Tulbaghia violacea and Allium ursinum Extracts Exhibit Anti-Parasitic and Antimicrobial Activities
Abstract
:1. Introduction
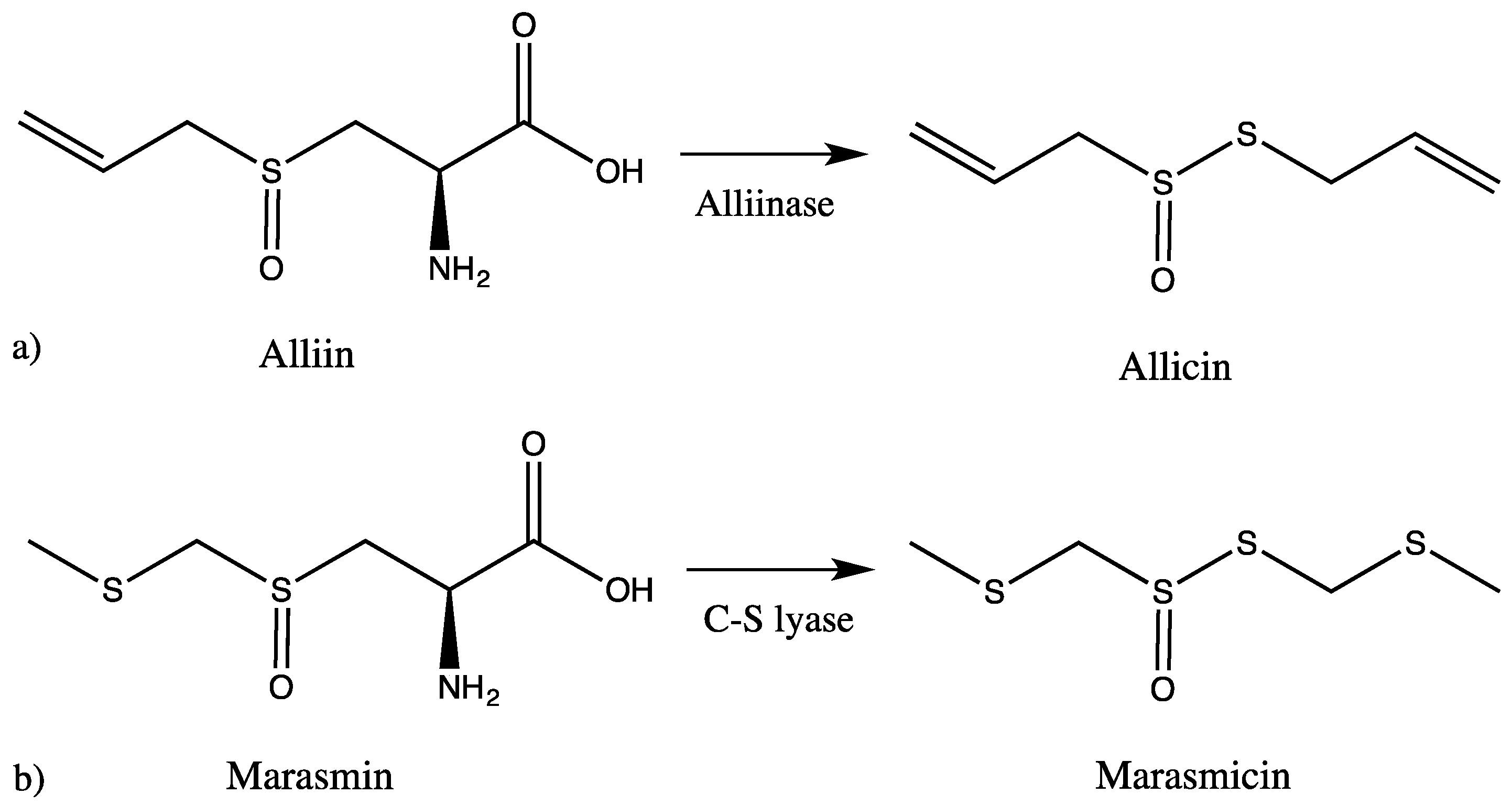
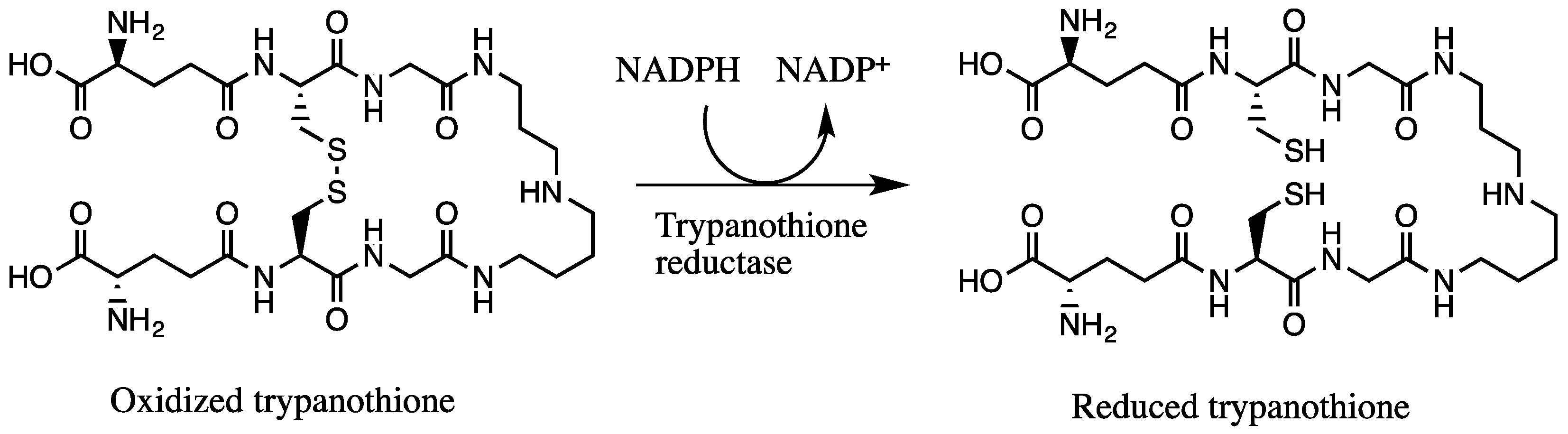
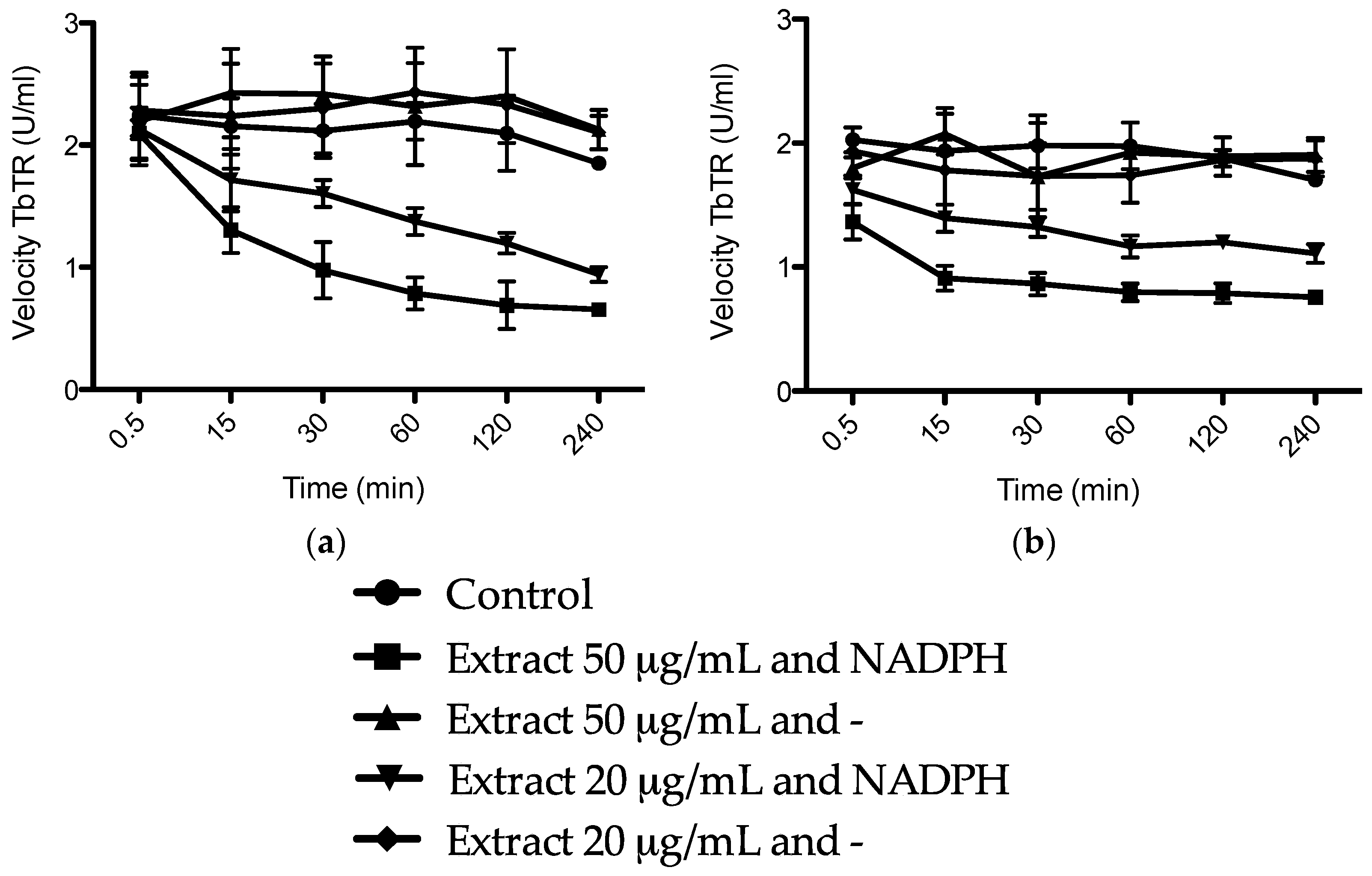
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Extract Preparation
3.3. Cell Lines
3.4. Screening of Trypanocidal, Leishmanicidal and Cytotoxic Activities
3.5. Selectivity Index
3.6. Trypanosoma brucei Trypanothione Reductase (TbTR) Inhibition Assay
3.7. Antimicrobial Tests
3.8. HPLC-MS/MS Analysis
Reference
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Petrovska, B.B.; Cekovska, S. Extracts from the history and medical properties of garlic. Pharmacogn. Rev. 2010, 4, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Garlic (Herbs/Suppl), Medscape. Available online: http://reference.medscape.com/drug/ail-ajo-garlic-344474#2 (accessed on 11 April 2016).
- Moodley, K.; Joseph, K.; Naidoo, Y.; Islam, S.; Mackraj, I. Antioxidant, antidiabetic and hypolipidemic effects of Tulbaghia violacea Harv. (wild garlic) rhizome methanolic extract in a diabetic rat model. BMC Complement. Altern. Med. 2015, 15, 408. [Google Scholar] [CrossRef] [PubMed]
- Nok, A.J.; Williams, S.; Onyenekwe, P.C. Allium sativum-induced death of African trypanosomes. Parasitol. Res. 1996, 82, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Sendl, A.; Elbl, G.; Steinke, B.; Redl, K.; Breu, W.; Wagner, H. Comparative pharmacological investigations of Allium ursinum and Allium sativum. Planta Med. 1992, 58, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.-E.; Wink, M. Medicinal Plants of the World; Briza Publications: Pretoria, South Africa, 2017. [Google Scholar]
