Food-Grade Synthesis of Maillard-Type Taste Enhancers Using Natural Deep Eutectic Solvents (NADES)
Abstract
:1. Introduction
2. Results and Discussion
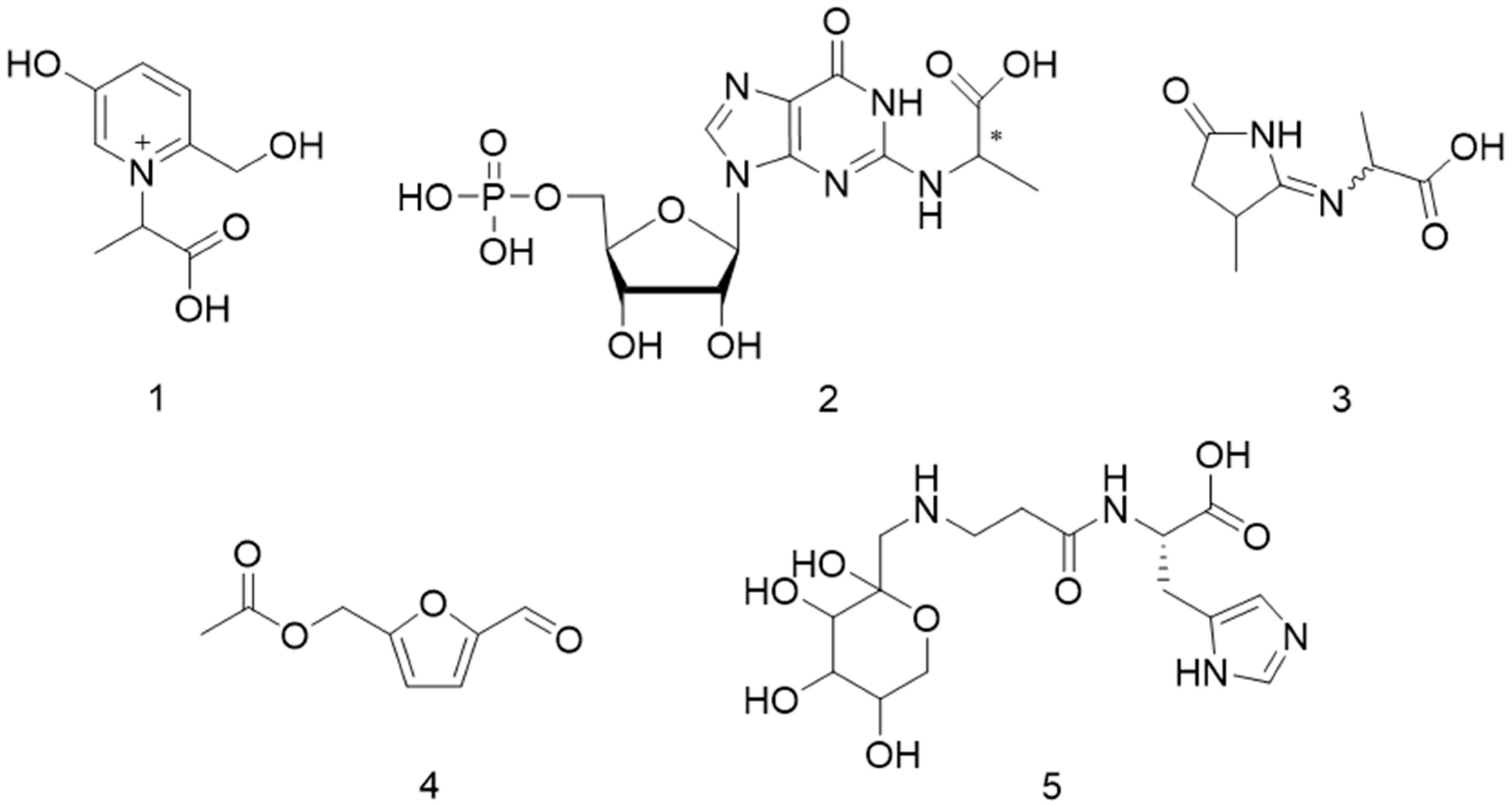
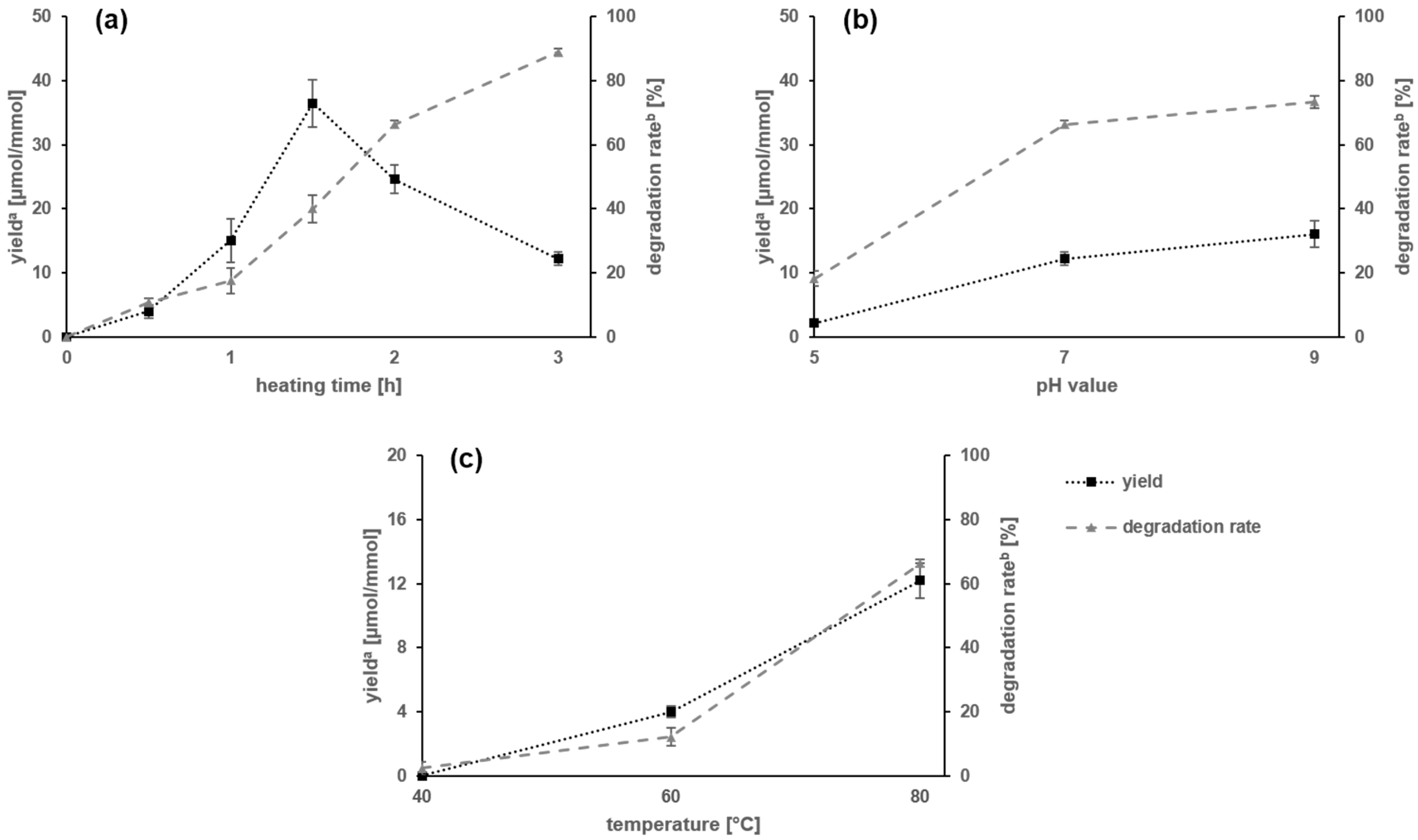
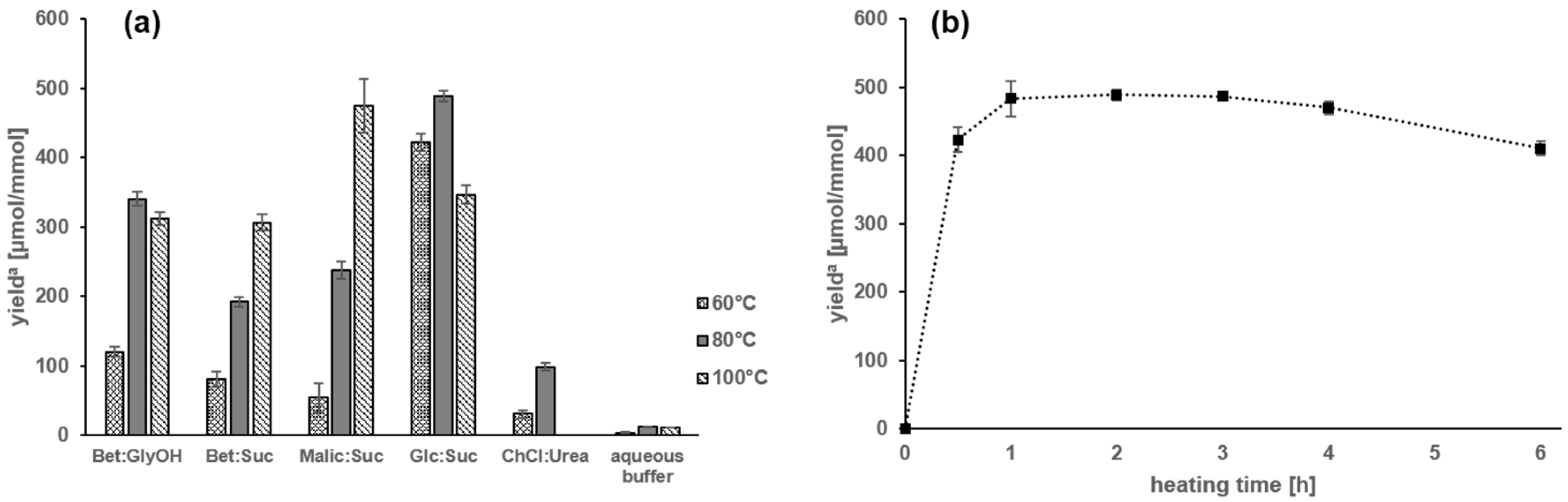
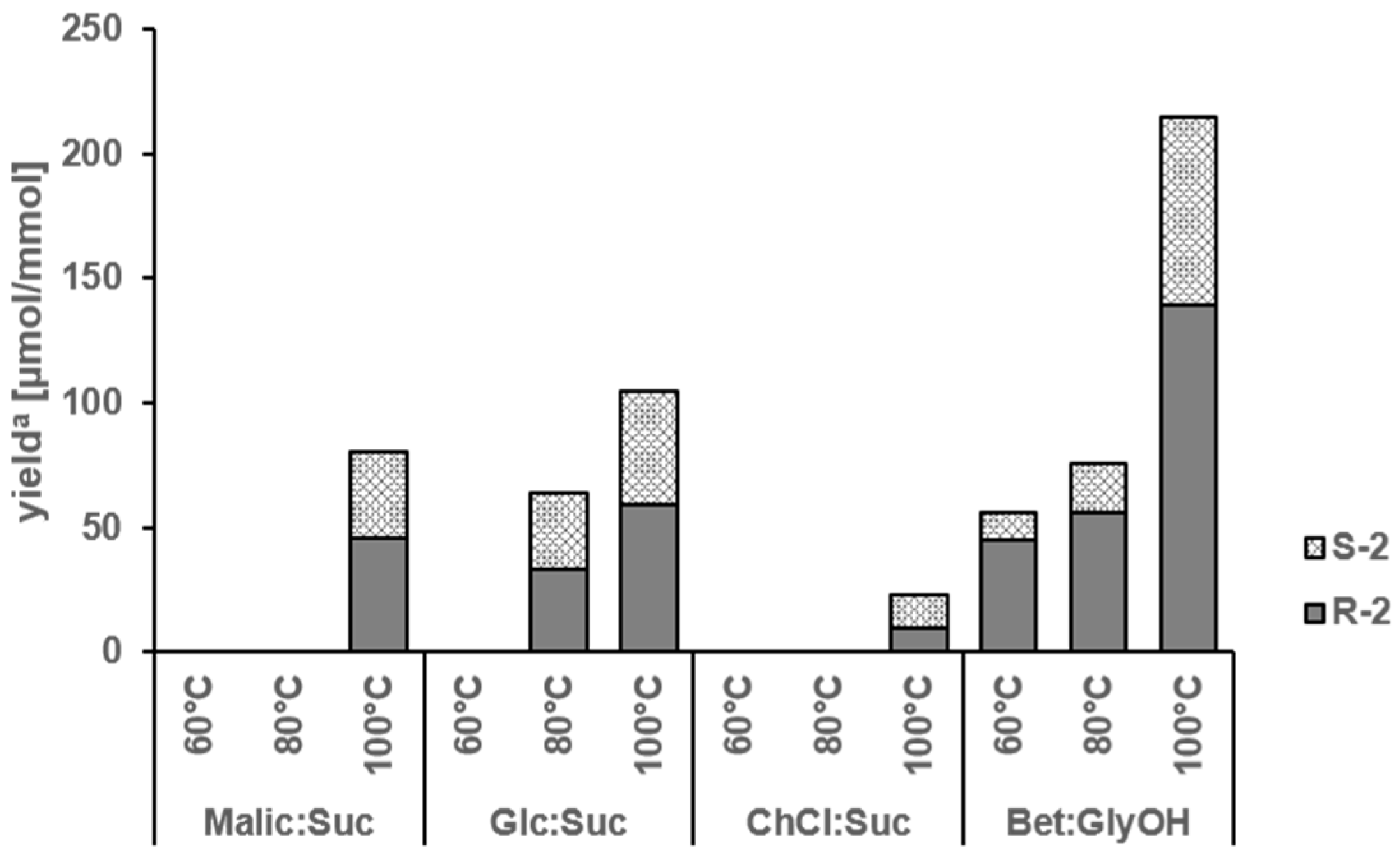
2.1. Amadori Rearrangement
2.2. Carboxyethylation of Amino Compounds
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of NADES
3.3. Experimental Setup
3.4. High-Performance Liquid Chromatography–Mass Spectrometry (HPLC-MS/MS)
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Resconi, V.C.; Escudero, A.; Campo, M.M. The development of aromas in ruminant meat. Molecules 2013, 18, 6748–6781. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Henle, T. Baking, ageing, diabetes: A short history of the maillard reaction. Angew. Chem. Int. Ed. Engl. 2014, 53, 10316–10329. [Google Scholar] [CrossRef] [PubMed]
- Frank, O.; Ottinger, H.; Hofmann, T. Characterization of an intense bitter-tasting 1 H,4 H-quinolizinium-7-olate by application of the taste dilution analysis, a novel bioassay for the screening and identification of taste-active compounds in foods. J. Agric. Food Chem. 2001, 49, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Frank, O.; Hofmann, T. Reinvestigation of the chemical structure of bitter-tasting quinizolate and homoquinizolate and studies on their maillard-type formation pathways using suitable13c-labeling experiments. J. Agric. Food Chem. 2002, 50, 6027–6036. [Google Scholar] [CrossRef] [PubMed]
