Nutritional Value, Chemical Characterization and Bulb Morphology of Greek Garlic Landraces
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material and Sampling
3.2. Standards and Reagents
3.3. Morphological and Quality Features
3.4. Nutritional and Chemical Composition
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brewster, J.L. Onions and othe vegetable Alliums, 2nd ed.; CABI: Wallingford, UK, 2008. [Google Scholar]
- Kimbaris, A.C.; Siatis, N.G.; Daferera, D.J.; Tarantilis, P.A.; Pappas, C.S.; Polissiou, M.G. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum). Ultrason. Sonochem. 2006, 13, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Lanzotti, V.; Scala, F.; Bonanomi, G. Compounds from Allium species with cytotoxic and antimicrobial activity. Phytochem. Rev. 2014, 13, 769–791. [Google Scholar] [CrossRef]
- Lanzotti, V.; Bonanomi, G.; Scala, F. What makes Allium species effective against pathogenic microbes? Phytochem. Rev. 2013, 12, 751–772. [Google Scholar] [CrossRef]
- Lu, X.; Ross, C.F.; Powers, J.R.; Aston, D.E.; Rasco, B.A. Determination of total phenolic content and antioxidant activity of garlic (Allium sativum) and elephant garlic (Allium ampeloprasum) by attenuated total reflectancefourier transformed infrared spectroscopy. J. Agric. Food Chem. 2011, 59, 5215–5221. [Google Scholar] [CrossRef] [PubMed]
- Rekowska, E.; Skupień, K. The influence of selected agronomic practices on the yield and chemical composition of winter garlic. Veg. Crop Res. Bull. 2009, 70, 173–182. [Google Scholar] [CrossRef]
- Koch HP, L.L. Garlic: The Science and Therapeutic Application of Allium sativum L. and Related Species; Koch HP, L.L., Ed.; Williams & Wilkins Publishing Co.: Baltimore, MD, USA, 1996. [Google Scholar]
- Figliuolo, G.; Candido, V.; Logozzo, G.; Miccolis, V.; Zeuli, P.L.S. Genetic evaluation of cultivated garlic germplasm (Allium sativum L. and A. ampeloprasum L.). Euphytica 2001, 121, 325–334. [Google Scholar] [CrossRef]
- Mohammadi, B.; Khodadadi, M.; Karami, E.; Shaaf, S. Variation in agro-morphological characters in Iranian garlic landraces. Int. J. Veg. Sci. 2014, 20, 202–215. [Google Scholar] [CrossRef]
- González, R.E.; Soto, V.C.; Sance, M.M.; Camargo, A.B.; Galmarini, C.R. Variability of solids, organosulfur compounds, pungency and health-enhancing traits in garlic (Allium sativum L.) cultivars belonging to different ecophysiological groups. J. Agric. Food Chem. 2009, 57, 10282–10288. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Kaul, V.; Gohil, R.N. Analysis of morphological variability in the Indian germplasm of Allium sativum L. Plant Syst. Evol. 2014, 300, 245–254. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, Â.; Barros, L.; Ciric, A.; Sokovic, M.; Ferreira, I.C.F.R. Chemical composition, nutritional value and antioxidant properties of Mediterranean okra genotypes in relation to harvest stage. Food Chem. 2018, 245, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Fanaei, H.; Narouirad, M.; Farzanjo, M.; Ghasemi, M. Evaluation of yield and some agronomical traits in garlic genotypes (Allium sativum L.). Annu. Res. Rev. Biol. 2014, 4, 3386–3391. [Google Scholar] [CrossRef]