- Van Wyk, B.-E.; Wink, M. Phytomedicines, Herbal Drugs, and Poisons; University of Chicago Press: Chicago, IL, USA, 2015. [Google Scholar]
- Watt, J.M.; Breyer-Brandwijk, M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa Being an Account of Their Medicinal and other Uses, Chemical Composition, Pharmacological Effects and Toxicology in Man and Animal, 2nd ed.; E&S Livingston: Edinburgh, UK, 1962. [Google Scholar]
- Tulbaghia violacea, The Plant List, 2013. Available online: http://www.theplantlist.org/tpl1.1/record/kew-289698 (accessed on 13 June 2016).
- Aremu, A.O.; Van Staden, J. The genus Tulbaghia (Alliaceae)—A review of its ethnobotany, pharmacology, phytochemistry and conservation needs. J. Ethnopharmacol. 2013, 149, 387–400. [Google Scholar] [CrossRef] [PubMed]
- McGaw, L.J.; Jager, A.K.; van Staden, J. Antibacterial, anthelmintic and anti-amoebic activity in South African medicinal plants. J. Ethnopharmacol. 2000, 72, 247–263. [Google Scholar] [CrossRef]
- Ncube, B.; Ngunge, V.N.; Finnie, J.F.; Van Staden, J. A comparative study of the antimicrobial and phytochemical properties between outdoor grown and micropropagated Tulbaghia violacea Harv. plants. J. Ethnopharmacol. 2011, 134, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.-E.; Wink, C.; Wink, M. Handbuch der Arzneipflanzen, 3rd ed.; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2015. [Google Scholar]
