Preliminary Characterization, Antioxidant and Hepatoprotective Activities of Polysaccharides from Taishan Pinus massoniana Pollen
Abstract
:1. Introduction
2. Results
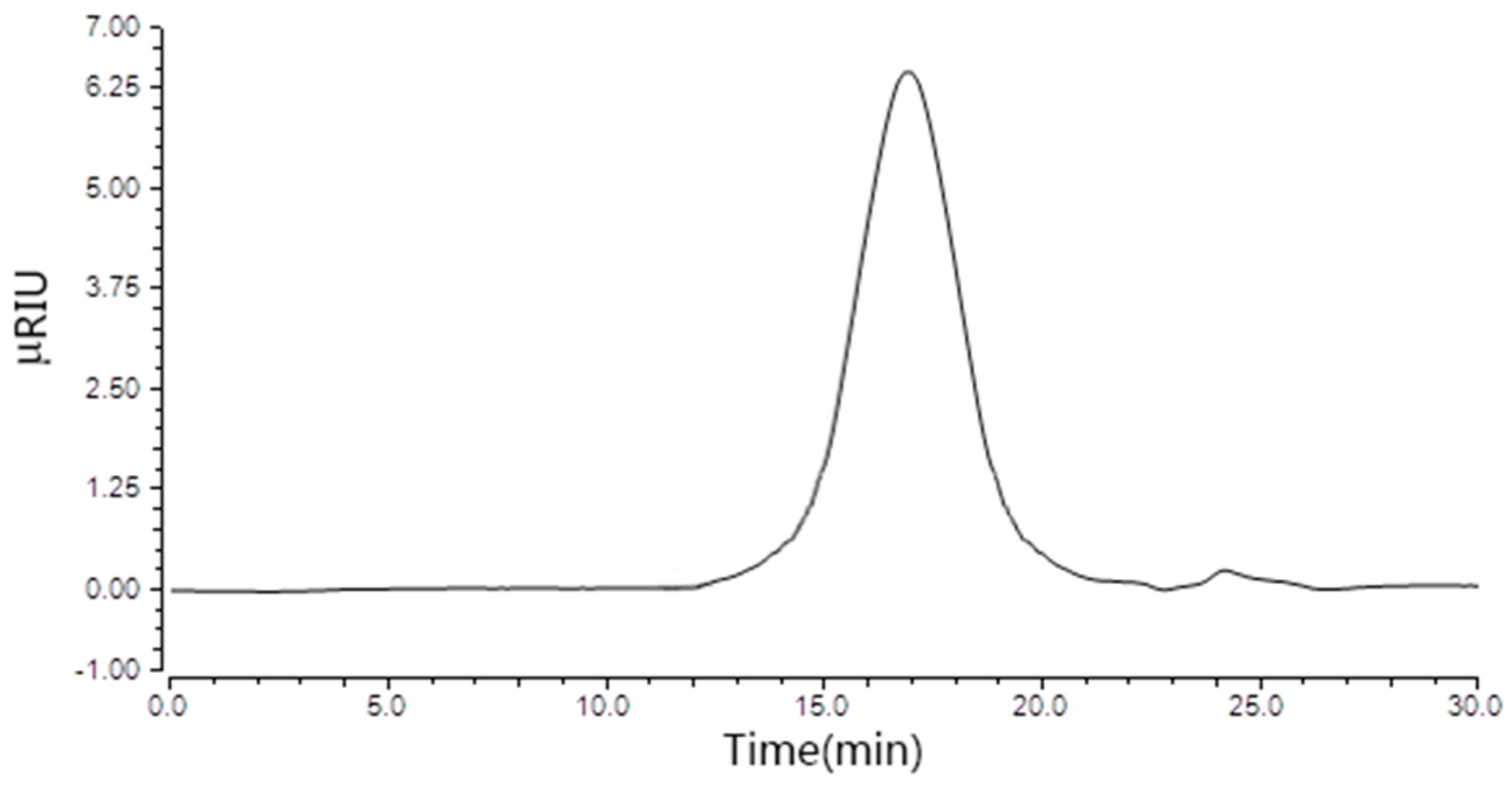
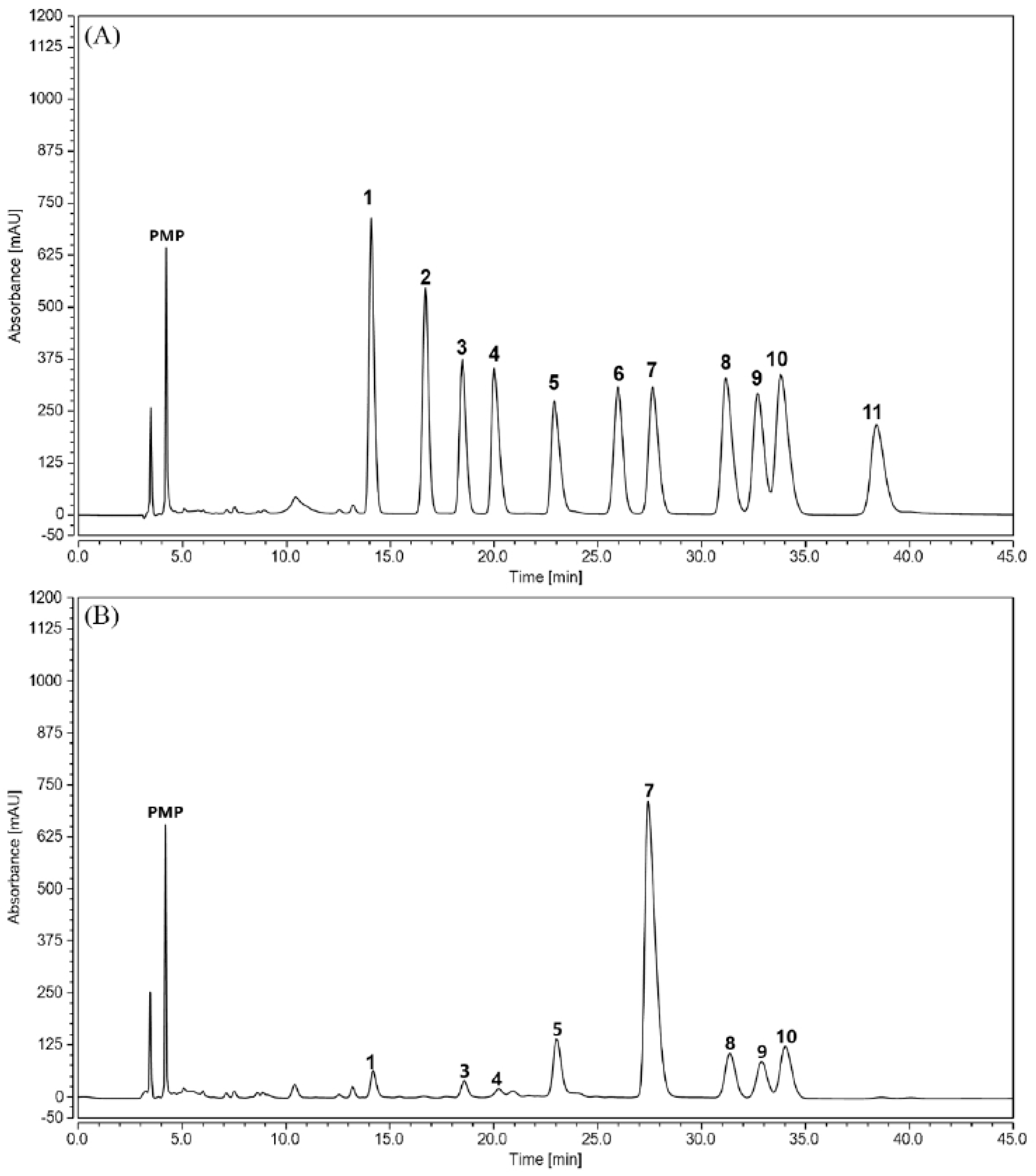
2.1. Physicochemical Properties of TPPPS
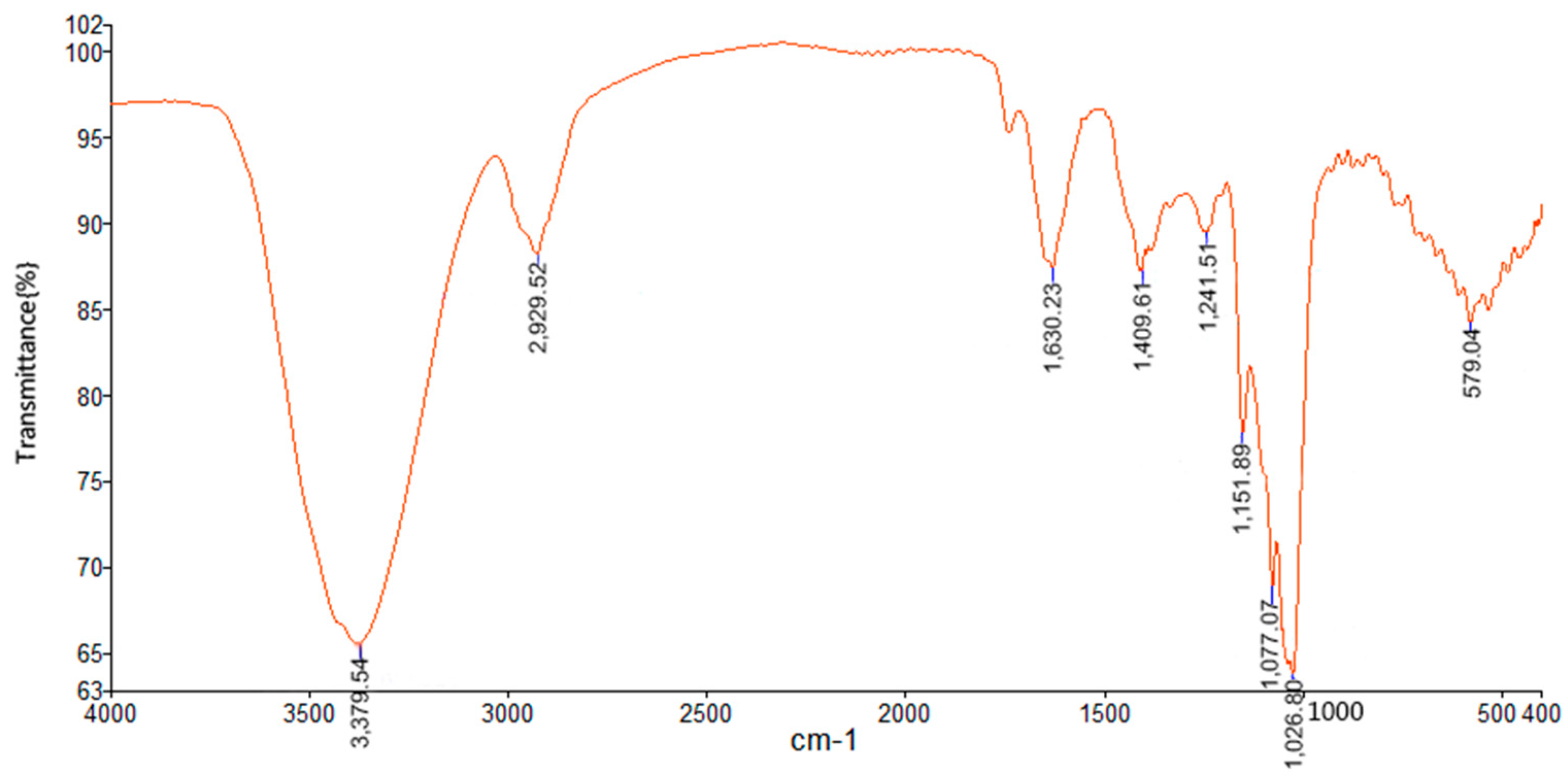
2.2. FT-IR Spectrum of TPPPS
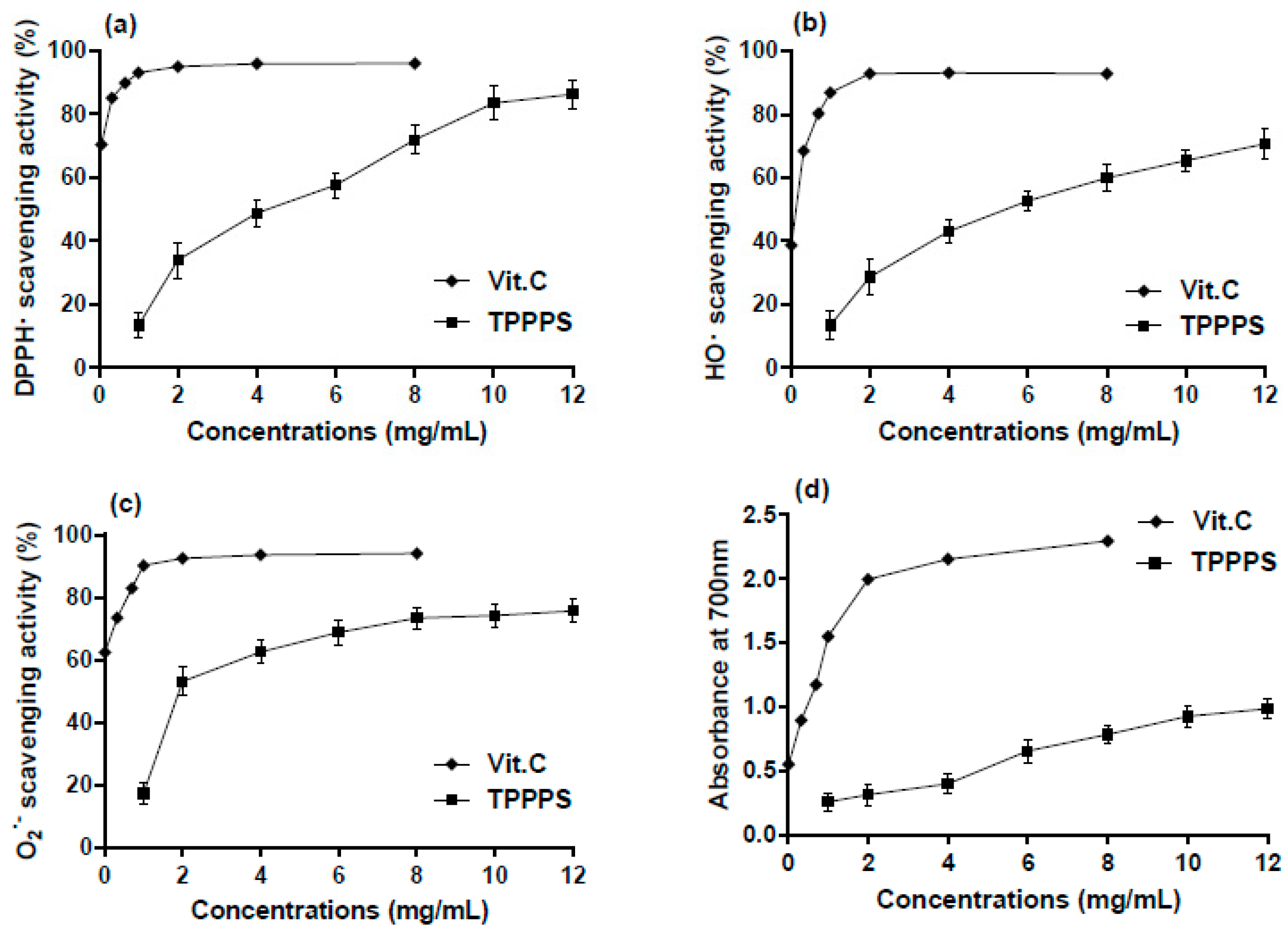
2.3. In Vitro Antioxidant Activity of TPPPS
2.4. The Toxicity of TPPPS
2.5. Effects of TPPPS on Body Weight, Liver Weight and Hepatosomatic Index (HI)
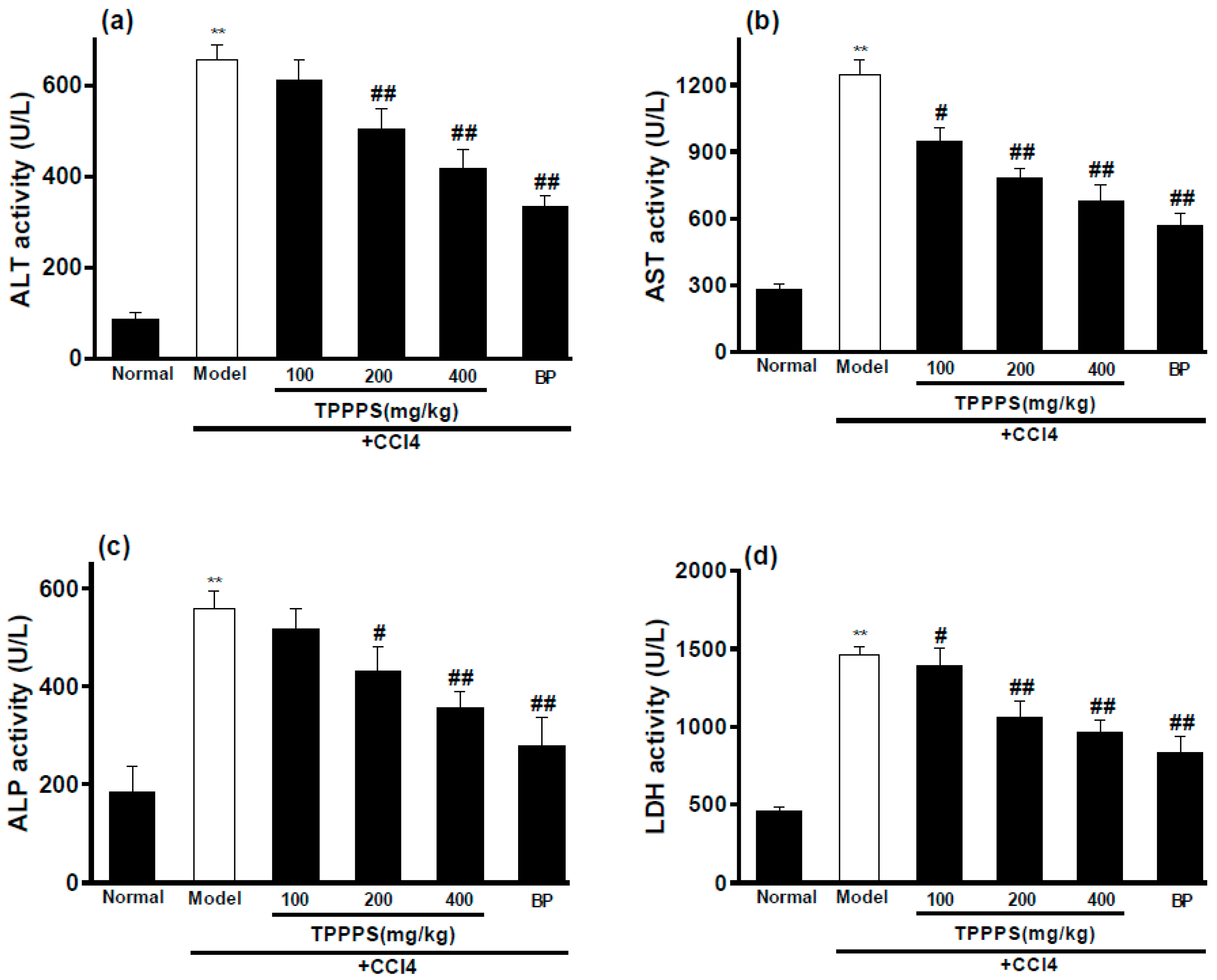
2.6. Effects of TPPPS on Activities of ALT, AST, ALP and LDH in Serum
2.7. Effects of TPPPS on the Levels of MDA, SOD and GSH-Px in Hepatic Tissue
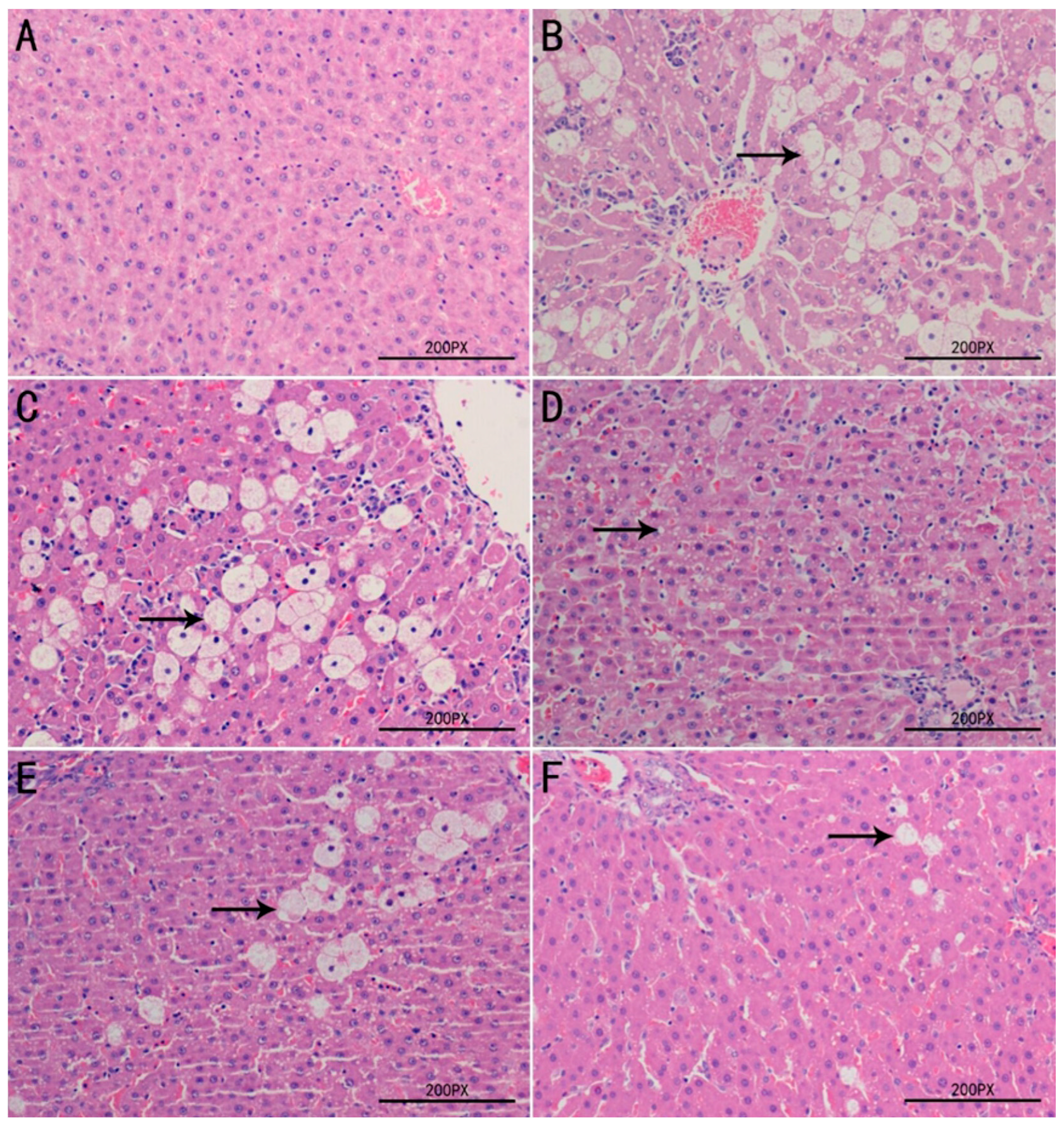
2.8. Histopathological Examination of Liver Tissues
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Animals
4.3. Preparation of TPPPS
4.4. Preliminary Characterization of TPPPS
4.5. Determination of Antioxidant Activity In Vitro
4.5.1. Scavenging Activity on DPPH•
4.5.2. Scavenging Activity on HO•
4.5.3. Scavenging Activity on O2•−
4.5.4. Measurement of Reducing Power
4.6. Determination of the Toxicity of TPPPS
4.7. Determination of Hepatoprotective Activity In Vivo
4.7.1. CCl4-induced Hepatotoxicity Experiment
4.7.2. Serum Biochemical Indicators Assay
4.7.3. Antioxidative Indicators Assay of Liver Homogenate
4.7.4. Histopathological Examination of Liver
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, L.G. Oxidative stress in aging: The oretical outcomes and clinical evidences in humans. Biomed. Aging Pathol. 2011, 1, 1–7. [Google Scholar] [CrossRef]