- Jabbes, N.; Arnault, I.; Auger, J.; Al Mohandes Dridi, B.; Hannachi, C. Agro-morphological markers and organo-sulphur compounds to assess diversity in Tunisian garlic landraces. Sci. Hortic. (Amsterdam) 2012, 148, 47–54. [Google Scholar] [CrossRef]
- Diriba-Shiferaw, G.; Nigussie-Dechassa, R.; Woldetsadik, K.; Tabor, G.; Sharma, J.J. Bulb quality of Garlic (Allium sativum L.) as influenced by the application of inorganic fertilizers. African J. Agric. Res. 2014, 9, 778–790. [Google Scholar] [CrossRef]
- Gadel-Hak, S.-N.H.; Moustafa, Y.M.M.; Abdel-Naem, G.F.; Abdel-Wahab, I. Studying different quantitative and qualitative traits of some white- and colored-bulb garlic genotypes grown under a drip irrigation system. Aust. J. Basic Appl. Sci. 2015, 5, 1415–1427. [Google Scholar]
- Hoon, H.G.; Koo, L.S.; Won, M. Alliin and fructan contents in garlics, by cultivars and cultivating areas. J. Korean Soc. Hortic. Sci. 1997, 38, 483–488. [Google Scholar]
- IPGRI. Descriptors for Allium spp.; International Plant Genetic Resources Institute: Rome, Italy, 2001. [Google Scholar]
- Argüello, J.A.; Ledesma, A.; Núñez, S.B.; Rodríguez, C.H.; Díaz Goldfarb, M.D.C. Vermicompost effects on bulbing dynamics, nonstructural carbohydrate content, yield, and quality of “Rosado Paraguayo” garlic bulbs. HortScience 2006, 41, 589–592. [Google Scholar]
- Baghalian, K.; Naghavi, M.R.; Ziai, S.A.; Badi, H.N. Post-planting evaluation of morphological characters and allicin content in Iranian garlic (Allium sativum L.) ecotypes. Sci. Hortic. 2006, 107, 405–410. [Google Scholar] [CrossRef]
- Minard, H.R.G. Effect of clove size, spacing, fertilisers, and lime on yield and nutrient content of garlic (Allium sativum). N. Z. J. Exp. Agric. 1978, 6, 139–143. [Google Scholar] [CrossRef]
- Rosen, C.J.; Tong, C.B.S. Yield, dry matter partitioning, and storage quality of hardneck garlic as affected by soil amendments and scape removal. HortScience 2001, 36, 1235–1239. [Google Scholar]
- Morales-Soto, A.; García-Salas, P.; Rodríguez-Pérez, C.; Jiménez-Sánchez, C.; Cádiz-Gurrea, M.; de la, L.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Genetic divergence among Brazilian garlic cultivars based on morphological characters and AFLP markers. Food Res. Int. 2014, 58, 35–46. [Google Scholar]
- Hacıseferoğulları, H.; Özcan, M.; Demir, F.; Çalışır, S. Some nutritional and technological properties of garlic (Allium sativum L.). J. Food Eng. 2005, 68, 463–469. [Google Scholar] [CrossRef]
