Crystallographic and Computational Characterization of Methyl Tetrel Bonding in S-Adenosylmethionine-Dependent Methyltransferases
Abstract
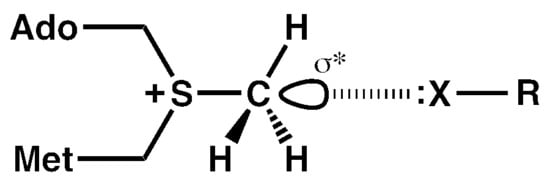
:1. Introduction
2. Material and Methods
2.1. PDB Survey
2.2. QM Calculations
3. Results
3.1. Methyltransferase Structural Survey
3.2. Computational Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schubert, H.L.; Blumenthal, R.M.; Cheng, X. Many paths to methyltransfer: A chronicle of convergence. Trends Biochem. Sci. 2003, 28, 329–335. [Google Scholar] [CrossRef]
- Petrossian, T.C.; Clarke, S.G. Uncovering the Human Methyltransferasome. Mol. Cell. Proteom. 2011, 10, M110.000976. [Google Scholar] [CrossRef] [PubMed]
- Petrossian, T.; Clarke, S. Bioinformatic Identification of Novel Methyltransferases. Epigenomics 2009, 1, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Woodard, R.W.; Tsai, M.D.; Floss, H.G.; Crooks, P.A.; Coward, J.K. Stereochemical course of the transmethylation catalyzed by catechol O-methyltransferase. J. Biol. Chem. 1980, 255, 9124–9127. [Google Scholar] [PubMed]
- Hegazi, M.F.; Borchard, R.T.; Schowen, R.L. Letter: SN2-like transition state for methyl transfer catalyzed by catechol-O-methyl-transferase. J. Am. Chem. Soc. 1976, 98, 3048–3049. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.H.; Coward, J.K.; Schowen, K.B.; Schowen, R.L. Alpha-Deuterium and C-13 Isotope Effects for a Simple, Inter-Molecular Sulfur-to-Oxygen Methyl-Transfer Reaction–Transition-State Structures and Isotope Effects in Transmethylation and Transalkylation. J. Am. Chem. Soc. 1979, 101, 4351–4358. [Google Scholar] [CrossRef]
- Mihel, I.; Knipe, J.O.; Coward, J.K.; Schowen, R.L. Alpha-Deuterium Isotope Effects and Transition-State Structure in an Intra-Molecular Model System for Methyl-Transfer Enzymes. J. Am. Chem. Soc. 1979, 101, 4349–4351. [Google Scholar] [CrossRef]
- Zhang, J.; Klinman, J.P. Enzymatic methyl transfer: Role of an active site residue in generating active site compaction that correlates with catalytic efficiency. J. Am. Chem. Soc. 2011, 133, 17134–17137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kulik, H.J.; Martinez, T.J.; Klinman, J.P. Mediation of donor-acceptor distance in an enzymatic methyl transfer reaction. Proc. Natl. Acad. Sci. USA 2015, 112, 7954–7959. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.Y.; Kahn, K.; Bash, P.A.; Bruice, T.C. The importance of reactant positioning in enzyme catalysis: A hybrid quantum mechanics/molecular mechanics study of a haloalkane dehalogenase. Proc. Natl. Acad. Sci. USA 2000, 97, 9937–9942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, K.; Bruice, T.C. Transition-state and ground-state structures and their interaction with the active-site residues in catechol-O-methyl transferase. J. Am. Chem. Soc. 2000, 122, 46–51. [Google Scholar] [CrossRef]
- Lau, E.Y.; Bruice, T.C. Importance of correlated motions in forming highly reactive near attack conformations in catechol O-methyltransferase. J. Am. Chem. Soc. 1998, 120, 12387–12394. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Bruice, T.C. A theoretical examination of the factors controlling the catalytic efficiency of a transmethylation enzyme: Catechol O-methyltransferase. J. Am. Chem. Soc. 1997, 119, 8137–8145. [Google Scholar] [CrossRef]
- Lameira, J.; Bora, R.P.; Chu, Z.T.; Warshel, A. Methyltransferases do not work by compression, cratic, or desolvation effects, but by electrostatic preorganization. Proteins 2015, 83, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Roca, M.; Marti, S.; Andres, J.; Moliner, V.; Tunon, I.; Bertran, J.; Williams, I.H. Theoretical modeling of enzyme catalytic power: Analysis of “cratic” and electrostatic factors in catechol O-methyltransferase. J. Am. Chem. Soc. 2003, 125, 7726–7737. [Google Scholar] [CrossRef] [PubMed]