- Sánchez-Valle, V.; Chavez-Tapia, N.C.; Uribe, M.; Méndez-Sánchez, N. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef] [PubMed]
- Marczak, A.; Bukowska, B. ROS production and their influence on the cellular antioxidative system in human erythrocytes incubated with daunorubicin and glutaraldehyde. Environ. Toxicol. Pharmacol. 2013, 36, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Bruck, R.; Aeed, H.; Avni, Y.; Shirin, H.; Matas, Z.; Shahmurov, M.; Avinoach, I.; Zozulya, G.; Weizman, N.; Hochman, A. Melatonin inhibits nuclear factor kappa B activation and oxidative stress and protects against thioacetamide induced liver damage in rats. J. Hepatol. 2004, 40, 86–93. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Kardošová, A.; Machova, E. Antioxidant activity of medicinal plant polysaccharides. Fitoterapia 2006, 77, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wu, Z.; Zhao, T.; Sun, Y.; Ye, H.; Xu, R.; Zeng, X. Characterization, antioxidant and hepatoprotective activities of polysaccharides from Ilex latifolia Thunb. Carbohydr. Polym. 2014, 101, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ju, Y.; Li, J.; Yu, M. Antioxidant activities of polysaccharides from Lentinus edodes and their significance for disease prevention. Int. J. Biol. Macromol. 2012, 50, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wei, K.; Jia, F.; Zhao, X.; Cui, G.; Guo, F.; Zhu, R. Characterization and Biological Activity of Taishan Pinus massoniana Pollen Polysaccharide In Vitro. PLoS ONE 2015, 10, e0115638. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Sun, X.; Yu, Z. Effective Components and Pharmacological Function of Pine Pollen. J. Northeast For. Univ. 2007, 35, 78–80. [Google Scholar]
