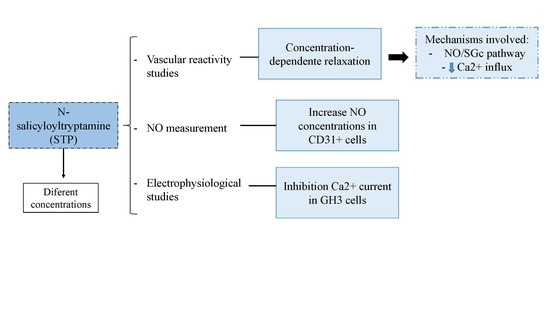
N-Salicyloyltryptamine, an N-Benzoyltryptamine Analogue, Induces Vasorelaxation through Activation of the NO/sGC Pathway and Reduction of Calcium Influx
Abstract
:1. Introduction
2. Results
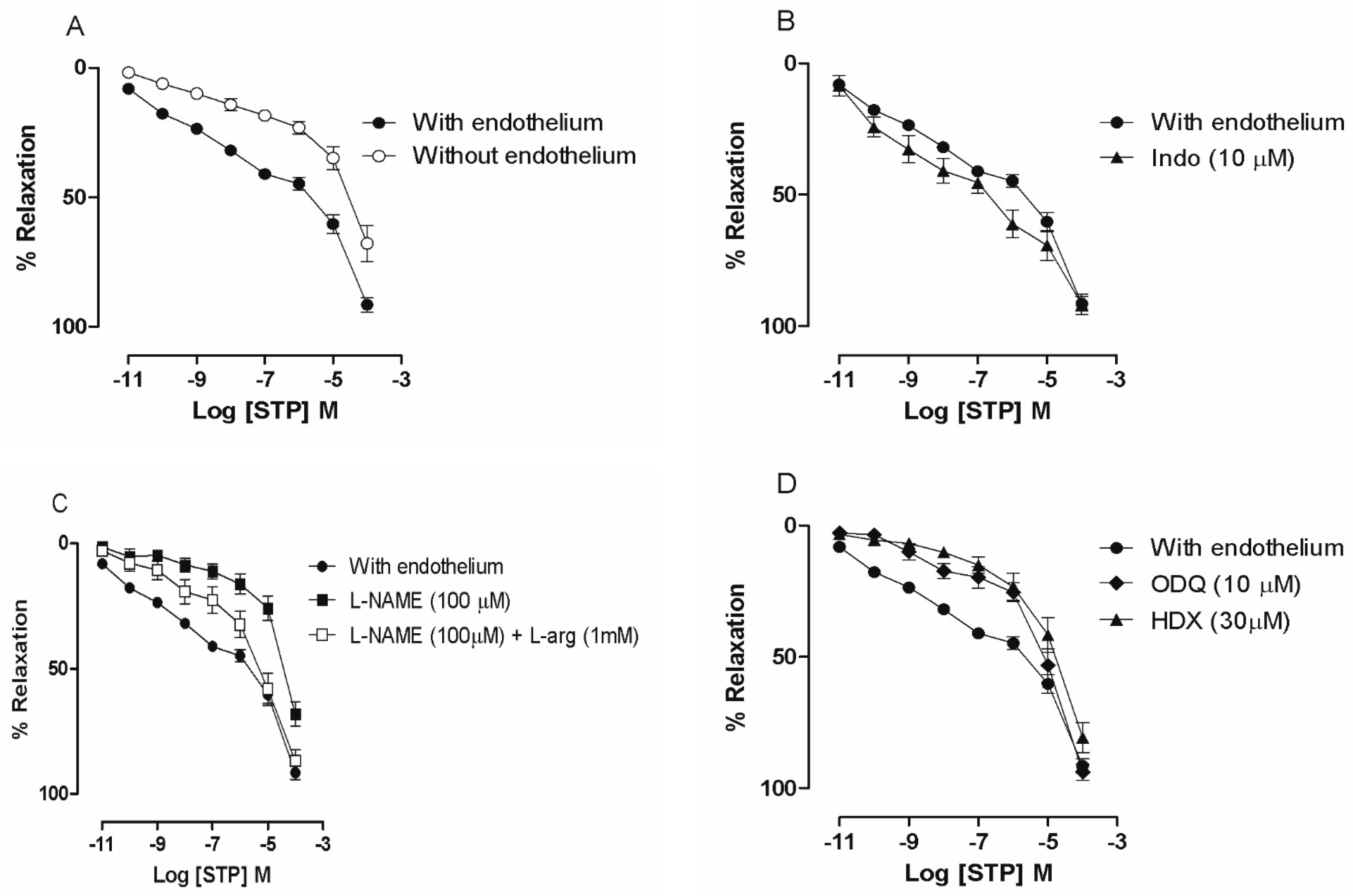
2.1. The Endothelium Participated in STP-Induced Relaxation
2.2. NO Production
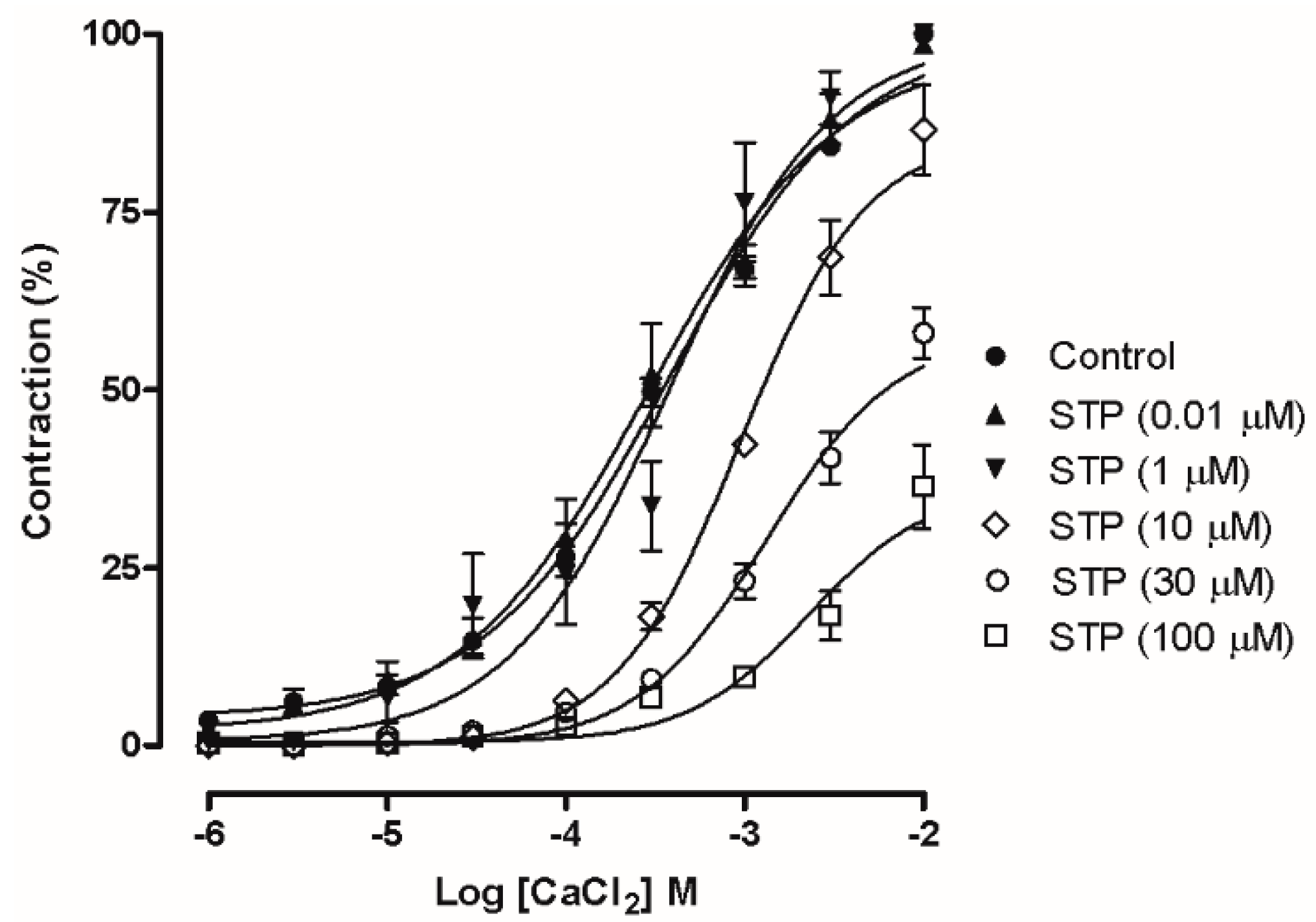
2.3. Ca2+ Influx Attenuation Mediated Endothelium-Independent, STP-Induced Relaxation
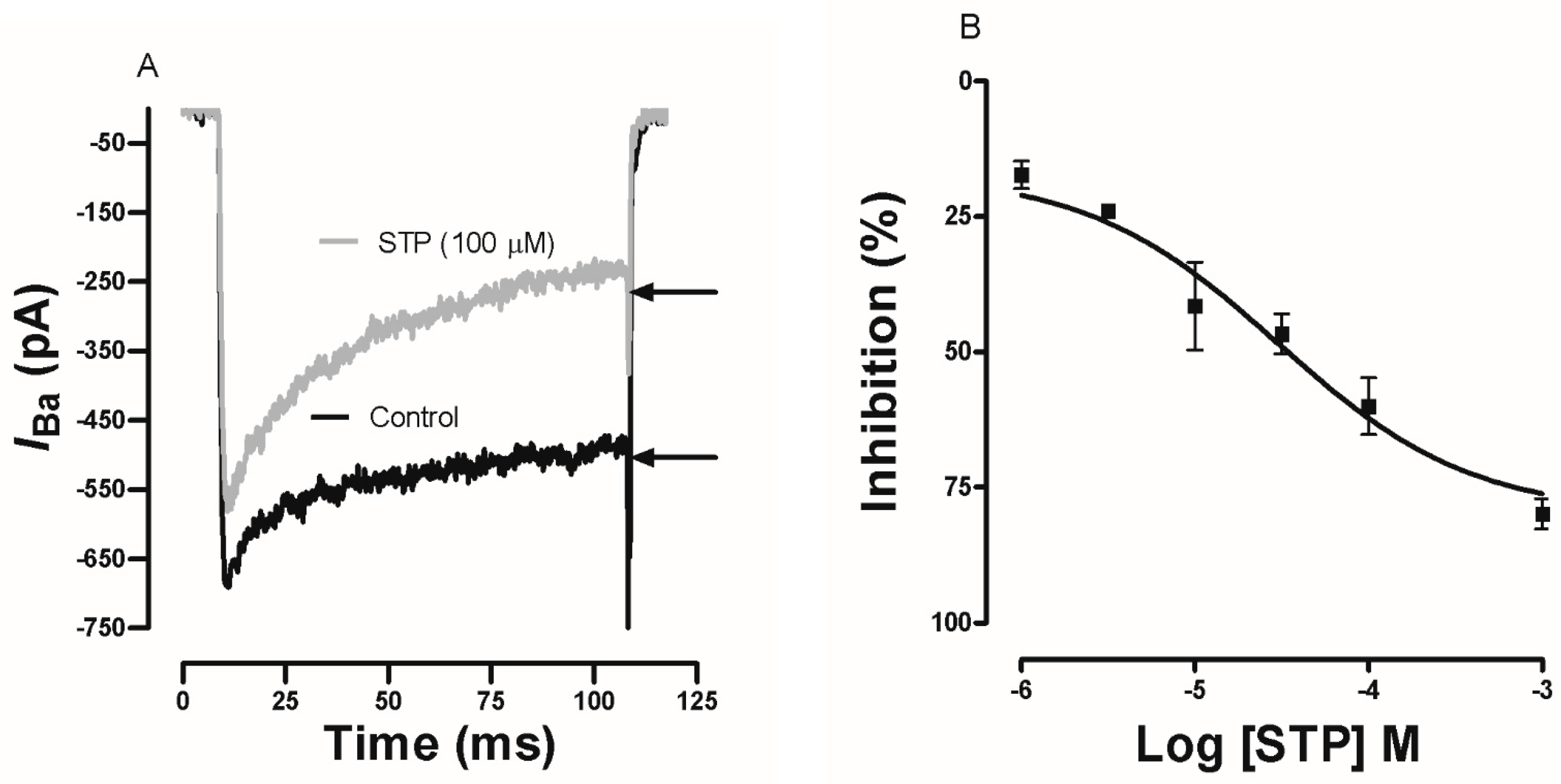
2.4. Effect of STP on Ca2+ Currents
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Drugs and Solutions
4.3. Vascular Reactivity Studies
4.4. NO Measurement by Amperometry in Endothelial Cells (ECs)
4.4.1. EC Isolation
4.4.2. EC Detection and Viability
4.4.3. NO Determination by Amperometry
4.5. Electrophysiology
4.5.1. GH3 Cell Culture
4.5.2. Electrophysiological Studies
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 esh/esc guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the european society of hypertension (esh) and of the european society of cardiology (esc). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [PubMed]
- Banik, R.; Berger, J. Peripheral vasodilators. In Essentials of Pharmacology for Anesthesia, Pain Medicine, and Critical Care; Kaye, A.D., Kaye, A.M., Urman, R.D., Eds.; Springer: New York, NY, USA, 2015; pp. 257–273. [Google Scholar]
- Krum, H.; Sobotka, P.; Mahfoud, F.; Bohm, M.; Esler, M.; Schlaich, M. Device-based antihypertensive therapy: Therapeutic modulation of the autonomic nervous system. Circulation 2011, 123, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Steckelings, U.M.; Unger, T. Angiotensin ii type 2 receptor agonists—Where should they be applied? Expert Opin. Investig. Drugs 2012, 21, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Moncada, S.; Higgs, A. The l-arginine-nitric oxide pathway. NEJM 1993, 329, 2002–2012. [Google Scholar] [PubMed]