- Kollman, P.A.; Kuhn, B.; Donini, O.; Perakyla, M.; Stanton, R.; Bakowies, D. Elucidating the nature of enzyme catalysis utilizing a new twist on an old methodology: Quantum mechanical-free energy calculations on chemical reactions in enzymes and in aqueous solution. Acc. Chem. Res. 2001, 34, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, B.; Kollman, P.A. QM-FE and molecular dynamics calculations on catechol O-methyltransferase: Free energy of activation in the enzyme and in aqueous solution and regioselectivity of the enzyme-catalyzed reaction. J. Am. Chem. Soc. 2000, 122, 2586–2596. [Google Scholar] [CrossRef]
- Horowitz, S.; Dirk, L.M.; Yesselman, J.D.; Nimtz, J.S.; Adhikari, U.; Mehl, R.A.; Scheiner, S.; Houtz, R.L.; Al-Hashimi, H.M.; Trievel, R.C. Conservation and functional importance of carbon–oxygen hydrogen bonding in AdoMet-dependent methyltransferases. J. Am. Chem. Soc. 2013, 135, 15536–15548. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, U.; Scheiner, S. Magnitude and mechanism of charge enhancement of CH··O hydrogen bonds. J. Phys. Chem. A 2013, 117, 10551–10562. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, S.; Yesselman, J.D.; Al-Hashimi, H.M.; Trievel, R.C. Direct evidence for methyl group coordination by carbon–oxygen hydrogen bonds in the lysine methyltransferase SET7/9. J. Biol. Chem. 2011, 286, 18658–18663. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, S.; Adhikari, U.; Dirk, L.M.; Del Rizzo, P.A.; Mehl, R.A.; Houtz, R.L.; Al-Hashimi, H.M.; Scheiner, S.; Trievel, R.C. Manipulating unconventional CH-based hydrogen bonding in a methyltransferase via noncanonical amino acid mutagenesis. ACS Chem. Biol. 2014, 9, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Poulin, M.B.; Schneck, J.L.; Matico, R.E.; McDevitt, P.J.; Huddleston, M.J.; Hou, W.; Johnson, N.W.; Thrall, S.H.; Meek, T.D.; Schramm, V.L. Transition state for the NSD2-catalyzed methylation of histone H3 lysine 36. Proc. Natl. Acad. Sci. USA 2016, 113, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Linscott, J.A.; Kapilashrami, K.; Wang, Z.; Senevirathne, C.; Bothwell, I.R.; Blum, G.; Luo, M. Kinetic isotope effects reveal early transition state of protein lysine methyltransferase SET8. Proc. Natl. Acad. Sci. USA 2016, 113, E8369–E8378. [Google Scholar] [CrossRef] [PubMed]
- Swiderek, K.; Tunon, I.; Williams, I.H.; Moliner, V. Insights on the Origin of Catalysis on Glycine N-Methyltransferase from Computational Modeling. J. Am. Chem. Soc. 2018, 140, 4327–4334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fick, R.J.; Kroner, G.M.; Nepal, B.; Magnani, R.; Horowitz, S.; Houtz, R.L.; Scheiner, S.; Trievel, R.C. Sulfur-Oxygen Chalcogen Bonding Mediates AdoMet Recognition in the Lysine Methyltransferase SET7/9. ACS Chem. Biol. 2016, 11, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Z.; Ji, B.M.; Zhang, Y. Chalcogen Bond: A Sister Noncovalent Bond to Halogen Bond. J. Phys. Chem. A 2009, 113, 8132–8135. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Comparison of CH∙∙∙O, SH∙∙∙O Chalcogen, and Tetrel Bonds Formed by Neutral and Cationic Sulfur-Containing Compounds. J. Phys. Chem. A 2015, 119, 9189–9199. [Google Scholar] [CrossRef] [PubMed]
- Bauza, A.; Mooibroek, T.J.; Frontera, A. Tetrel-bonding interaction: Rediscovered supramolecular force? Angew. Chem. 2013, 52, 12317–12321. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Lane, P.; Politzer, P. Expansion of the sigma-hole concept. J. Mol. Model. 2009, 15, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Bauza, A.; Frontera, A. RCH3∙∙∙O Interactions in Biological Systems: Are They Trifurcated H-Bonds or Noncovalent Carbon Bonds? Crystals 2016, 6, 26. [Google Scholar] [CrossRef]