- Lee, K.H.; Kim, A.J.; Choi, E.M. Antioxidant and antiinflammatory activity of pine pollen extract In Vitro. Phytother. Res. 2009, 23, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Sun, Z.; Yan, Z.; Tan, Y.; Zhu, X.; Wang, X.; Sheng, P.; Zhu, R. Effects of Taishan Pinus massoniana pollen polysaccharide on immune response of rabbit haemorrhagic disease tissue inactivated vaccine and on production performance of Rex rabbits. Vaccine 2011, 29, 2530–2536. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Zhong, S.; Yang, S.; Zuo, X.; Liang, M.; Sun, J.; Liu, J.; Zhu, R. Effects of Taishan Pinus massoniana pollen polysaccharide on the subunit vaccine of Proteus mirabilis in birds. Int. J. Biol. Macromol. 2013, 56, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liang, M.; Yang, P.; Guo, F.; Pan, D.; Huang, X.; Li, Y.; Wu, C.; Qu, T.; Zhu, R. Taishan Pinus massoniana pollen polysaccharides promote immune responses of recombinant Bordetella avium ompA in BALB/c mice. Int. Immunopharmacol. 2013, 17, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Xue, C.; Wu, C.; Zhao, X.; Qu, T.; He, X.; Guo, Z.; Zhu, R. Immunoregulatory effects of Taishan Pinus massoniana pollen polysaccharide on chicks co-infected with avian leukosis virus and Bordetella avium early in ovo. Res. Vet. Sci. 2014, 96, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yang, N.; Yang, B.; Jiang, Y.; Zhang, G. Structural