- Moncada, S.; Higgs, E.A. The discovery of nitric oxide and its role in vascular biology. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S193–S201. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, T.A.; da Silva, R.S.; Miranda, K.M.; Switzer, C.H.; Wink, D.A.; Fukuto, J.M. Biological nitric oxide signalling: Chemistry and terminology. Br. J. Pharmacol. 2013, 169, 1417–1429. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, D.R.; Schwartz, J.B. Calcium-antagonist drugs. NEJM 1999, 341, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Rampe, D.; Su, C.M.; Yousif, F.; Triggle, D.J. Calcium channel antagonists: Pharmacological considerations. Br. J. Clin. Pharmacol. 1985, 20, 247S–253S. [Google Scholar] [CrossRef] [PubMed]
- Hockerman, G.H.; Peterson, B.Z.; Johnson, B.D.; Catterall, W.A. Molecular determinants of drug binding and action on L-type calcium channels. Ann. Rev. Pharm. Toxicol. 1997, 37, 361–396. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Tenorio, M.; Porras-Gonzalez, C.; Castellano, A.; Lopez-Barneo, J.; Urena, J. Tonic arterial contraction mediated by L-type Ca2+ channels requires sustained Ca2+ influx, g protein-associated Ca2+ release, and rhoa/rock activation. Eur. J. Pharmacol. 2012, 697, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D.; Clarke, D.E.; Fozard, J.R.; Hartig, P.R.; Martin, G.R.; Mylecharane, E.J.; Saxena, P.R.; Humphrey, P.P. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol. Rev. 1994, 46, 157–203. [Google Scholar] [PubMed]
- Jensen, N.; Gartz, J.; Laatsch, H. Aeruginascin, a trimethylammonium analogue of psilocybin from the hallucinogenic mushroom inocybe aeruginascens. Planta Med. 2006, 72, 665–666. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.A.; de Almeida, R.N.; Sousa, M.F.; Barbosa-Filho, J.M.; Diniz, S.A.; de Medeiros, I.A. Anticonvulsant properties of n-salicyloyltryptamine in mice. Pharmacol. Biochem. Behav. 2001, 68, 199–202. [Google Scholar] [CrossRef]