- Ottinger, H.; Bareth, A.; Hofmann, T. Characterization of natural “cooling” compounds formed from glucose and l-proline in dark malt by application of taste dilution analysis. J. Agric. Food Chem. 2001, 49, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Ottinger, H.; Soldo, T.; Hofmann, T. Systematic studies on structure and physiological activity of cyclic α-keto enamines, a novel class of “cooling” compounds. J. Agric. Food Chem. 2001, 49, 5383–5390. [Google Scholar] [CrossRef] [PubMed]
- Beksan, E.; Schieberle, P.; Robert, F.; Blank, I.; Fay, L.B.; Schlichtherle-Cerny, H.; Hofmann, T. Synthesis and sensory characterization of novel umami-tasting glutamate glycoconjugates. J. Agric. Food Chem. 2003, 51, 5428–5436. [Google Scholar] [CrossRef] [PubMed]
- Toelstede, S.; Dunkel, A.; Hofmann, T. A series of kokumi peptides impart the long-lasting mouthfulness of matured gouda cheese. J. Agric. Food Chem. 2009, 57, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Toelstede, S.; Hofmann, T. Quantitative studies and taste re-engineering experiments toward the decoding of the nonvolatile sensometabolome of gouda cheese. J. Agric. Food Chem. 2008, 56, 5299–5307. [Google Scholar] [CrossRef] [PubMed]
- Toelstede, S.; Hofmann, T. Sensomics mapping and identification of the key bitter metabolites in gouda cheese. J. Agric. Food Chem. 2008, 56, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, J.C.; Hofmann, T. Quantitative reconstruction of the nonvolatile sensometabolome of a red wine. J. Agric. Food Chem. 2008, 56, 9190–9199. [Google Scholar] [CrossRef] [PubMed]
- Ottinger, H.; Hofmann, T. Identification of the taste enhancer alapyridaine in beef broth and evaluation of its sensory impact by taste reconstitution experiments. J. Agric. Food Chem. 2003, 51, 6791–6796. [Google Scholar] [CrossRef] [PubMed]