characterization of water-soluble polysaccharides from Opuntia monacantha cladodes in relation to their anti-glycated activities. Food Chem. 2007, 105, 1480–1486. [Google Scholar] [CrossRef]
- Maciel, J.S.; Chaves, L.S.; Souza, B.W.; Teixeira, D.I.; Freitas, A.L.; Feitosa, J.P.; de Paula, R.C. Structural characterization of cold extracted fraction of soluble sulfated polysaccharide from red seaweed Gracilaria birdiae. Carbohydr. Polym. 2008, 71, 559–565. [Google Scholar] [CrossRef]
- Tian, L.; Zhao, Y.; Guo, C.; Yang, X. A comparative study on the antioxidant activities of an acidic polysaccharide and various solvent extracts derived from herbal Houttuynia cordata. Carbohydr. Polym. 2011, 83, 537–544. [Google Scholar] [CrossRef]
- Wu, M.; Xu, L.; Zhao, L.; Xiao, C.; Gao, N.; Luo, L.; Yang, L.; Li, Z.; Chen, L.; Zhao, J. Structural analysis and anticoagulant activities of the novel sulfated fucan possessing a regular well-defined repeating unit from sea cucumber. Mar. Drugs 2015, 13, 2063–2084. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-C.; Ho, J.-A.; Shieh, M.-C.; Lu, I.-W. Antioxidant and antiproliferative activities of Spirulina and Chlorella water extracts. J. Agric. Food. Chem. 2005, 53, 4207–4212. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahhab, M.A.; Abdel-Galil, M.M.; El-Lithey, M. Melatonin counteracts oxidative stress in rats fed an ochratoxin A contaminated diet. J. Pineal Res. 2005, 38, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Hiraganahalli, B.D.; Chinampudur, V.C.; Dethe, S.; Mundkinajeddu, D.; Pandre, M.K.; Balachandran, J.; Agarwal, A. Hepatoprotective and antioxidant activity of standardized herbal extracts. Pharmacogn. Mag. 2012, 8, 116–123. [Google Scholar] [PubMed]