- Vadalà, R.; Mottese, A.; Bua, G.; Salvo, A.; Mallamace, D.; Corsaro, C.; Vasi, S.; Giofrè, S.; Alfa, M.; Cicero, N.; Dugo, G. Statistical analysis of mineral concentration for the geographic identification of garlic samples from Sicily (Italy), Tunisia and Spain. Foods 2016, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Akinwande, B.A.; Olatunde, S. Comparative evaluation of the mineral profile and other selected components of onion and garlic. Int. Food Res. J. 2015, 22, 332–336. [Google Scholar]
- Põldma, P.; Tõnutare, T.; Viitak, A.; Luik, A.; Moor, U. Effect of selenium treatment on mineral nutrition, bulb size, and antioxidant properties of garlic (Allium sativum L.). J. Agric. Food Chem. 2011, 59, 5498–5503. [Google Scholar] [CrossRef] [PubMed]
- Ritota, M.; Casciani, L.; Han, B.-Z.; Cozzolino, S.; Leita, L.; Sequi, P.; Valentini, M. Traceability of Italian garlic (Allium sativum L.) by means of HRMAS-NMR spectroscopy and multivariate data analysis. Food Chem. 2012, 135, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Soto Vargas, V.C.; Elizabeth González, R.; Mirta Sance, M.; Luis Burba, J.; Beatriz Camargo, A. Genotype-environment interaction on the expression of allicin and pyruvic acid in garlic (Allium sativum L.). Rev. La Fac. Ciencias Agrar. 2010, 42, 15–22. [Google Scholar]
- Chhokar, V.; Sood, D.R.; Siwach, P.; Rani, M. Fatty acid composition of some promising genotypes of garlic (Allium sativum L.). Ann. Biol. 2006, 22, 23–26. [Google Scholar]
- Cardelle-Cobas, A.; Costo, R.; Corzo, N.; Villamiel, M. Fructo-oligosaccharide changes during the storage of dehydrated commercial garlic and onion samples. Int. J. Food Sci. Technol. 2009, 44, 947–952. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Yoon, M.K.; Kwak, J.H. Contents of phytochemical constituents and antioxidant activity of 19 garlic (Allium sativum L.) parental lines and cultivars. Hortic. Environ. Biotechnol. 2014, 55, 138–147. [Google Scholar] [CrossRef]
- Pardo, E.J.; Escribano, J.; Gómez, R.; Alvarruiz, A. Physical-chemical and sensory quality evaluation of garlic cultivars. J. Food Qual. 2007, 30, 607–622. [Google Scholar] [CrossRef]
- Imen, A.; Najjaa, H.; Neffati, M. Influence of sulfur fertilization on S-containing, phenolic, and carbohydrate metabolites in rosy garlic (Allium roseum L.): A wild edible species in North Africa. Eur. Food Res. Technol. 2013, 237, 521–527. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists (AOAC). Official Methods of Analysis of AOAC International, 18th ed.; Horwitz, W., Latimer, G., Eds.; MD: AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Association of Official Agricultural Chemists (AOAC). Official Methods of Analysis of AOAC International, 20th ed.; Horwitz, W., Latimer, G., Eds.; MD: AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Pereira, C.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Use of UFLC-PDA for the analysis of organic acids in thirty-five species of food and medicinal plants. Ind. Crops Prod. 2013, 49, 61–65. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Carvalho, A.M.; Santos-Buelga, C.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Nutrients, phytochemicals and bioactivity of wild Roman chamomile: A comparison between the herb and its preparations. Food Chem. 2013, 136, 718–725. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