- Ottinger, H.; Soldo, T.; Hofmann, T. Discovery and structure determination of a novel maillard-derived sweetness enhancer by application of the comparative taste dilution analysis (ctda). J. Agric. Food Chem. 2003, 51, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Soldo, T.; Blank, I.; Hofmann, T. (+)-(S)-Alapyridaine—A general taste enhancer? Chem. Sens. 2003, 28, 371–379. [Google Scholar] [CrossRef]
- Villard, R.; Robert, F.; Blank, I.; Bernardinelli, G.; Soldo, T.; Hofmann, T. Racemic and enantiopure synthesis and physicochemical characterization of the novel taste enhancer n-(1-carboxyethyl)-6-(hydroxymethyl)pyridinium-3-ol inner salt. J. Agric. Food Chem. 2003, 51, 4040–4045. [Google Scholar] [CrossRef] [PubMed]
- Festring, D.; Hofmann, T. Discovery of n(2)-(1-carboxyethyl)guanosine 5′-monophosphate as an umami-enhancing maillard-modified nucleotide in yeast extracts. J. Agric. Food Chem. 2010, 58, 10614–10622. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, T.; Kunert, C.; Dunkel, A.; Hofmann, T. Sensory-guided identification of n-(1-methyl-4-oxoimidazolidin-2-ylidene)-alpha-amino acids as contributors to the thick-sour and mouth-drying orosensation of stewed beef juice. J. Agric. Food Chem. 2010, 58, 6341–6350. [Google Scholar] [CrossRef] [PubMed]
- Kunert, C.; Walker, A.; Hofmann, T. Taste modulating n-(1-methyl-4-oxoimidazolidin-2-ylidene) alpha-amino acids formed from creatinine and reducing carbohydrates. J. Agric. Food Chem. 2011, 59, 8366–8374. [Google Scholar] [CrossRef] [PubMed]
- Hillmann, H.; Mattes, J.; Brockhoff, A.; Dunkel, A.; Meyerhof, W.; Hofmann, T. Sensomics analysis of taste compounds in balsamic vinegar and discovery of 5-acetoxymethyl-2-furaldehyde as a novel sweet taste modulator. J. Agric. Food Chem. 2012, 60, 9974–9990. [Google Scholar] [CrossRef] [PubMed]