- Thomas, S.P.; Pavan, M.S.; Guru Row, T.N. Experimental evidence for ‘carbon bonding’ in the solid state from charge density analysis. Chem. Commun. 2014, 50, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.X.; Bauza, A.; Seth, S.K.; Frontera, A. Importance of R–CF3∙∙∙O Tetrel Bonding Interactions in Biological Systems. J. Phys. Chem. A 2017, 121, 5371–5376. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Tetrel bond-sigma-hole bond as a preliminary stage of the SN2 reaction. Phys. Chem. Chem. Phys. 2014, 16, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Kennard, O. Crystallographic Evidence for the Existence of C-H∙∙∙O, C-H∙∙∙N, and C-H∙∙∙C1 Hydrogen-Bonds. J. Am. Chem. Soc. 1982, 104, 5063–5070. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef] [Green Version]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. D 2010, 66, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular Interactions from a Natural Bond Orbital, Donor-Acceptor Viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinhold, F.; Curtiss, L.A.; Pochatko, D.J. Natural Bond Orbital Analysis of Molecular-Interactions–Theoretical-Studies of Binary Complexes of HF, H2O, NH3, N2, O2, F2, CO, and CO2 with HF, H2O, and NH3. J. Chem. Phys. 1986, 84, 5687–5705. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Ditchfield, R. Self-Consistent Perturbation-Theory of Diamagnetism 1. Gauge-Invariant Lcao Method for Nmr Chemical-Shifts. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- Cheeseman, J.R.; Trucks, G.W.; Keith, T.A.; Frisch, M.J. A comparison of models for calculating nuclear magnetic resonance shielding tensors. J. Chem. Phys. 1996, 104, 5497–5509. [Google Scholar] [CrossRef]
- Couture, J.F.; Hauk, G.; Thompson, M.J.; Blackburn, G.M.; Trievel, R.C. Catalytic roles for carbon–oxygen hydrogen bonding in SET domain lysine methyltransferases. J. Biol. Chem. 2006, 281, 19280–19287. [Google Scholar] [CrossRef] [PubMed]
- Kaniskan, H.U.; Martini, M.L.; Jin, J. Inhibitors of Protein Methyltransferases and Demethylases. Chem. Rev. 2018, 118, 989–1068. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Liu, H.; Wu, B. Structure-based drug design of catechol-O-methyltransferase inhibitors for CNS disorders. Br. J. Clin. Pharmacol. 2014, 77, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Kiss, L.E.; Soares-da-Silva, P. Medicinal chemistry of catechol O-methyltransferase (COMT) inhibitors and their therapeutic utility. J. Med. Chem. 2014, 57, 8692–8717. [Google Scholar] [CrossRef] [PubMed]
- Gnyszka, A.; Jastrzebski, Z.; Flis, S. DNA methyltransferase inhibitors and their emerging role in epigenetic therapy of cancer. Anticancer. Res. 2013, 33, 2989–2996. [Google Scholar] [PubMed]
- Morera, L.; Lubbert, M.; Jung, M. Targeting histone methyltransferases and demethylases in clinical trials for cancer therapy. Clin. Epigenetics 2016, 8, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonifacio, M.J.; Palma, P.N.; Almeida, L.; Soares-da-Silva, P. Catechol-O-methyltransferase and its inhibitors in Parkinson’s disease. CNS Drug Rev. 2007, 13, 352–379. [Google Scholar] [CrossRef] [PubMed]
- Ellermann, M.; Lerner, C.; Burgy, G.; Ehler, A.; Bissantz, C.; Jakob-Roetne, R.; Paulini, R.; Allemann, O.; Tissot, H.; Grunstein, D.; et al. Catechol-O-methyltransferase in complex with substituted 3’-deoxyribose bisubstrate inhibitors. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.T.; Poslusney, M.S.; Mulhearn, J.J.; Zhao, Z.; Kett, N.R.; Schubert, J.W.; Melamed, J.Y.; Allison, T.J.; Patel, S.B.; Sanders, J.M.; et al. Synthesis and Evaluation of Heterocyclic Catechol Mimics as Inhibitors of Catechol-O-methyltransferase (COMT). ACS Med. Chem. Lett. 2015, 6, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.N.; Rodrigues, M.L.; Archer, M.; Bonifacio, M.J.; Loureiro, A.I.; Learmonth, D.A.; Carrondo, M.A.; Soares-da-Silva, P. Comparative study of ortho- and meta-nitrated inhibitors of catechol-O-methyltransferase: Interactions with the active site and regioselectivity of O-methylation. Mol. Pharmacol. 2006, 70, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Vidgren, J.; Svensson, L.A.; Liljas, A. Crystal structure of catechol O-methyltransferase. Nature 1994, 368, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Spellmon, N.; Holcomb, J.; Trescott, L.; Sirinupong, N.; Yang, Z. Structure and function of SET and MYND domain-containing proteins. Int. J. Mol. Sci. 2015, 16, 1406–1428. [Google Scholar] [CrossRef] [PubMed]