- Sobolewska, D.; Podolak, I.; Makowska-Was, J. Allium ursinum: Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2015, 14, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Song, G.Q.; Yu, Y.Q.; Ma, H.Y.; Ma, L.; Jin, Y.N. Apoptosis and G2/M arrest induced by Allium ursinum (ramson) watery extract in an AGS gastric cancer cell line. OncoTargets Ther. 2013, 6, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavallito, C.; Bailey, J.; Buck, J. The antibacterial principle of Allium sativum. III. Its precursor and “essential oil of garlic”. J. Am. Chem. Soc. 1945, 67, 1032–1033. [Google Scholar] [CrossRef]
- Cavallito, C.; Bailey, J. Allicin, the antibacterial principle of Allium sativum. I. Isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 1944, 66, 1950–1951. [Google Scholar] [CrossRef]
- Kubec, R.; Krejcova, P.; Mansur, L.; Garcia, N. Flavor precursors and sensory-active sulfur compounds in Alliaceae species native to South Africa and South America. J. Agric. Food Chem. 2013, 6, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Medicinal plants: A source of anti-parasitic secondary metabolites. Molecules 2012, 17, 12771–12791. [Google Scholar] [CrossRef] [PubMed]
- Fairlamb, A.H.; Cerami, A. Metabolism and functions of trypanothione in the Kinetoplastida. Annu. Rev. Microbiol. 1992, 46, 695–729. [Google Scholar] [CrossRef] [PubMed]
- Irigoin, F.; Cibils, L.; Comini, M.A.; Wilkinson, S.R.; Flohe, L.; Radi, R. Insights into the redox biology of Trypanosoma cruzi: Trypanothione metabolism and oxidant detoxification. Free Radic. Biol. Med. 2008, 45, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Hiromu, K.; Seiji, H. Two sulfur constituents from Allium Schoenoprasum. Phytochemistry 1983, 22, 294–295. [Google Scholar] [CrossRef]