- Smarrito-Menozzi, C.M.; Viton, F.; Hofmann, T.; Kranz, M. Sugar-Dipeptide Conjugates as Flavor Molecules. Patent WO2016120250A1, 4 August 2016. [Google Scholar]
- Kranz, M.; Viton, F.; Smarrito-Menozzi, C.; Hofmann, T. Sensomics-based molecularization of the taste of pot-au-feu, a traditional meat/vegetable broth. J. Agric. Food Chem. 2018, 66, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Garti, N. Microemulsions as microreactors for food applications. Curr. Opin. Colloid Interface Sci. 2003, 8, 197–211. [Google Scholar] [CrossRef]
- Troise, A.D.; Berton-Carabin, C.C.; Fogliano, V. Amadori products formation in emulsified systems. Food Chem. 2016, 199, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Sagalowicz, L.; Moccand, C.; Davidek, T.; Ghanbari, R.; Martiel, I.; Negrini, R.; Mezzenga, R.; Leser, M.E.; Blank, I.; Michel, M. Lipid self-assembled structures for reactivity control in food. Philos. Trans. R. Soc. A 2016, 374, 20150136. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.; Witkamp, G.J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Wang, Y.; Huang, Y.; Ding, X.; Chen, J.; Xu, K. Deep eutectic solvents as novel extraction media for protein partitioning. Analyst 2014, 139, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Gorke, J.T.; Srienc, F.; Kazlauskas, R.J. Hydrolase-catalyzed biotransformations in deep eutectic solvents. Chem. Commun. (Camb.) 2008, 14, 1235–1237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; de Oliveira Vigier, K.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Ruß, C.; König, B. Low melting mixtures in organic synthesis—An alternative to ionic liquids? Green Chem. 2012, 14, 2969. [Google Scholar] [CrossRef]