- Araújo, D.A.M.; Mafra, R.A.; Rodrigues, A.L.P.; Silva, V.M.; Beirão, P.S.L.; Almeida, R.N.; Quitans, L., Jr.; Souza, M.F.V.; Cruz, J.S. N-salicyloyltryptamine, a new anticonvulsant drug, acts on voltage-dependent Na+, Ca2+ and K+ ion channels. Br. J. Pharm. 2003, 140, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Quintans, L.J., Jr.; Silva, D.A.; Siqueira, J.S.; Araujo, A.A.; Barreto, R.S.; Bonjardim, L.R.; Desantana, J.M.; De Lucca, W., Jr.; Souza, M.F.; Gutierrez, S.J.; et al. Bioassay-guided evaluation of antinociceptive effect of n-salicyloyltryptamine: A behavioral and electrophysiological approach. J. Biomed. Biotechnol. 2010, 2010, 230745. [Google Scholar] [PubMed]
- Gutierrez, S.J.; de Claudino, S.F.; Da Silva, B.A.; Camara, C.A.; de Almeida, R.N.; de Fatima, V.d.S.M.; Da Silva, M.S.; Da-Cunha, E.V.; Barbosa-Filho, J.M. Nb-benzoyltryptamine derivatives with relaxant activity in guinea-pig ileum. Farmaco 2005, 60, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto, J.; de Bittencourt Pasquali, M.A.; Somensi, N.; Vasques, L.M.; Moreira, J.C.; de Almeida, R.N.; Barbosa-Filho, J.M.; de Fatima Vanderlei de Souza, M.; Gutierrez, S.J.; Junior, L.J.; et al. Effect of N-salicyloyltryptamine (stp), a novel tryptamine analogue, on parameters of cell viability, oxidative stress, and immunomodulation in raw 264.7 macrophages. Cell Biol. Toxicol. 2013, 29, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Hagensen, M.K.; Vanhoutte, P.M.; Bentzon, J.F. Arterial endothelial cells: Still the craftsmen of regenerated endothelium. Cardiovasc. Res. 2012, 95, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Feletou, M.; Kohler, R.; Vanhoutte, P.M. Nitric oxide: Orchestrator of endothelium-dependent responses. Ann. Med. 2012, 44, 694–716. [Google Scholar] [CrossRef] [PubMed]
- Schrammel, A.; Behrends, S.; Schmidt, K. Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol. Pharmacol. 1996, 50, 1–5. [Google Scholar] [PubMed]