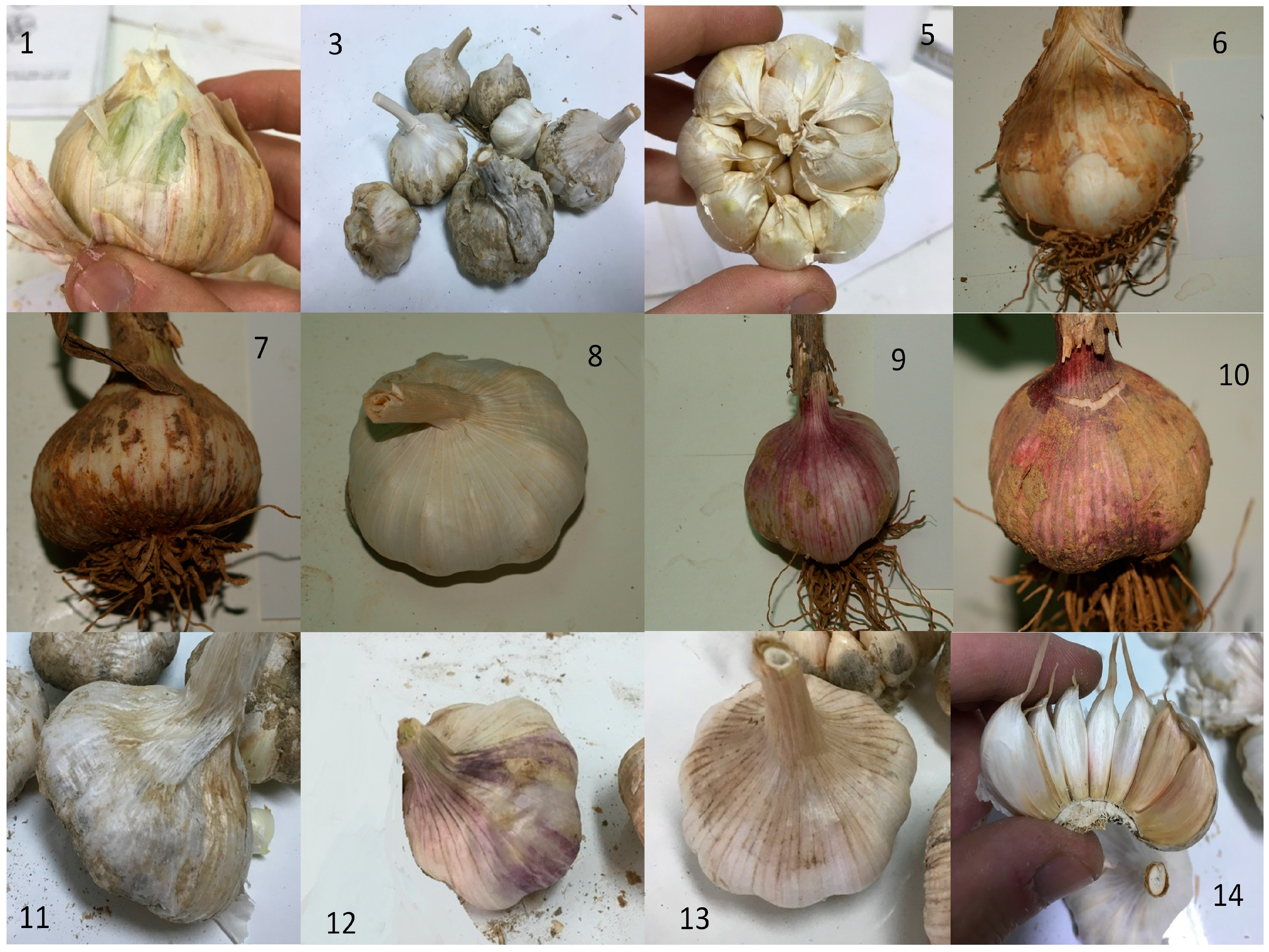
| Genotypes | Bulb * Shape | Bulb Skin Colour | Clove Skin Colour | Number of Cloves | Bulb Structure Type | Shape of the Compound Bulb in Horizontal Section | Average Weight of 10 Cloves | Dry Matter (%) | TSS (Brix°) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 1–6 | 2 | 4 | 1 | 1 | 4 | 37.60 | 37.9 |
| 2 | 3 | 1–6 | 2 | 4 | 1 | 1 | 4 | 39.91 | 36.4 |
| 3 | 2–3 | 1 | 1–2 | 6 | 1 | 1 | 1 | 38.86 | 39.4 |
| 4 | 3 | 1–6 | 3–5 | 4 | 1–2 | 1 | 4 | 35.80 | 35.2 |
| 5 | 3 | 1 | 1–2 | 4 | 1–2 | 1 | 3 | 32.61 | 33.7 |
| 6 | 2 | 2–3 | 2 | 4 | 1 | 1 | 4 | 34.02 | 34.2 |
| 7 | 3 | 2–3 | 2 | 4 | 1 | 1 | 2 | 32.60 | 34.3 |
| 8 | 3 | 1 | 2 | 5 | 1 | 1 | 4 | 35.71 | 37.7 |
| 9 | 3 | 1–6 | 2–5 | 4 | 1–2 | 1 | 2 | 31.67 | 33.6 |
| 10 | 3 | 1–6 | 2 | 4 | 1–2 | 1 | 3 | 32.27 | 31.8 |
| 11 | 2 | 2–3–5 | 3–5 | 4 | 1–2 | 1 | 4 | 42.64 | 40.4 |
| 12 | 2 | 2–3–5 | 3–5 | 6 | 1–2 | 1 | 4 | 40.52 | 37.6 |
| 13 | 2 | 2–3 | 3–5 | 5 | 1–2 | 1 | 5 | 36.65 | 36.8 |
| 14 | 2–3 | 1 | 1–2 | 6 | 1 | 1 | 1 | 33.94 | 39.0 |
| Genotypes | Humidity (%) | Ash (g/100 g f.w.) | Proteins (g/100 g f.w.) | Fat (g/100 g f.w.) | Carbohydrates (g/100 g f.w.) | Energy (kcal/100 g f.w.) |
|---|---|---|---|---|---|---|
| 1 | 62.38 ± 1.79 defg | 1.44 ± 0.02 fg | 6.44 ± 0.07 c | 0.21 ± 0.01 g | 29.53 ± 1.76 bcd | 145.76 ± 7.25 bcd |
| 2 | 60.09 ± 1.78 fgh | 1.47 ± 0.03 ef | 6.25 ± 0.05 d | 0.27 ± 0.01 e | 31.92 ± 1.77 bc | 155.08 ± 7.17 ab |
| 3 | 61.14 ± 0.96 efgh | 1.40 ± 0.03 g | 5.15 ± 0.07 g | 0.32 ± 0.01 bc | 31.99 ± 1.03 bc | 151.44 ± 3.87 abc |
| 4 | 64.20 ± 0.93 bcde | 1.65 ± 0.02 c | 6.23 ± 0.02 d | 0.30 ± 0.01 d | 27.62 ± 0.93 def | 138.16 ± 3.72 cdef |
| 5 | 67.39 ± 2.16 abc | 1.87 ± 0.01 a | 7.27 ± 0.05 b | 0.33 ± 0.02 b | 23.13 ± 2.19 h | 124.60 ± 8.72 f |
| 6 | 65.33 ± 1.13 abcd | 1.52 ± 0.03 de | 7.34 ± 0.05 ab | 0.28 ± 0.01 e | 25.53 ± 1.14 efgh | 133.96 ± 4.44 def |
| 7 | 67.39 ± 0.78 abc | 1.25 ± 0.03 h | 7.45 ± 0.18 a | 0.36 ± 0.01 a | 23.54 ± 0.90 gh | 127.20 ± 3.20 ef |
| 8 | 64.29 ± 1.57 bcde | 1.55 ± 0.04 d | 6.42 ± 0.02 c | 0.37 ± 0.01 a | 27.38 ± 1.58 defg | 138.48 ± 6.40 cdef |
| 9 | 68.33 ± 1.00 a | 1.26 ± 0.03 h | 6.27 ± 0.03 d | 0.26 ± 0.01 e | 23.87 ± 0.97 fgh | 122.94 ± 3.94 f |
| 10 | 67.73 ± 0.96 ab | 1.16 ± 0.03 i | 5.63 ± 0.03 f | 0.24 ± 0.02 f | 25.24 ± 0.96 efgh | 125.64 ± 3.85 ef |
| 11 | 57.36 ± 0.40 h | 1.87 ± 0.03 a | 4.62 ± 0.06 h | 0.12 ± 0.01 h | 36.03 ± 0.35 a | 163.69 ± 1.51 a |
| 12 | 59.48 ± 1.14 gh | 1.75 ± 0.04 b | 6.07 ± 0.02 e | 0.12 ± 0.01 h | 32.58 ± 1.15 ab | 155.69 ± 4.57 ab |
| 13 | 63.65 ± 1.85 cdef | 1.45 ± 0.03 efg | 6.42 ± 0.02 c | 0.20 ± 0.01 g | 28.28 ± 1.85 cde | 140.58 ± 7.39 bcde |