- Cardellini, F.; Tiecco, M.; Germani, R.; Cardinali, G.; Corte, L.; Roscini, L.; Spreti, N. Novel zwitterionic deep eutectic solvents from trimethylglycine and carboxylic acids: Characterization of their properties and their toxicity. RSC Adv. 2014, 4, 55990–56002. [Google Scholar] [CrossRef]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionisio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and thermal behavior of natural deep eutectic solvents. J. Mol. Liq. 2016, 215, 534–540. [Google Scholar] [CrossRef]
- Abbott, A.P.; Cullis, P.M.; Gibson, M.J.; Harris, R.C.; Raven, E. Extraction of glycerol from biodiesel into a eutectic based ionic liquid. Green Chem. 2007, 9, 868–872. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J. Chromatogr. A 2013, 1285, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.-F.; Wang, X.-Q.; Peng, X.; Wang, W.; Zhao, C.-J.; Zu, Y.-G.; Fu, Y.-J. Fast and green extraction and separation of main bioactive flavonoids from radix scutellariae. Ind. Crops Prod. 2015, 63, 175–181. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Rodriguez-Juan, E.; Rodriguez-Gutierrez, G.; Rios, J.J.; Fernandez-Bolanos, J. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (dess). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Krystof, M.; Perez-Sanchez, M.; Dominguez de Maria, P. Lipase-catalyzed (trans)esterification of 5-hydroxy-methylfurfural and separation from hmf esters using deep-eutectic solvents. ChemSusChem 2013, 6, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Baker, G.A.; Holmes, S. Protease activation in glycerol-based deep eutectic solvents. J. Mol. Catal. B Enzym. 2011, 72, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Azizi, N.; Batebi, E.; Bagherpour, S.; Ghafuri, H. Natural deep eutectic salt promoted regioselective reduction of epoxides and carbonyl compounds. RSC Adv. 2012, 2, 2289. [Google Scholar] [CrossRef]