- Lian, L.-H.; Wu, Y.-L.; Song, S.-Z.; Wan, Y.; Xie, W.-X.; Li, X.; Bai, T.; Ouyang, B.-Q.; Nan, J.-X. Gentiana manshurica Kitagawa reverses acute alcohol-induced liver steatosis through blocking sterol regulatory element-binding protein-1 maturation. J. Agric. Food. Chem. 2010, 58, 13013–13019. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.H.; El-Banna, S.G.; Elhusseiny, A.F.; Mansour, E.-S.M. Antioxidant Activity of New Aramide Nanoparticles Containing Redox-Active N-phthaloyl Valine Moieties in the Hepatic Cytochrome P450 System in Male Rats. Molecules 2012, 17, 8255–8275. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.E.; Kroening, C. Mechanism of early carbon tetrachloride toxicity in cultured rat hepatocytes. Basic Clin. Pharmacol. Toxicol. 1998, 83, 231–239. [Google Scholar] [CrossRef]
- Singh, B.; Saxena, A.; Chandan, B.; Anand, K.; Suri, O.; Suri, K.; Satti, N. Hepatoprotective activity of verbenalin on experimental liver damage in rodents. Fitoterapia 1998, 69, 135–140. [Google Scholar]
- Cheng, D.; Kong, H. The effect of Lycium barbarum polysaccharide on alcohol-induced oxidative stress in rats. Molecules 2011, 16, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- Karakus, E.; Karadeniz, A.; Simsek, N.; Can, I.; Kara, A.; Yildirim, S.; Kalkan, Y.; Kisa, F. Protective effect of Panax ginseng against serum biochemical changes and apoptosis in liver of rats treated with carbon tetrachloride (CCl4). J. Hazard. Mater. 