- Feletou, M.; Vanhoutte, P.M. Edhf: An update. Clin. Sci. 2009, 117, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Feletou, M.; Huang, Y.; Vanhoutte, P.M. Endothelium-mediated control of vascular tone: Cox-1 and cox-2 products. Br. J. Pharmacol. 2011, 164, 894–912. [Google Scholar] [CrossRef] [PubMed]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Poulos, T.L. Structure-function studies on nitric oxide synthases. J. Inorg. Biochem. 2005, 99, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Rand, M. Differential effects of hydroxocobalamin on relaxations induced by nitrosothiols in rat aorta and anoccygeus muscle. Eur. J. Pharmacol. 1993, 241, 249–254. [Google Scholar]
- Kruszyna, H.; Magyar, J.S.; Rochelle, L.G.; Russell, M.A.; Smith, R.P.; Wilcox, D.E. Spectroscopic studies of nitric oxide (no) interactions with cobalamins: Reaction of no with superoxocobalamin(iii) likely accounts for cobalamin reversal of the biological effects of no. J. Pharmacol. Exp. Ther. 1998, 285, 665–671. [Google Scholar] [PubMed]
- Evora, P.R.; Evora, P.M.; Celotto, A.C.; Rodrigues, A.J.; Joviliano, E.E. Cardiovascular therapeutics targets on the no-sgc-cgmp signaling pathway: A critical overview. Curr. Drug Targets 2012, 13, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E.R.; Marletta, M.A. Structure and regulation of soluble guanylate cyclase. Annu. Rev. Biochem. 2012, 81, 533–559. [Google Scholar] [CrossRef] [PubMed]
- Hoenicka, M.; Schmid, C. Cardiovascular effects of modulators of soluble guanylyl cyclase activity. Cardiovasc. Hematol. Agents Med. Chem. 2008, 6, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Walter, U. Physiological role of cgmp and cgmp-dependent protein kinase in the cardiovascular system. Rev. Physiol. Biochem. Pharmacol. 1989, 113, 41–88. [Google Scholar] [PubMed]
- Karaki, K.; Weiss, G.B. Calcium channels in smoth muscle. Gastroenterology 1984, 87, 960–970. [Google Scholar] [PubMed]
- Karaki, H.; Ozaki, H.; Hori, M.; Saito, M. Calcium movements, distribution, and functions in smooth muscle. Pharmacol. Rev. 1997, 49, 157–230. [Google Scholar] [PubMed]