| 14 | 66.06 ± 0.71 abcd | 1.31 ± 0.02 h | 5.12 ± 0.04 g | 0.31 ± 0.01 cd | 27.19 ± 0.69 defg | 132.05 ± 2.80 def |
| G * | K | Na | Ca | Mg | Mn | Fe | Zn |
|---|---|---|---|---|---|---|---|
| 1 | 529 ± 16 de | 18.1 ± 1.5 d | 359 ± 47 c | 23.1 ± 4.3 e | 1.39 ± 0.08 abc | 3.52 ± 0.64 efg | 0.81 ± 0.08 cde |
| 2 | 492 ± 5 ef | 12.0 ± 1.6 e | 551 ± 76 b | 49.0 ± 5.2 b | 1.39 ± 0.20 abc | 5.12 ± 0.32 abc | 0.69 ± 0.18 defg |
| 3 | 446 ± 47 g | 36.0 ± 3.5 a | 321 ± 78 cd | 33.9 ± 2.7 cde | 1.26 ± 0.16 bc | 3.45 ± 0.36 efg | 0.55 ± 0.06 g |
| 4 | 508 ± 7 ef | 12.4 ± 2.1 e | 332 ± 49 cd | 37.5 ± 13.0 bc | 1.31 ± 0.19 bc | 5.43 ± 0.57 ab | 0.86 ± 0.06 cd |
| 5 | 583 ± 29 bc | 23.9 ± 2.1 b | 259 ± 42 d | 31.7 ± 1.9 cde | 1.23 ± 0.20 c | 4.62 ± 0.81 bcd | 1.52 ± 0.21 a |
| 6 | 605 ± 48 b | 12.1 ± 1.4 e | 347 ± 73 cd | 32.9 ± 9.3 cde | 1.35 ± 0.06 abc | 2.89 ± 0.24 g | 0.82 ± 0.08 cde |
| 7 | 472 ± 4 fg | 20.0 ± 1.4 cd | 163 ± 30 e | 30.2 ± 7.0 cde | 1.44 ± 0.12 ab | 3.37 ± 0.92 fg | 0.83 ± 0.04 cde |
| 8 | 552 ± 25 cd | 12.1 ± 1.2 e | 404 ± 22 c | 31.4 ± 5.0 cde | 1.37 ± 0.07 abc | 4.04 ± 0.85 def | 1.28 ± 0.21 b |
| 9 | 469 ± 13 fg | 22.8 ± 2.9 bc | 361 ± 37 c | 25.5 ± 1.3 de | 1.29 ± 0.07 bc | 2.88 ± 0.21 g | 0.89 ± 0.06 c |
| 10 | 480 ± 21 fg | 19.4 ± 1.7 d | 322 ± 57 cd | 34.4 ± 8.2 cde | 1.30 ± 0.07 bc | 4.69 ± 0.55 bcd | 1.19 ± 0.07 b |
| 11 | 585 ± 13 bc | 12.8 ± 1.7 e | 264 ± 95 d | 26.4 ± 11.2 cde | 1.54 ± 0.04 a | 4.38 ± 0.65 cde | 0.63 ± 0.04 efg |
| 12 | 675 ± 19 a | 7.0 ± 0.5 f | 333 ± 19 cd | 25.4 ± 6.9 de | 1.52 ± 0.04 a | 5.78 ± 0.59 a | 0.76 ± 0.03 cdef |
| 13 | 603 ± 26 b | 7.8 ± 1.1 f | 5557 ± 22 b | 23.5 ± 5.5 e | 1.36 ± 0.13 abc | 3.75 ± 0.66 defg | 0.72 ± 0.12 cdefg |
| 14 | 505 ± 27 ef | 18.8 ± 0.4 d | 963 ± 38 a | 63.1 ± 2.0 a | 1.31 ± 0.06 bc | 3.27 ± 0.46 fg | 0.58 ± 0.06 fg |
| Genotypes | Oxalic Acid | Malic Acid | Pyruvic Acid | Citric Acid | Total |
|---|---|---|---|---|---|
| 1 | 0.20 ± 0.01 h | 0.48 ± 0.01 c | 1.67 ± 0.01 c | 0.61 ± 0.01 i | 2.96 ± 0.01 e |
| 2 | 0.14 ± 0.01 k | 0.36 ± 0.01 d | 1.09 ± 0.01 j | 0.61 ± 0.01 i | 2.20 ± 0.01 i |
| 3 | 0.30 ± 0.01 b | 0.60 ± 0.01 a | 1.30 ± 0.01 i | 1.07 ± 0.01 c | 3.27 ± 0.01 c |
| 4 | 0.23 ± 0.01 g | 0.44 ± 0.01 c | 1.55 ± 0.01 e | 0.85 ± 0.01 e | 3.07 ± 0.01 d |
| 5 | 0.35 ± 0.01 a | 0.54 ± 0.01 b | 1.77 ± 0.01 b | 1.20 ± 0.01 a | 3.86 ± 0.01 a |
| 6 | 0.29 ± 0.01 c | 0.36 ± 0.01 d | 1.41 ± 0.01 g | 0.68 ± 0.03 g | 2.74 ± 0.04 f |
| 7 | 0.27 ± 0.01 e | 0.31 ± 0.01 e | 1.53 ± 0.01 e | 0.60 ± 0.02 i | 2.72 ± 0.01 f |
| 8 | 0.28 ± 0.01 d | 0.32 ± 0.01 de | 1.91 ± 0.01 a | 1.15 ± 0.01 b | 3.66 ± 0.02 b |
| 9 | 0.27 ± 0.01 e | 0.54 ± 0.08 b | 1.06 ± 0.01 k | 0.89 ± 0.01 d | 2.77 ± 0.06 f |
| 10 | 0.24 ± 0.01 f | 0.19 ± 0.02 f | 1.33 ± 0.03 h | 0.84 ± 0.02 e | 2.60 ± 0.03 g |
| 11 | 0.11 ± 0.01 l | 0.20 ± 0.01 f | 1.46 ± 0.01 f | 0.60 ± 0.01 i | 2.38 ± 0.01 h |