2011, 195, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zhang, Q.Y.; Jiao, L.; Han, T.; Zhang, H.; Qin, L.P.; Khalid, R. Synergistic hepatoprotective effect of Schisandrae lignans with Astragalus polysaccharides on chronic liver injury in rats. Phytomed. Int. J. Phytother. Phytopharmacol. 2009, 16, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.L.; Huang, T.C. Sweet cassava polysaccharide extracts protects against CCl4 liver injury in Wistar rats. Food Hydrocoll. 2009, 23, 1494–1500. [Google Scholar] [CrossRef]
- Maheshwari, D.; Kumar, M.Y.; Verma, S.K.; Singh, V.K.; Singh, S.N. Antioxidant and hepatoprotective activities of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L.) leaves. Food Chem. Toxicol. 2011, 49, 2422–2428. [Google Scholar] [CrossRef] [PubMed]
- Sigala, F.; Kostopanagiotou, G.; Andreadou, I.; Kavatzas, N.; Felekouras, E.; Sigalas, P.; Bastounis, E.; Papalambros, E. Histological and lipid peroxidation changes after administration of 2-acetylaminofluorene in a rat liver injury model following selective periportal and pericentral damage. Toxicology 2004, 196, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.-Q.; Xiao, J.-J.; Zhang, H.-N.; Wang, J.-H.; Pan, L.-H.; Yang, X.-F.; Luo, J.-P. Polysaccharides in Laminaria japonica (LP): Extraction, physicochemical properties and their hypolipidemic activities in diet-induced mouse model of atherosclerosis. Food Chem. 2012, 134, 244–252. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Lott, J.A.; Stephan, V.A.; Pritchard, K.A., Jr. Evaluation of the Coomassie Brilliant blue G-250 method for urinary protein. Clin. Chem. 1983, 29, 1946–1950. [Google Scholar] [PubMed]