- Ferary, S.; Auger, J.; Touche, A. Trace identification of plant substances by combining gas chromatography-mass spectrometry and direct deposition gas chromatography-Fourier transform infrared spectrometry. Talanta 1996, 43, 349–357. [Google Scholar] [CrossRef]
- Calvey, E.M.; White, K.D.; Matusik, J.E.; Sha, D.; Block, E. Allium chemistry: Identification of organosulfur compounds in ramp (Allium tricoccum) homogenates. Phytochemistry 1998, 49, 359–364. [Google Scholar] [CrossRef]
- Mondy, N.; Naudin, A.; Christides, J.P.; Mandon, N.; Auger, J. Comparison of GC-MS and HPLC for the analysis of Allium volatiles. Chromatographia 2001, 53, 356–360. [Google Scholar] [CrossRef]
- Kusterer, J.; Keusgen, M. Cysteine sulfoxides and volatile sulfur compounds from Allium tripedale. J. Agric. Food Chem. 2009, 58, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Kucerova, P.; Kubec, R.; Simek, P.; Vaclavik, L.; Schraml, J. Allium discoloration: The precursor and formation of the red pigment in giant onion (Allium giganteum Regel) and some other subgenus Melanocrommyum species. J. Agric. Food Chem. 2011, 59, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Sobolewska, D.; Janeczko, Z.; Kisiel, W.; Podolak, I.; Galanty, A.; Trojanowska, D. Steroidal glycosides from the underground parts of Allium ursinum L. and their cytostatic and antimicrobial activity. Acta Pol. Pharm. 2006, 63, 219–223. [Google Scholar] [PubMed]
- Bagiu, R.V.; Vlaicu, B.; Butnariu, M. Chemical composition and in vitro antifungal activity screening of the Allium ursinum L. (Liliaceae). Int. J. Mol. Sci. 2012, 13, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Motsei, M.L.; Lindsey, K.L.; van Staden, J.; Jager, A.K. Screening of traditionally used South African plants for antifungal activity against Candida albicans. J. Ethnopharmacol. 2003, 86, 235–241. [Google Scholar] [CrossRef]