| 12 | 0.18 ± 0.01 i | 0.10 ± 0.01 g | 1.07 ± 0.01 jk | 0.81 ± 0.01 f | 2.16 ± 0.01 i |
| 13 | 0.23 ± 0.01 g | 0.30 ± 0.01 e | 1.58 ± 0.01 d | 0.65 ± 0.02 h | 2.75 ± 0.02 f |
| 14 | 0.15 ± 0.01 j | 0.18 ± 0.01 f | 0.95 ± 0.01 l | 0.68 ± 0.01 g | 1.96 ± 0.01 j |
| Genotypes | |||||||
| Fatty Acids | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| C6 : 0 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.95 ± 0.06 | 0.75 ± 0.01 | 0.92 ± 0.01 |
| C8 : 0 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.07 ± 0.01 | 0.97 ± 0.07 | 0.73 ± 0.01 | 0.99 ± 0.01 |
| C10 : 0 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.07 ± 0.01 | 2.59 ± 0.02 | 1.87 ± 0.05 | 2.42 ± 0.03 |
| C12 : 0 | 0.17 ± 0.01 | 0.17 ± 0.01 | 0.13 ± 0.01 | 0.14 ± 0.01 | 1.69 ± 0.02 | 1.09 ± 0.04 | 1.18 ± 0.01 |
| C13 : 0 | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.15 ± 0.01 | 0.08 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.15 ± 0.01 |
| C14 : 0 | 0.61 ± 0.03 | 0.55 ± 0.01 | 0.49 ± 0.01 | 0.40 ± 0.01 | 3.50 ± 0.05 | 2.35 ± 0.07 | 2.47 ± 0.01 |
| C14 : 1 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.36 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| C15 : 0 | 0.42 ± 0.01 | 0.45 ± 0.01 | 0.38 ± 0.01 | 0.49 ± 0.01 | 0.59 ± 0.01 | 0.47 ± 0.02 | 0.50 ± 0.01 |
| C16 : 0 | 15.93 ± 0.28 | 16.43 ± 0.07 | 14.25 ± 0.03 | 13.62 ± 0.12 | 18.46 ± 0.21 | 17.61 ± 0.27 | 16.62 ± 0.06 |
| C16 : 1 | 0.58 ± 0.04 | 0.63 ± 0.01 | 0.53 ± 0.01 | 0.46 ± 0.02 | 0.47 ± 0.02 | 0.67 ± 0.06 | 0.54 ± 0.03 |
| C17 : 0 | 0.62 ± 0.01 | 0.71 ± 0.01 | 0.66 ± 0.01 | 0.72 ± 0.01 | 0.87 ± 0.01 | 0.67 ± 0.02 | 0.73 ± 0.01 |
| C18 : 0 | 4.84 ± 0.01 | 4.35 ± 0.05 | 3.39 ± 0.04 | 2.85 ± 0.01 | 8.34 ± 0.05 | 5.87 ± 0.05 | 6.26 ± 0.01 |
| C18 : 1n9 | 14.44 ± 0.05 | 12.64 ± 0.01 | 13.64 ± 0.04 | 6.75 ± 0.05 | 14.74 ± 0.06 | 11.80 ± 0.07 | 11.40 ± 0.01 |
| C18 : 2n6 | 49.99 ± 0.16 | 49.48 ± 0.18 | 51.58 ± 0.12 | 52.14 ± 0.12 | 32.54 ± 0.10 | 44.93 ± 0.23 | 43.39 ± 0.04 |
| C18 : 3n3 | 6.38 ± 0.07 | 7.52 ± 0.06 | 8.41 ± 0.01 | 9.14 ± 0.02 | 7.07 ± 0.01 | 6.55 ± 0.06 | 6.70 ± 0.01 |
| C20 : 0 | 0.92 ± 0.04 | 1.02 ± 0.01 | 1.18 ± 0.04 | 2.56 ± 0.01 | 1.47 ± 0.04 | 0.71 ± 0.03 | 0.94 ± 0.07 |
| C20 : 1 | 0.29 ± 0.01 | 0.28 ± 0.02 | 0.22 ± 0.01 | 0.37 ± 0.03 | 0.32 ± 0.01 | 0.17 ± 0.02 | 0.15 ± 0.01 |
| C20 : 2 | 0.08 ± 0.01 | 0.06 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.10 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 |
| C20 : 3 | 0.31 ± 0.01 | 0.33 ± 0.03 | 0.34 ± 0.01 | 0.39 ± 0.01 | 0.29 ± 0.02 | 0.24 ± 0.01 | 0.25 ± 0.02 |
| C20 : 5n3 | 0.38 ± 0.03 | 0.48 ± 0.02 | 0.48 ± 0.01 | 3.50 ± 0.01 | 1.23 ± 0.01 | 0.33 ± 0.01 | 0.81 ± 0.01 |
| C22 : 0 | 2.23 ± 0.04 | 2.68 ± 0.02 | 2.21 ± 0.02 | 3.73 ± 0.03 | 2.43 ± 0.13 | 1.70 ± 0.08 | 2.04 ± 0.01 |
| C22 : 1n9 | 0.32 ± 0.01 | 0.35 ± 0.01 | 0.24 ± 0.01 | 0.32 ± 0.01 | 0.28 ± 0.01 | 0.18 ± 0.01 | 0.22 ± 0.01 |
| C23 : 0 | 0.55 ± 0.03 | 0.72 ± 0.03 | 0.61 ± 0.04 | 0.78 ± 0.02 | 0.44 ± 0.01 | 0.51 ± 0.01 | 0.49 ± 0.02 |