- Yan, Z.H.; Fan, R.F.; Yin, S.J.; Zhao, X.N.; Liu, J.Z.; Li, L.H.; Zhang, W.Q.; Ge, L.J. Protective effects of Ginkgo biloba leaf polysaccharide on nonalcoholic fatty liver disease and its mechanisms. Int. J. Biol. Macromol. 2015, 80, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Zhao, Y.; Jiao, Y.D.; Yu, L.H.; Yang, S.; Yang, X.B. Antioxidative and hepatoprotective effects of the polysaccharides from Zizyphus jujube cv. Shaanbeitanzao. Carbohydr. Polym. 2012, 88, 1453–1459. [Google Scholar] [CrossRef]
- Huang, X.Q.; Tu, Z.C.; Jiang, Y.; Xiao, H.; Zhang, Q.T.; Wang, H. Dynamic high pressure microfluidization-assisted extraction and antioxidant activities of lentinan. Int. J. Biol. Macromol. 2012, 51, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.K.; Tsai, M.L.; Huang, J.R.; Chen, R.H. In vitro antioxidant activities of low-molecular-weight polysaccharides with various functional groups. J. Agric. Food Chem. 2009, 57, 2699–2704. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
| Treatments | Doses | Body Weight (g) | Liver Weight (g) | HI (%) |
|---|---|---|---|---|
| Normal | - | 285 ± 17 | 13.2 ± 2.7 | 4.62 ± 0.48 |
| CCl4 | - | 317 ± 15 * | 18.3 ± 2.4 ** | 5.77 ± 0.23 ** |
| CCl4 + TPPPS | 100 mg/kg | 305 ± 18 | 16.4 ± 1.9 | 5.36 ± 0.29 # |
| CCl4 + TPPPS | 200 mg/kg | 299 ± 17 | 14.8 ± 2.4 # | 4.95 ± 0.36 ## |
| CCl4 + TPPPS | 400 mg/kg | 300 ± 16 | 14.8 ± 2.6 # | 4.92 ± 0.22 ## |