- Koch, W.J.; Ellinor, P.T.; Schwartz, A. Cdna cloning of a dihydropyridine-sensitive calcium channel from rat aorta. Evidence for the existence of alternatively spliced forms. J. Biol. Chem. 1990, 265, 17786–17791. [Google Scholar] [PubMed]
- Liévano, A.; Boldem, A.; Horn, R. Calcium channels in excitable cells: Divergent genotypic and phenotypic expression of α1-subunits. Am. J. Physiol. 1994, 36, C411–C424. [Google Scholar] [CrossRef] [PubMed]
- Meza, U.; Avila, G.; Felix, R.; Gomora, J.C.; Cota, G. Longterm regulation of calcium channels in clonal pituitary cells by epidermal growth factor, insulin, and glucocorticoids. J. Gen. Physiol. 1994, 104, 1019–1038. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.; Barbosa Filho, J.M.; Souza, M.F.V.; Gutierrez, J.C.; Silva, D.A.; Quintans, L.J., Jr. Novo Derivado Benzoiltriptamínico e Processo Para Sua Obtenção. Patent PI 0304393-2 A2, 7 June 2005. [Google Scholar]
- Ribeiro, T.P.; Porto, D.L.; Menezes, C.P.; Antunes, A.A.; Silva, D.F.; De Sousa, D.P.; Nakao, L.S.; Braga, V.A.; Medeiros, I.A. Unravelling the cardiovascular effects induced by alpha-terpineol: A role for the nitric oxide-cgmp pathway. Clin. Exp. Pharmacol. Physiol. 2010, 37, 811–816. [Google Scholar] [PubMed]
- Moore, P.K.; Al-Swayeh, O.A.; Chong, N.W.S.; Evans, R.; Gibson, A. l-ng-nitro arginine (l-arginine reversible inhibitor of endothelium-dependent vasodilatation in vitro). Br. J. Pharmacol. 1990, 99, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Friebe, A.; Koesling, D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ. Res. 2003, 93, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Garthwaite, J.; Southam, E.; Boulton, C.L.; Nielsen, E.B.; Schmidt, K.; Mayer, B. Potent and selective-inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-1,2,4 oxadiazolo 4,3-a quinoxalin-1-one. Mol. Pharm. 1995, 48, 184–188. [Google Scholar]
