a metalloestrogen, cadmium. J. Pineal Res. 2006, 40, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Campa, C.M.; Alonso-González, C.; Mediavilla, M.D.; Cos, S.; González, A.; Sanchez-Barcelo, E.J. Melatonin down-regulates hTERT expression induced by either natural estrogens (17beta-estradiol) or metalloestrogens (cadmium) in MCF-7 human breast cancer cells. Cancer Lett. 2008, 268, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Alonso-González, C.; González, A.; Mazarrasa, O.; Güezmes, A.; Sánchez-Mateos, S.; Martínez-Campa, C.; Cos, S.; Sánchez-Barceló, E.J.; Mediavilla, M.D. Melatonin prevents the estrogenic effects of sub-chronic administration of cadmium on mice mammary glands and uterus. J. Pineal Res. 2007, 42, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Gonzalez, C.; Mediavilla, D.; Martinez-Campa, C.; Gonzalez, A.; Cos, S.; Sanchez-Barcelo, E.J. Melatonin modulates the cadmium-induced expression of MT-2 and MT-1 metallothioneins in three lines of human tumor cells (MCF-7, MDA-MB-231 and HeLa). Toxicol. Lett. 2008, 181, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Bay, B.H.; Jin, R.; Huang, J.; Tan, P.H. Metallothionein as a prognostic biomarker in breast cancer. Exp. Biol. Med. 2006, 231, 1516–1521. [Google Scholar] [CrossRef]
- El-Sokkary, G.H.; Nafady, A.A.; Shabash, E.H. Melatonin administration ameliorates cadmium-induced oxidative stress and morphological changes in the liver of rat. Ecotoxicol. Environ. Saf. 2010, 73, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Pi, H.; Xu, S.; Reiter, R.J.; Guo, P.; Zhang, L.; Li, Y.; Li, M.; Cao, Z.; Tian, L.; Xie, J.; et al. SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy 2015, 11, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.L.; Ross, R.K.; Paganini-Hill, A.; Bernstein, L. Effect of family history, obesity and exercise on breast cancer risk among postmeno-pausal women. Int. J. Cancer 2003, 106, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, L.M.; White, E.; Chen, Z.; Chlebowski, R.T.; Hays, J.; Kuller, L.; Lopez, A.M.; Manson, J.; Margolis, K.L.; Muti, P.C.; et al. Obesity, body size, and risk of postmenopausal breast cancer: The Women’s Health Initiative (United States). Cancer Causes Control 2002, 13, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L.; Aragaki, A.K.; Prentice, R.L.; Manson, J.E.; Chlebowski, R.; Carty, C.L.; Ochs-Balcom, H.M.; Thomson, C.A.; Caan, B.J.; Tinker, L.F.; et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: A secondary analysis of the Women’s Health Initiative Randomized Clinical Trials. JAMA Oncol. 2015, 1, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Jiang, L.; Stefanick, M.L.; Johnson, K.C.; Lane, D.S.; LeBlanc, E.S.; Prentice, R.; Rohan, T.E.; Snively, B.M.; Vitolins, M.; et al. Duration of Adulthood Overweight, Obesity, and Cancer Risk in the Women’s Health Initiative: A Longitudinal Study from the United States. PLoS Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Welti, L.M.; Beavers, D.P.; Caan, B.J.; Sangi-Haghpeykar, H.; Vitolins, M.-Z.; Beavers, K.M. Weight Fluctuation and Cancer Risk in Postmeno-pausal Women: The Women’s Health Initiative. Cancer Epidemiol. Biomark. Prev. 2017, 26, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Kotsopoulos, J.; Olopado, O.I.; Ghadirian, P.; Lubinski, J.; Lynch, H.T.; Isaacs, C.; Weber, B.; Kim-Sing, C.; Ainsworth, P.; Foulkes, W.D.; et al. Changes in body weight and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2005, 7, 833–843. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Noncommunicable Diseases 2014; WHO Press: Geneva, Switzerland, 2014. [Google Scholar]