| C24 : 0 | 0.53 ± 0.02 | 0.77 ± 0.01 | 0.73 ± 0.01 | 0.91 ± 0.01 | 0.46 ± 0.01 | 0.49 ± 0.01 | 0.68 ± 0.06 |
| Total SFA (% of total FA) | 27.19 ± 0.21 g | 28.21 ± 0.16 e | 24.40 ± 0.08 k | 26.46 ± 0.14 h | 42.91 ± 0.01 a | 34.98 ± 0.32 c | 36.39 ± 0.02 b |
| Total MUFA (% of total FA) | 15.65 ± 0.01 b | 13.91 ± 0.04 d | 14.66 ± 0.04 c | 8.25 ± 0.05 k | 15.86 ± 0.05 a | 12.85 ± 0.03 e | 12.34 ± 0.03 f |
| Total PUFA (% of total FA) | 57.16 ± 0.20 h | 57.87 ± 0.20 g | 60.94 ± 0.12 f | 65.29 ± 0.10 d | 41.23 ± 0.05 k | 52.16 ± 0.29 i | 51.27 ± 0.06 j |
| Genotypes | |||||||
| Fatty Acids | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| C6 : 0 | 0.14 ± 0.01 | 0.27 ± 0.01 | 0.22 ± 0.01 | 0.22 ± 0.01 | 0.09 ± 0.01 | 0.15 ± 0.01 | 0.02 ± 0.01 |
| C8 : 0 | 0.16 ± 0.01 | 0.27 ± 0.01 | 0.22 ± 0.01 | 0.24 ± 0.01 | 0.08 ± 0.01 | 0.19 ± 0.01 | 0.47 ± 0.03 |
| C10 : 0 | 0.36 ± 0.01 | 0.75 ± 0.01 | 0.57 ± 0.02 | 0.56 ± 0.02 | 0.21 ± 0.01 | 0.47 ± 0.02 | 0.17 ± 0.01 |
| C12 : 0 | 0.48 ± 0.04 | 0.59 ± 0.01 | 0.51 ± 0.02 | 0.55 ± 0.01 | 0.40 ± 0.01 | 0.48 ± 0.01 | 0.29 ± 0.01 |
| C13 : 0 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | nd | nd | nd | 0.07 ± 0.01 |
| C14 : 0 | 0.79 ± 0.03 | 1.15 ± 0.01 | 0.99 ± 0.02 | 1.01 ± 0.01 | 0.74 ± 0.04 | 1.06 ± 0.01 | 0.50 ± 0.01 |
| C14 : 1 | 0.09 ± 0.01 | 0.16 ± 0.01 | 0.40 ± 0.01 | 0.50 ± 0.01 | 0.41 ± 0.01 | 0.37 ± 0.01 | 0.40 ± 0.01 |
| C15 : 0 | 0.42 ± 0.01 | 0.47 ± 0.01 | 0.52 ± 0.01 | 0.43 ± 0.01 | 0.39 ± 0.01 | 0.40 ± 0.01 | 0.41 ± 0.01 |
| C16 : 0 | 14.66 ± 0.14 | 14.33 ± 0.02 | 15.76 ± 0.08 | 14.85 ± 0.01 | 14.52 ± 0.07 | 15.78 ± 0.12 | 13.73 ± 0.05 |
| C16 : 1 | 0.61 ± 0.02 | 0.56 ± 0.03 | 0.64 ± 0.02 | 0.57 ± 0.04 | 0.60 ± 0.01 | 0.67 ± 0.01 | 0.60 ± 0.01 |
| C17 : 0 | 0.69 ± 0.01 | 0.78 ± 0.02 | 0.77 ± 0.06 | 0.58 ± 0.01 | 0.61 ± 0.01 | 0.61 ± 0.01 | 0.76 ± 0.02 |
| C18 : 0 | 3.31 ± 0.01 | 4.39 ± 0.01 | 4.15 ± 0.01 | 3.75 ± 0.05 | 3.42 ± 0.01 | 4.84 ± 0.01 | 2.62 ± 0.01 |
| C18 : 1n9 | 8.65 ± 0.01 | 10.31 ± 0.01 | 9.10 ± 0.05 | 6.34 ± 0.07 | 6.21 ± 0.02 | 10.63 ± 0.02 | 7.01 ± 0.02 |
| C18 : 2n6 | 49.37 ± 0.21 | 42.48 ± 0.01 | 50.23 ± 0.28 | 58.15 ± 0.26 | 58.97 ± 0.01 | 52.76 ± 0.05 | 54.66 ± 0.05 |
| C18 : 3n3 | 10.45 ± 0.05 | 9.59 ± 0.01 | 9.65 ± 0.01 | 6.36 ± 0.09 | 6.77 ± 0.06 | 6.54 ± 0.01 | 9.96 ± 0.02 |
| C20 : 0 | 2.00 ± 0.04 | 2.78 ± 0.01 | 0.97 ± 0.02 | 0.89 ± 0.04 | 1.01 ± 0.03 | 0.72 ± 0.02 | 1.38 ± 0.03 |
| C20 : 1 | 0.34 ± 0.01 | 0.26 ± 0.01 | 0.19 ± 0.01 | 0.17 ± 0.01 | 0.18 ± 0.01 | 0.13 ± 0.01 | 0.16 ± 0.01 |
| C20 : 2 | 0.16 ± 0.01 | 0.14 ± 0.01 | 0.19 ± 0.01 | 0.20 ± 0.01 | 0.15 ± 0.01 | 0.12 ± 0.01 | 0.180.01 |
| C20 : 3 | 0.31 ± 0.01 | 0.28 ± 0.02 | 0.27 ± 0.01 | 0.31 ± 0.01 | 0.32 ± 0.01 | 0.24 ± 0.01 | 0.45 ± 0.01 |
| C20 : 5n3 | 1.86 ± 0.11 | 4.59 ± 0.01 | 0.70 ± 0.01 | 1.11 ± 0.02 | 1.36 ± 0.01 | 1.08 ± 0.01 | 1.45 ± 0.14 |