- Veras, R.C.; Rodrigues, K.G.; Alustau, M.d.C.; Araújo, I.G.A.; de Barros, A.L.B.; Alves, R.J.; Nakao, L.S.; Braga, V.A.; Silva, D.F.; de Medeiros, I.A. Participation of nitric oxide pathway in the relaxation response induced by e-cinnamaldehyde oxime in superior mesenteric artery isolated from rats. J. Cardiovasc. Pharmacol. 2013, 62, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Lagaud, G.J.; Randriamboavonjy, R.G.; Stoclet, J.G.; Andriatsitohaina, R. Mechanism of Ca2+ release and entry during contraction elicited by norepinephrine in rat resistance arteries. Am. J. Physiol. 1999, 276, H300–H308. [Google Scholar] [CrossRef] [PubMed]
- Van Beijnum, J.R.; Rousch, M.; Castermans, K.; van der Linden, E.; Griffioen, A.W. Isolation of endothelial cells from fresh tissues. Nat. Protoc. 2008, 3, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.T.; Hipolito, U.V.; Callera, G.E.; Yogi, A.; Neto Filho Mdos, A.; Bendhack, L.M.; Touyz, R.M.; Tirapelli, C.R. Ethanol induces vascular relaxation via redox-sensitive and nitric oxide-dependent pathways. Vasc. Pharmacol. 2012, 56, 74–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baudin, B.; Bruneel, A.; Bosselut, N.; Vaubourdolle, M. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat. Protoc. 2007, 2, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Hewett, P.W.; Murray, J.C. Immunomagnetic purification of human microvessel endothelial cells using dynabeads coated with monoclonal antibodies to pecam-1. Eur. J. Cell Biol. 1993, 62, 451–454. [Google Scholar] [PubMed]
- Ozdogu, H.; Sozer, O.; Boga, C.; Kozanoglu, L.; Maytalman, E.; Guzey, M. Flow cytometric evaluation of circulating endothelial cells: A new protocol for identifying endothelial cells at several stages of differentiation. Am. J. Hematol. 2007, 82, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Roerig, D.L.; Bosnjak, Z.J.; Stowe, D.F. Effects of vasodilators and perfusion pressure on coronary flow and simultaneous release of nitric oxide from guinea pig isolated hearts. Cardiovasc. Res. 1998, 38, 655–667. [Google Scholar] [CrossRef]