- Nduhirabandi, F.; Du Toit, E.F.; Blackhurst, D.; Marais, D.; Lochner, A. Chronic melatonin consumption prevents obesity-related metabolic abnormalities and protects the heart against myocardial ischemia and reperfusion injury in a prediabetic model of diet-induced obesity. J. Pineal Res. 2011, 50, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Mateos, S.; Alonso-Gonzalez, C.; Gonzalez, A.; Martinez-Campa, C.M.; Mediavilla, M.D.; Cos, S.; Sanchez-Barcelo, E.J. Melatonin and estradiol effects on food intake, body weight, and leptin in ovariectomized rats. Maturitas 2007, 58, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Amstrup, A.K.; Sikjaer, T.; Pedersen, S.B.; Heickendorff, L.; Mosekilde, L.; Rejnmark, L. Reduced fat mass and increased lean mass in response to 1 year of melatonin treatment in postmenopausal women: A randomized placebo-controlled trial. Clin. Endocrinol. 2016, 84, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk-Golec, K.; Rajewski, P.; Gackowski, M.; Mila-Kierzenkowska, C.; Wesołowski, R.; Sutkowy, P.; Pawłowska, M.; Woźniak, A. Melatonin supplementation lowers oxidative stress and regulates adipokines in obese patients on a calorie-restricted diet. Oxid. Med. Cell. Longev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.A.; Thompson, S.; Raboud, J.M.; Hoffman, B.R. Light and exercise and melatonin production in women. Am. J. Epidemiol. 2005, 162, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.L.; Spiegel, K.; Penev, P.; Van Cauter, E. The metabolic consequences of sleep deprivation. Sleep Med. Rev. 2007, 11, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Markwald, R.R.; Melanson, E.L.; Smith, M.R.; Higgins, J.; Perreault, L.; Eckel, R.H.; Wright, K.P., Jr. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc. Natl. Acad. Sci. USA 2013, 110, 5695–5700. [Google Scholar] [CrossRef] [PubMed]
- Bayon, V.; Leger, D.; Gomez-Merino, D.; Vecchierini, M.F.; Chennaoui, M. Sleep debt and obesity. Ann. Med. 2014, 46, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal Res. 2014, 56, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk-Golec, K.; Woźniak, A.; Reiter, R.J. Inter-relationships of the chronobiotic, melatonin, with leptin and adiponectin: Implications for obesity. J. Pineal Res. 2015, 59, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R. Aromatase and the breast: Regulation and clinical aspects. Maturitas 2006, 54, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Lønning, P.E.; Helle, H.; Duong, N.K.; Ekse, D.; Aas, T.; Geisler, J. Tissue estradiol is selectively elevated in receptor positive breast cancers while tumour estrone is reduced independent of receptor status. J. Steroid Biochem. Mol. Biol. 2009, 117, 31–41. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A.; Wu, L.; Chen, C.; Chlebowski, R.; Mossavar-Rahmani, Y.; Modugno, F.; Perri, M.G.; Stanczyk, F.Z.; Van Horn, L.; Wang, C.Y.; et al. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity 2006, 14, 1662–1677. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Sáez-López, C.; Barbosa-Desongles, A.; Hernández, C.; Selva, D.M. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol. Metab. 2015, 26, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Brown, K.A. Obesity and breast cancer—Role of estrogens and the molecular underpinnings of aromatase regulation in breast adipose tissue. Mol. Cell. Endocrinol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Macis, D.; Guerrieri-Gonzaga, A.; Gandini, S. Circulating adiponectin and breast cancer risk: A systematic review and meta-analysis. Int. J. Epidemiol. 2014, 43, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Vona-Davis, L.; Rose, D.P. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr. Relat. Cancer 2007, 14, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Pan, Q.; Chen, X.; Xu, S.; Luo, X.; Chen, L. The association between obesity related adipokines and risk of breast cancer: A meta-analysis. Oncotarget 2017, 8, 75389–75399. [Google Scholar] [CrossRef] [PubMed]