- Thamburan, S.; Klaasen, J.; Mabusela, W.T.; Cannon, J.F.; Folk, W.; Johnson, Q. Tulbaghia alliacea phytotherapy: A potential anti-infective remedy for candidiasis. Phytother. Res. 2006, 20, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Leroux, A.E.; Krauth-Siegel, R.L. Thiol redox biology of trypanosomatids and potential targets for chemotherapy. Mol. Biochem. Parasitol. 2015, 206, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Gallwitz, H.; Bonse, S.; Martinez-Cruz, A.; Schlichting, I.; Schumacher, K.; Krauth-Siegel, R.L. Ajoene is an inhibitor and subversive substrate of human glutathione reductase and Trypanosoma cruzi trypanothione reductase: Crystallographic, kinetic, and spectroscopic studies. J. Med. Chem. 1999, 42, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Baltz, T.; Baltz, D.; Giroud, C.; Crockett, J. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 1985, 4, 1273–1277. [Google Scholar] [PubMed]
- Wallbanks, K.R.; Maazoun, R.; Canning, E.U.; Rioux, J.A. The identity of Leishmania tarentolae Wenyon 1921. Parasitology 1985, 90, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Jockers-Scherubl, M.C.; Schirmer, R.H.; Krauth-Siegel, R.L. Trypanothione reductase from Trypanosoma cruzi. Catalytic properties of the enzyme and inhibition studies with trypanocidal compounds. Eur. J. Biochem. 1989, 180, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, F.X.; Walsh, C.T. Cloning, sequencing, overproduction and purification of trypanothione reductase from Trypanosoma cruzi. Mol. Biochem. Parasitol. 1991, 44, 145–147. [Google Scholar] [CrossRef]