- Zhang, X.; Cardosa, L.; Broderick, M.; Fein, H.; Davies, I.R. Novel calibration method for nitric oxide microsensors by stoichiometrical generation of nitric oxide from snap. Electroanalysis 2000, 12, 425–428. [Google Scholar] [CrossRef]
- Serpe, M.; Zhang, X. The principles, development and application of microelectrodes for the in vivo determination of nitric oxide. In Electrochemical Methods for Neuroscience; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Schmidt, K.; Mayer, B. Determination of NO with a clark-type electrode. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 1998; pp. 101–109. [Google Scholar]
Sample Availability: Samples of the compounds used in this study are available from the authors. |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veras, R.C.; Silva, D.F.; Bezerra, L.S.; Assis, V.L.d.; Vasconcelos, W.P.d.; Alustau, M.D.C.; Albuquerque, J.G.F.d.; Furtado, F.F.; Araújo, I.G.d.A.; Azevedo, F.D.L.A.A.d.; et al. N-Salicyloyltryptamine, an N-Benzoyltryptamine Analogue, Induces Vasorelaxation through Activation of the NO/sGC Pathway and Reduction of Calcium Influx. Molecules 2018, 23, 253. https://doi.org/10.3390/molecules23020253
Veras RC, Silva DF, Bezerra LS, Assis VLd, Vasconcelos WPd, Alustau MDC, Albuquerque JGFd, Furtado FF, Araújo IGdA, Azevedo FDLAAd, et al. N-Salicyloyltryptamine, an N-Benzoyltryptamine Analogue, Induces Vasorelaxation through Activation of the NO/sGC Pathway and Reduction of Calcium Influx. Molecules. 2018; 23(2):253. https://doi.org/10.3390/molecules23020253
Chicago/Turabian StyleVeras, Robson Cavalcante, Darizy Flávia Silva, Lorena Soares Bezerra, Valéria Lopes de Assis, Walma Pereira de Vasconcelos, Maria Do Carmo Alustau, José George Ferreira de Albuquerque, Fabíola Fialho Furtado, Islania Giselia de Albuquerque Araújo, Fátima De Lourdes Assunção Araújo de Azevedo, and et al. 2018. "N-Salicyloyltryptamine, an N-Benzoyltryptamine Analogue, Induces Vasorelaxation through Activation of the NO/sGC Pathway and Reduction of Calcium Influx" Molecules 23, no. 2: 253. https://doi.org/10.3390/molecules23020253