- Yunusova, N.V.; Kondakova, I.V.; Kolomiets, L.A.; Afanas’ev, S.G.; Chernyshova, A.L.; Kudryavtsev, I.V.; Tsydenova, A.A. Molecular targets for the therapy of cancer associated with metabolic syndrome (transcription and growth factors). Asia Pac. J. Clin. Oncol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Arditi, J.D.; Venihaki, M.; Karalis, K.P.; Chrousos, G.P. Antiproliferative effect of adiponectin on MCF7 breast cancer cells: A potential hormonal link between obesity and cancer. Horm. Metab. Res. 2007, 39, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, A.; Ohta, Y.; Ohashi, K. Melatonin improves metabolic syndrome induced by high fructose intake in rats. J. Pineal Res. 2012, 52, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Nduhirabandi, F.; Huisamen, B.; Strijdom, H.; Blackhurst, D.; Lochner, A. Short-term melatonin consumption protects the heart of obese rats independent of body weight change and visceral adiposity. J. Pineal Res. 2014, 57, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Smith-Kirwin, S.M.; O’Connor, D.M.; De Johnston, J.; Lancey, E.D.; Hassink, S.G.; Funanage, V.L. Leptin expression in human mammary epithelial cells and breast milk. J. Clin. Endocrinol. Metab. 1998, 83, 1810–1813. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Juneja, S.C.; Maihle, N.J.; Cleary, M.P. Leptin—A growth factor in normal and malignant breast cells and for normal mammary gland development. J. Natl. Cancer Inst. 2002, 94, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Dieudonne, M.N.; Machinal-Quelin, F.; Serazin-Leroy, V.; Leneveu, M.C.; Pecquery, R.; Giudicelli, Y. Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem. Biophys. Res. Commun. 2002, 293, 622–628. [Google Scholar] [CrossRef]
- Karaduman, M.; Bilici, A.; Ozet, A.; Sengul, A.; Musabak, U.; Alomeroglu, M. Tissue leptin levels in patients with breast cancer. J. BUON 2010, 15, 369–372. [Google Scholar] [PubMed]
- Catalano, S.; Marsico, S.; Giordano, C.; Mauro, L.; Rizza, P.; Panno, M.L.; Andò, S. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J. Biol. Chem. 2003, 278, 28668–28676. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, R.R.; Xu, Y.; Guo, S.; Watters, A.; Zhou, W.; Leibovich, S.J. Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFkappaB/HIF-1alpha activation. Cell. Signal. 2010, 22, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhao, T.; Wang, X.; Gao, C.; Wang, J.; Yu, M.; Hao, J. Leptin upregulates telomerase activity and transcription of human telomerase reverse transcriptase in MCF-7 breast cancer cells. Biochem. Biophys. Res. Commun. 2010, 394, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Perks, C.M.; Holly, J.M. Hormonal mechanisms underlying the relationship between obesity and breast cancer. Endocrinol. Metab. Clin. N. Am. 2011, 40, 485–507. [Google Scholar] [CrossRef] [PubMed]
- Cagnacci, A.; Malmusi, S.; Zanni, A.; Arangino, S.; Cagnacci, P.; Volpe, A. Acute modifications in the levels of daytime melatonin do not influenceleptin in postmenopausal women. J. Pineal Res. 2002, 33, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Joseph, N.A.; Greenberg, A.S. Adipocytokines and insulin resistance. J. Clin. Endocrinol. Metab. 2004, 89, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Endogenous Hormones and Breast Cancer Collaborative Group; Key, T.J.; Appleby, P.N.; Reeves, G.K.; Roddam, A.W. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: Pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010, 11, 530–542. [Google Scholar] [PubMed]
- Cardinali, D.P.; Vigo, D.E. Melatonin, mitochondria, and the metabolic syndrome. Cell. Mol. Life Sci. 2017, 74, 3941–3954. [Google Scholar] [CrossRef] [PubMed]