- Comini, M.A.; Dirdjaja, N.; Kaschel, M.; Krauth-Siegel, R.L. Preparative enzymatic synthesis of trypanothione and trypanothione analogues. Int. J. Parasitol. 2009, 39, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Krstin, S.; Mohamed, T.; Wang, X.; Wink, M. How do the alkaloids emetine and homoharringtonine kill trypanosomes? An insight into their molecular modes of action. Phytomedicine 2016, 23, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Sobeh, M.; Esmat, A.; Petruk, G.; Abdelfattah, M.A.O.; Dmirieh, M.; Monti, D.M.; Abdel-Naim, A.B.; Wink, M. Phenolic compounds from Syzygium jambos (Myrtaceae) exhibit distinct antioxidant and hepatoprotective activities in vivo. J. Funct. Foods 2018, 41, 223–231. [Google Scholar] [CrossRef]
Sample Availability: Samples of the plant materials are not available from the authors. |
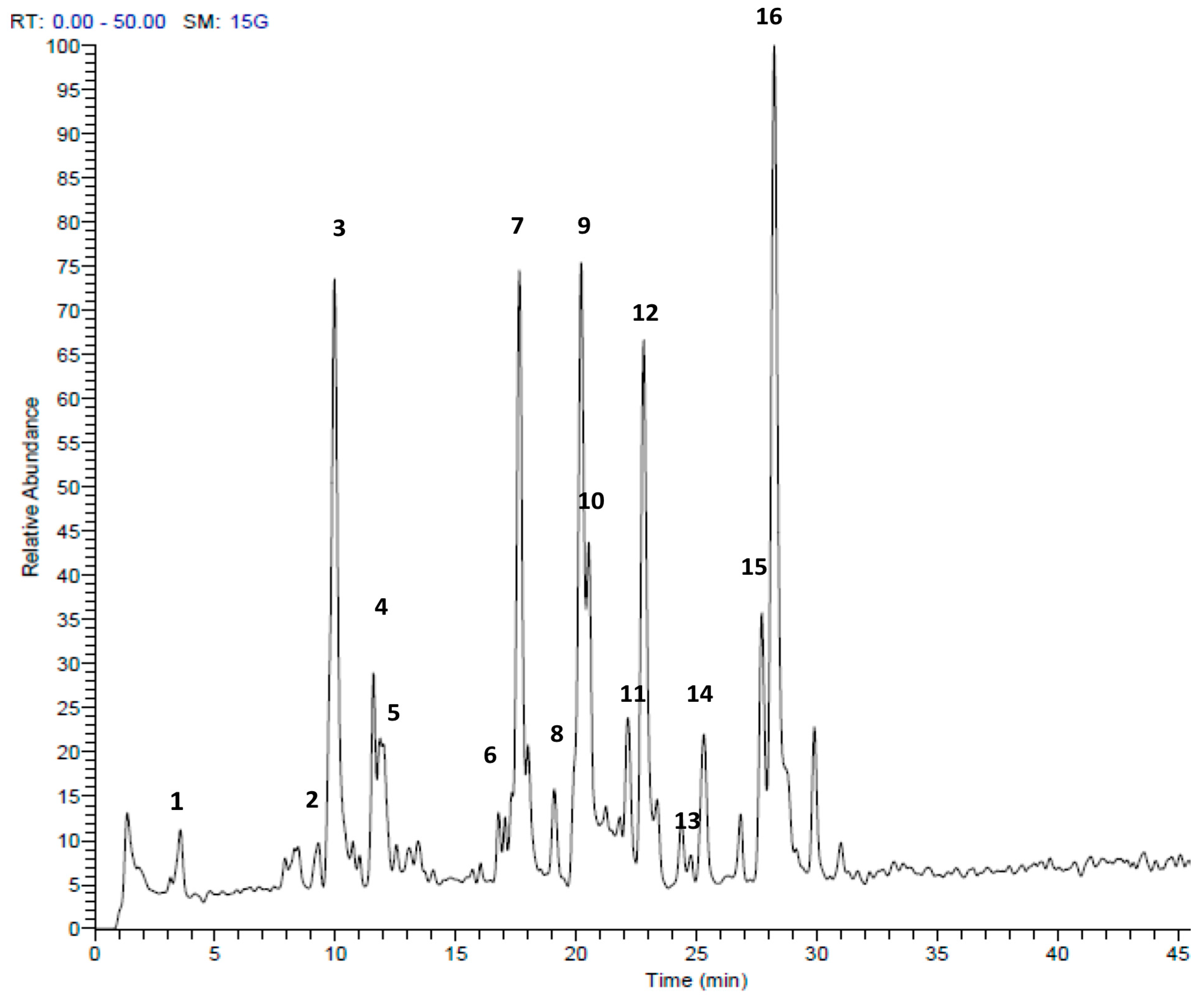
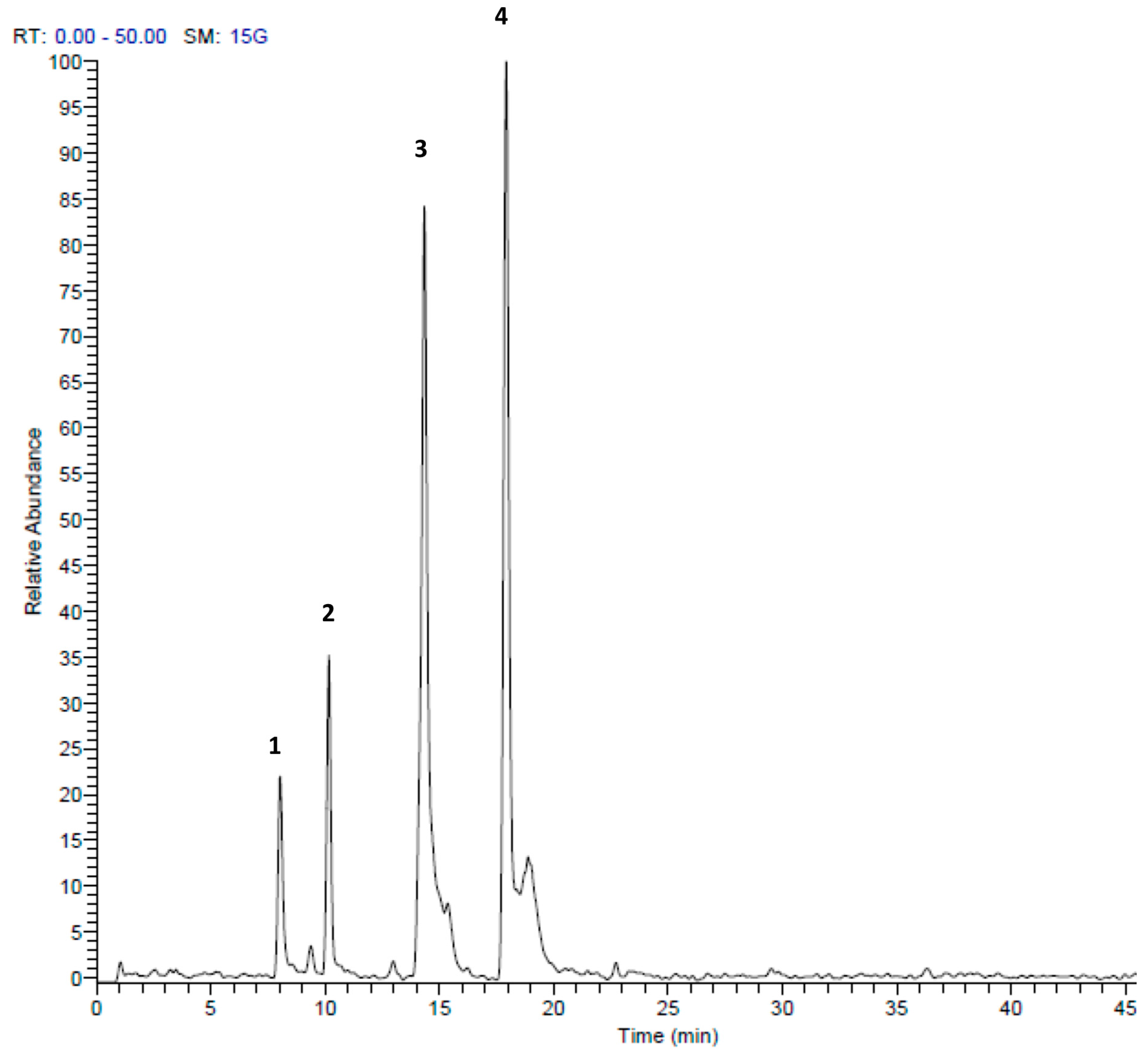
| Peak No. | tR | [M + H]+ | Area % | Proposed Compound | Reference |
|---|---|---|---|---|---|
| 1 | 3.56 | 151 | 1.67 | Methyl pentyl disulfide | [23] |
| 2 | 9.73 | 137 | 0.90 | Methanesulfinothioic acid S-(E)-1-propenyl ester | [24] |
| 3 | 9.93 | 137 | 14.02 | Methanesulfinothioic acid S-(Z)-1-propenyl ester | [24] |
| 4 | 11.57 | 137 | 2.96 | S-Methyl 1-propenesulfinothioate | [24] |
| 5 | 11.83 | 137 | 3.75 | S-1-Propenyl methanesulfinothioate | [24] |
| 6 | 16.77 | 185 | 1.07 | Methyl 1-(methylsulfinyl)propyl disulfide | [25] |
| 7 | 17.65 | 163 | 12.83 | Allicin | [26] |
| 8 | 19.92 | 209 | 1.67 | (E)-1-allyl-2-(3-(methylesulfinyl)prop-1-en-1-yl)disulfane | Tentative |