- Mobinikhaledi, A.; Amiri, A.K. Natural eutectic salts catalyzed one-pot synthesis of 5-arylidene-2-imino-4-thiazolidinones. Res. Chem. Intermed. 2012, 39, 1491–1498. [Google Scholar] [CrossRef]
- Cammerer, B.; Wedzicha, B.L.; Kroh, L.W. Nonenzymatic browning reactions of retro-aldol degradation products of carbohydrates. Eur. Food Res. Technol. 1999, 209, 261–265. [Google Scholar] [CrossRef]
- Festring, D.; Hofmann, T. Systematic studies on the chemical structure and umami enhancing activity of maillard-modified guanosine 5′-monophosphates. J. Agric. Food Chem. 2011, 59, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Festring, D.; Brockhoff, A.; Meyerhof, W.; Hofmann, T. Stereoselective synthesis of amides sharing the guanosine 5′-monophosphate scaffold and umami enhancement studies using human sensory and ht1r1/rt1r3 receptor assays. J. Agric. Food Chem. 2011, 59, 8875–8885. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sabag-Daigle, A.; Metz, T.O.; Deatherage Kaiser, B.; Gopalan, V.; Behrman, E.J.; Wysocki, V.H.; Ahmer, B. Measurement of fructose-asparagine concentrations in human and animal foods. J. Agric. Food Chem. 2018, 66, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Finot, P. The Maillard Reaction in Food Processing, Human Nutrition and Physiology; Springer Science & Business Media: New York, NY, USA, 1990. [Google Scholar]
- Amrein, T.M.; Limacher, A.; Conde-Petit, B.; Amadò, R.; Escher, F. Influence of thermal processing conditions on acrylamide generation and browning in a potato model system. J. Agric. Food Chem. 2006, 54, 5910–5916. [Google Scholar] [CrossRef] [PubMed]