| CCl4 + BP | 100 mg/kg | 299 ± 16 | 14.8 ± 1.9 # | 4.94 ± 0.56 ## |
| Treatments | Dose | MDA (nmol/mg Protein) | SOD (U/mg Protein) | GSH-Px (U/mg Protein) |
|---|---|---|---|---|
| Normal | - | 2.23 ± 0.31 | 117.88 ± 16.20 | 67.20 ± 4.35 |
| CCl4 | - | 7.63 ± 1.17 ** | 45.58 ± 9.89 ** | 34.96 ± 3.76 ** |
| CCl4 + TPPPS | 100 mg/kg | 5.99 ± 1.31 # | 55.95 ± 8.64 # | 39.51 ± 4.62 |
| CCl4 + TPPPS | 200 mg/kg | 4.71 ± 0.92 # | 77.10 ± 5.34 ## | 45.56 ± 5.14 # |
| CCl4 + TPPPS | 400 mg/kg | 3.71 ± 0.48 ## | 94.74 ± 7.10 ## | 56.79 ± 8.36 ## |
| CCl4 + BP | 100 mg/kg | 4.40 ± 0.38 # | 79.27 ± 6.31 ## | 50.88 ± 3.47 ## |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Yin, S.; Yu, Z.; Feng, Y.; Wei, K.; Ma, W.; Ge, L.; Yan, Z.; Zhu, R. Preliminary Characterization, Antioxidant and Hepatoprotective Activities of Polysaccharides from Taishan Pinus massoniana Pollen. Molecules 2018, 23, 281. https://doi.org/10.3390/molecules23020281
Zhou C, Yin S, Yu Z, Feng Y, Wei K, Ma W, Ge L, Yan Z, Zhu R. Preliminary Characterization, Antioxidant and Hepatoprotective Activities of Polysaccharides from Taishan Pinus massoniana Pollen. Molecules. 2018; 23(2):281. https://doi.org/10.3390/molecules23020281
Chicago/Turabian StyleZhou, Changming, Shaojie Yin, Zhongfang Yu, Yuxiang Feng, Kai Wei, Weiming Ma, Lijiang Ge, Zhengui Yan, and Ruiliang Zhu. 2018. "Preliminary Characterization, Antioxidant and Hepatoprotective Activities of Polysaccharides from Taishan Pinus massoniana Pollen" Molecules 23, no. 2: 281. https://doi.org/10.3390/molecules23020281