| 9 | 20.20 | 163 | 12.39 | 2-Propene-1-sulfinothioic acid S-(E)-1-propenyl ester | [24] |
| 10 | 20.54 | 163 | 5.78 | Propene-1-sulfinothioic acid S-(Z)-1-propenyl ester | [24] |
| 11 | 22.16 | 211 | 2.45 | 1-(methylsulfinyl)propyl(E,Z)-1-propenyl disulfide | [25] |
| 12 | 22.81 | 211 | 10.88 | Methyl 1-(2-propenylsulfinyl)propyl disulfide | [25] |
| 13 | 23.37 | 211 | 1.22 | 1-(methysulfinyl) propyl 2-propenyl disulfide | [25] |
| 14 | 25.31 | 235 | 3.27 | Ajoene | [26] |
| 15 | 27.69 | 237 | 4.65 | (E)-1-propenyl 1-(1-propenylsulfinyl)propyl disulfide | [25] |
| 16 | 28.25 | 237 | 20.50 | 2-Propenyl 1(2-pro-penylsulfinyl) propyl disulfide | [25] |
| Peak No. | tR | [M + H]+ | Area % | Proposed Compound | Reference |
|---|---|---|---|---|---|
| 1 | 8.01 | 157 | 4.21 | S-Propyl thiosulfate | Tentative |
| 2 | 10.16 | 303 | 11.30 | Di-(1-S-sulfoxymethyl-butyl)-disulfide | [27] |
| 3 | 14.34 | 203 | 32.03 | S-(2-Pyrrolyl)cysteine S-oxide | [28] |
| 4 | 17.92 | 203 | 52.46 | 2,4,5,7-tetrathiaoctane4-oxide (marasmicin) | [19] |
| Gram Type | Sample Indicator Strain | A. ursinum | T. violacea | Ciprofloxacin | Ampicillin | Nystatin | ||
|---|---|---|---|---|---|---|---|---|
| MIC | MMC | MIC | MMC | MIC | MIC | MIC | ||
| + | Bacillus subtilis | 80 | 160 | 40 | 80 | ≤0.03 | ≤0.03 | NT |
| + | MRSA | 80 | >320 | 320 | >320 | 0.03 | 16 | NT |
| + | MRSA CI | 80 | >320 | 320 | >320 | 4 | 16 | NT |
| + | Staphylococcus epidermidis | 80 | >320 | 160 | >320 | 0.03 | 0.5 | NT |
| + | Enterococcus faecalis | 320 | >320 | >320 | >320 | 0.5 | 1 | NT |
| + | VRE | >320 | >320 | >320 | >320 | 0.5 | 1 | NT |
| + | Streptococcus pyogenes | 160 | 160 | 160 | 160 | 0.13 | <0.03 | NT |