- Koziróg, M.; Poliwczak, A.R.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Broncel, M. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J. Pineal Res. 2011, 50, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Terry, P.D.; Superak, H.M.; Nell-Dybdahl, C.L.; Chowdhury, R.; Phillips, L.S.; Kutner, M.H. Melatonin supplementation to treat the metabolic syndrome: A randomized controlled trial. Diabetol. Metab. Syndr. 2014. [Google Scholar] [CrossRef] [PubMed]
- Mesri Alamdari, N.; Mahdavi, R.; Roshanravan, N.; Lotfi Yaghin, N.; Ostadrahimi, A.R.; Faramarzi, E. A double-blind, placebo-controlled trial related to the effects of melatonin on oxidative stress and inflammatory parameters of obese women. Horm. Metab. Res. 2015, 47, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Sastre, P.; Scheer, F.A.; Gómez-Abellán, P.; Madrid, J.A.; Garaulet, M. Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep 2014. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, D.; McGowan, E.M. Recent advances in the use of metformin: Can treating diabetes prevent breast cancer? Biomed. Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Decensi, A.; Puntoni, M.; Goodwin, P.; Cazzaniga, M.; Gennari, A.; Bonanni, B.; Gandini, S. Metformin and cancer risk in diabetic patients: A systematic review and meta-analysis. Cancer Prev. Res. 2010, 3, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Lobanova, L.; Dawicki, W.; Groot, G.; Gordon, J.R.; Bowen, M.; Harkness, T.; Arnason, T. Metformin inhibits the development, and promotes the resensitization, of treatment-resistant breast cancer. PLoS ONE 2017. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska-Ogłaza, A.; Zarzycka-Lindner, G.; Olejniczak, H.; Polaszewska-Muszyńska, M.; Junik, R. Use of metformin is associatedwith lower incidence of cancer in patients with type 2 diabetes. Endokrynol. Pol. 2017. [Google Scholar] [CrossRef]
- Kusturica, J.; Kulo Ćesić, A.; Gušić, E.; Maleškić, S.; Rakanović-Todić, M.; Šečić, D. Metformin use associated with lower risk of cancer in patients with diabetes mellitus type 2. Med. Glas 2017, 14, 176–181. [Google Scholar]
- Anisimov, V.N.; Egormin, P.A.; Piskunova, T.S.; Popovich, I.G.; Tyndyk, M.L.; Yurova, M.N.; Zabezhinski, M.A.; Anikin, I.V.; Karkach, A.S.; Romanyukha, A.A. Metformin extends life span of HER-2/neu transgenic mice and in combination with melatonin inhibits growth of transplantable tumors in vivo. Cell Cycle 2010, 9, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Bojková, B.; Kajo, K.; Kisková, T.; Kubatka, P.; Žúbor, P.; Solár, P.; Péč, M.; Adamkov, M. Metformin and melatonin inhibit DMBA-induced mammary tumorigenesis in rats fed a high-fat diet. Anticancer Drugs 2017. [Google Scholar] [CrossRef] [PubMed]
- Kurhaluk, N.; Bojkova, B.; Radkowski, M.; Zaitseva, O.V.; Kyriienko, S.; Demkow, U.; Winklewski, P.J. Melatonin and metformin diminish oxidative stress in heart tissue in a rat model of high fat fiet and mammary carcinogenesis. Adv. Exp. Med. Biol. 2017. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-González, A.; Mediavilla, M.D.; Sánchez-Barceló, E.J. Melatonin: A Molecule for Reducing Breast Cancer Risk. Molecules 2018, 23, 336. https://doi.org/10.3390/molecules23020336
González-González A, Mediavilla MD, Sánchez-Barceló EJ. Melatonin: A Molecule for Reducing Breast Cancer Risk. Molecules. 2018; 23(2):336. https://doi.org/10.3390/molecules23020336
Chicago/Turabian StyleGonzález-González, Alicia, María Dolores Mediavilla, and Emilio J. Sánchez-Barceló. 2018. "Melatonin: A Molecule for Reducing Breast Cancer Risk" Molecules 23, no. 2: 336. https://doi.org/10.3390/molecules23020336
APA StyleGonzález-González, A., Mediavilla, M. D., & Sánchez-Barceló, E. J. (2018). Melatonin: A Molecule for Reducing Breast Cancer Risk. Molecules, 23(2), 336. https://doi.org/10.3390/molecules23020336