| C22 : 0 | 3.34 ± 0.10 | 3.88 ± 0.04 | 2.21 ± 0.02 | 1.95 ± 0.06 | 2.02 ± 0.01 | 1.45 ± 0.02 | 3.07 ± 0.01 |
| C22 : 1n9 | 0.32 ± 0.01 | 0.28 ± 0.01 | 0.18 ± 0.01 | 0.27 ± 0.01 | 0.25 ± 0.01 | 0.31 ± 0.01 | 0.17 ± 0.01 |
| C23 : 0 | 0.69 ± 0.01 | 0.66 ± 0.03 | 0.72 ± 0.06 | 0.52 ± 0.04 | 0.61 ± 0.05 | 0.49 ± 0.01 | 0.73 ± 0.01 |
| C24 : 0 | 0.80 ± 0.06 | 1.01 ± 0.04 | 0.83 ± 0.05 | 0.47 ± 0.01 | 0.70 ± 0.01 | 0.52 ± 0.04 | 0.75 ± 0.01 |
| Total SFA (% of total FA) | 27.85 ± 0.12 f | 31.36 ± 0.01 d | 28.46 ± 0.31 e | 26.03 ± 0.02 i | 24.79 ± 0.08 j | 27.16 ± 0.08 g | 24.97 ± 0.08 j |
| Total MUFA (% of total FA) | 10.01 ± 0.03 j | 11.55 ± 0.01 h | 10.51 ± 0.03 i | 7.85 ± 0.12 l | 7.64 ± 0.01 m | 12.11 ± 0.03 g | 8.34 ± 0.02 k |
| Total PUFA (% of total FA) | 62.13 ± 0.15 e | 57.08 ± 0.01 h | 61.03 ± 0.28 f | 66.12 ± 0.14 c | 67.56 ± 0.06 a | 60.74 ± 0.05 f | 66.70 ± 0.10 b |
| Genotypes | Fructose | Glucose | Sucrose | Total Sugars |
|---|---|---|---|---|
| 1 | 0.13 ± 0.01 e | 0.11 ± 0.01 b | 2.15 ± 0.04 d | 2.39 ± 0.04 f |
| 2 | 0.10 ± 0.01 fg | tr | 2.39 ± 0.04 c | 2.49 ± 0.05 ef |
| 3 | 0.41 ± 0.03 a | 0.27 ± 0.01 a | 2.76 ± 0.01 b | 3.45 ± 0.03 a |
| 4 | 0.22 ± 0.01 c | 0.11 ± 0.01 b | 2.73 ± 0.01 b | 3.06 ± 0.01 b |
| 5 | 0.32 ± 0.01 b | tr | 2.75 ± 0.02 b | 3.07 ± 0.01 b |
| 6 | 0.12 ± 0.01 ef | tr | 1.99 ± 0.03 e | 2.11 ± 0.03 h |
| 7 | 0.21 ± 0.01 c | tr | 2.05 ± 0.01 de | 2.25 ± 0.01 g |
| 8 | 0.17 ± 0.01 d | tr | 3.29 ± 0.06 a | 3.46 ± 0.06 a |
| 9 | 0.20 ± 0.02 c | tr | 2.72 ± 0.12 b | 2.91 ± 0.13 c |
| 10 | 0.09 ± 0.01 g | tr | 2.67 ± 0.03 b | 2.76 ± 0.03 d |
| 11 | 0.09 ± 0.01 g | tr | 2.34 ± 0.06 c | 2.43 ± 0.07 ef |
| 12 | 0.08 ± 0.02 g | tr | 2.34 ± 0.13 c | 2.42 ± 0.14 f |
| 13 | 0.08 ± 0.01 g | tr | 2.09 ± 0.02 de | 2.17 ± 0.03 gh |
| 14 | 0.13 ± 0.01 e | tr | 2.43 ± 0.01 c | 2.56 ± 0.01 e |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petropoulos, S.A.; Fernandes, Â.; Ntatsi, G.; Petrotos, K.; Barros, L.; Ferreira, I.C.F.R. Nutritional Value, Chemical Characterization and Bulb Morphology of Greek Garlic Landraces. Molecules 2018, 23, 319. https://doi.org/10.3390/molecules23020319
Petropoulos SA, Fernandes Â, Ntatsi G, Petrotos K, Barros L, Ferreira ICFR. Nutritional Value, Chemical Characterization and Bulb Morphology of Greek Garlic Landraces. Molecules. 2018; 23(2):319. https://doi.org/10.3390/molecules23020319
Chicago/Turabian StylePetropoulos, Spyridon A., Ângela Fernandes, Georgia Ntatsi, Konstantinos Petrotos, Lillian Barros, and Isabel C. F. R. Ferreira. 2018. "Nutritional Value, Chemical Characterization and Bulb Morphology of Greek Garlic Landraces" Molecules 23, no. 2: 319. https://doi.org/10.3390/molecules23020319
APA StylePetropoulos, S. A., Fernandes, Â., Ntatsi, G., Petrotos, K., Barros, L., & Ferreira, I. C. F. R. (2018). Nutritional Value, Chemical Characterization and Bulb Morphology of Greek Garlic Landraces. Molecules, 23(2), 319. https://doi.org/10.3390/molecules23020319