| − | Escherichia coli | 80 | >320 | 80 | 320 | ≤0.03 | 4 | NT |
| − | Escherichia coli EHEC | 160 | >320 | 160 | >320 | ≤0.03 | 4 | NT |
| − | Klebsiella pneumoniae | 80 | >320 | 160 | >320 | 0.125 | >64 | NT |
| − | Klebsiella pneumoniae CI | 160 | >320 | 160 | >320 | <0.03 | 32 | NT |
| − | Pseudomonas aeruginosa | 40 | >320 | 160 | >320 | ≤0.03 | >64 | NT |
| F | Candida albicans | 20 | 20 | 20 | 20 | NT | NT | 10 |
| F | Candida parapsilosis | 10 | 10 | 20 | 40 | NT | NT | 10 |
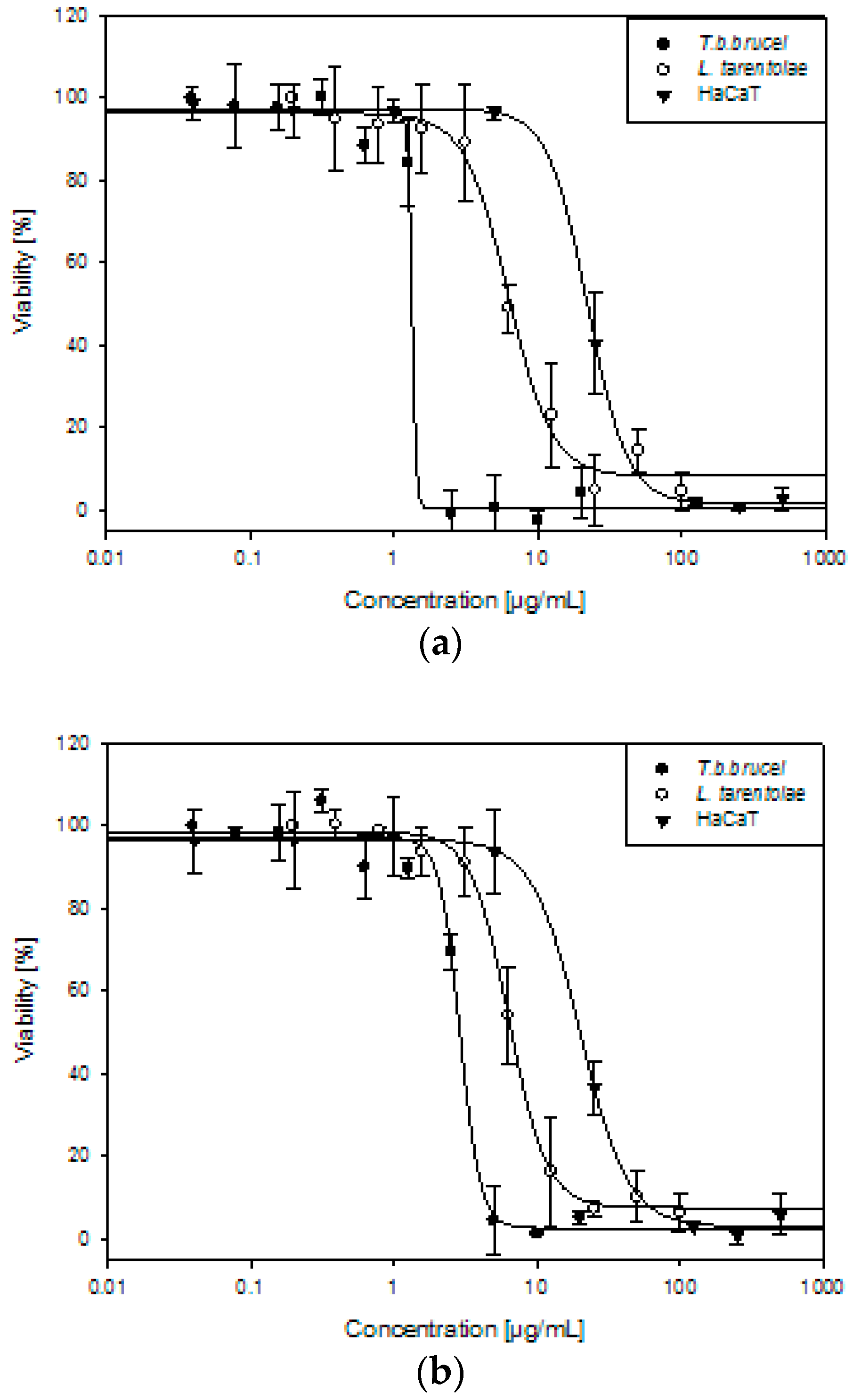
| T. b. brucei | L. tarentolae | HaCaT | SI | ||
|---|---|---|---|---|---|
| HaCaT/T. b. b. | HaCaT/L. t. | ||||
| Allium ursinum | 1.45 ± 0.14 | 5.87 ± 0.48 | 23.71 ± 2.66 | 16 | 4 |
| Tulbaghia violacea | 2.83 ± 0.23 | 6.29 ± 0.58 | 21.35 ± 2.54 | 8 | 3 |
| Suramin | 0.13 ± 0.01 | nt | nt | - | - |
| Amphotericin B | nt | 0.13 ± 0.02 | nt | - | - |
| Doxorubicin | nt | nt | 1.04 ± 0.35 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krstin, S.; Sobeh, M.; Braun, M.S.; Wink, M. Tulbaghia violacea and Allium ursinum Extracts Exhibit Anti-Parasitic and Antimicrobial Activities. Molecules 2018, 23, 313. https://doi.org/10.3390/molecules23020313
Krstin S, Sobeh M, Braun MS, Wink M. Tulbaghia violacea and Allium ursinum Extracts Exhibit Anti-Parasitic and Antimicrobial Activities. Molecules. 2018; 23(2):313. https://doi.org/10.3390/molecules23020313
Chicago/Turabian StyleKrstin, Sonja, Mansour Sobeh, Markus Santhosh Braun, and Michael Wink. 2018. "Tulbaghia violacea and Allium ursinum Extracts Exhibit Anti-Parasitic and Antimicrobial Activities" Molecules 23, no. 2: 313. https://doi.org/10.3390/molecules23020313