- Märk, J.; Pollien, P.; Lindinger, C.; Blank, I.; Märk, T. Quantitation of furan and methylfuran formed in different precursor systems by proton transfer reaction mass spectrometry. J. Agric. Food Chem. 2006, 54, 2786–2793. [Google Scholar] [CrossRef] [PubMed]
- Perez Locas, C.; Yaylayan, V.A. Isotope labeling studies on the formation of 5-(hydroxymethyl)-2-furaldehyde (hmf) from sucrose by pyrolysis-gc/ms. J. Agric. Food Chem. 2008, 56, 6717–6723. [Google Scholar] [CrossRef] [PubMed]
- Poisson, L.; Auzanneau, N.; Mestdagh, F.; Blank, I.; Davidek, T. New insight into the role of sucrose in the generation of α-diketones upon coffee roasting. J. Agric. Food Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 2, 3 and 5 are available from the authors. |

| NADES | Sample Name | Ratio a | |
|---|---|---|---|
| Component 1 | Component 2 | ||
| Choline chloride | Sucrose | ChCl:Suc | 4:1:4 |
| Choline chloride | Urea | ChCl:Urea | 1:2:1 |
| Malic acid | Sucrose | Malic:Suc | 1:1:5 |
| Glucose | Sucrose | Glc:Suc | 1:1:9 |
| Betaine | Sucrose | Bet:Suc | 2:1:9 |
| Betaine | Glycerol | Bet:GlyOH | 1:2:2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kranz, M.; Hofmann, T. Food-Grade Synthesis of Maillard-Type Taste Enhancers Using Natural Deep Eutectic Solvents (NADES). Molecules 2018, 23, 261. https://doi.org/10.3390/molecules23020261
Kranz M, Hofmann T. Food-Grade Synthesis of Maillard-Type Taste Enhancers Using Natural Deep Eutectic Solvents (NADES). Molecules. 2018; 23(2):261. https://doi.org/10.3390/molecules23020261
Chicago/Turabian StyleKranz, Maximilian, and Thomas Hofmann. 2018. "Food-Grade Synthesis of Maillard-Type Taste Enhancers Using Natural Deep Eutectic Solvents (NADES)" Molecules 23, no. 2: 261. https://doi.org/10.3390/molecules23020261