- Kudithipudi, S.; Jeltsch, A. Role of somatic cancer mutations in human protein lysine methyltransferases. BBA-Rev. Cancer 2014, 1846, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Tracy, C.; Warren, J.S.; Szulik, M.; Wang, L.; Garcia, J.; Makaju, A.; Russell, K.; Miller, M.; Franklin, S. The Smyd Family of Methyltransferases: Role in Cardiac and Skeletal Muscle Physiology and Pathology. Curr. Opin. Physiol. 2018, 1, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.H.; Boriack-Sjodin, P.A.; Smith, S.; Thomenius, M.; Rioux, N.; Munchhof, M.; Mills, J.E.; Klaus, C.; Totman, J.; Riera, T.V.; et al. Novel Oxindole Sulfonamides and Sulfamides: EPZ031686, the First Orally Bioavailable Small Molecule SMYD3 Inhibitor. ACS Med. Chem. Lett. 2016, 7, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Eggert, E.; Hillig, R.C.; Koehr, S.; Stockigt, D.; Weiske, J.; Barak, N.; Mowat, J.; Brumby, T.; Christ, C.D.; Ter Laak, A.; et al. Discovery and Characterization of a Highly Potent and Selective Aminopyrazoline-Based in Vivo Probe (BAY-598) for the Protein Lysine Methyltransferase SMYD2. J. Med. Chem. 2016, 59, 4578–4600. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.D.; Larsen, N.A.; Howard, T.; Pollard, H.; Green, I.; Grande, C.; Cheung, T.; Garcia-Arenas, R.; Cowen, S.; Wu, J.; et al. Structural basis of substrate methylation and inhibition of SMYD2. Structure 2011, 19, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Bae, B.; Kuemin, M.; Circello, B.T.; Metcalf, W.W.; Nair, S.K.; van der Donk, W.A. Characterization and structure of DhpI, a phosphonate O-methyltransferase involved in dehydrophos biosynthesis. Proc. Natl. Acad Sci. USA 2010, 107, 17557–17562. [Google Scholar] [CrossRef] [PubMed]
- Fick, R.J.; Clay, M.C.; Vander Lee, L.; Scheiner, S.; Al-Hashimi, H.; Trievel, R.C. Water-Mediated Carbon–oxygen Hydrogen Bonding Facilitates S-Adenosylmethionine Recognition in the Reactivation Domain of Cobalamin-Dependent Methionine Synthase. Biochemistry 2018, 57, 3733–3740. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Ability of IR and NMR Spectral Data to Distinguish between a Tetrel Bond and a Hydrogen Bond. J. Phys. Chem. A 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-M.; Chen, J.C.C.; Chen, H.-Y.; Lin, I.J.B. A triple helical structure supported solely by C–H∙∙∙O hydrogen bonding. Chem. Commun. 2012, 48, 1242–1244. [Google Scholar] [CrossRef] [PubMed]
- Simonson, T.; Perahia, D. Internal and Interfacial Dielectric-Properties of Cytochrome-C from Molecular-Dynamics in Aqueous-Solution. Proc. Natl. Acad. Sci. USA 1995, 92, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.J.; Gittis, A.G.; Karp, D.A.; Lattman, E.E.; Spencer, D.S.; Stites, W.E.; Garcia-Moreno, B. High apparent dielectric constants in the interior of a protein reflect water penetration. Biophys. J. 2000, 79, 1610–1620. [Google Scholar] [CrossRef]
- Smith, P.E.; Brunne, R.M.; Mark, A.E.; Vangunsteren, W.F. Dielectric-Properties of Trypsin-Inhibitor and Lysozyme Calculated from Molecular-Dynamics Simulations. J. Phys. Chem. 1993, 97, 2009–2014. [Google Scholar] [CrossRef]
- Roy, S.; Drew, M.G.B.; Bauza, A.; Frontera, A.; Chattopadhyay, S. Non-covalent tetrel bonding interactions in hemidirectional lead(ii) complexes with nickel(ii)-salen type metalloligands. New J. Chem. 2018, 42, 6062–6076. [Google Scholar] [CrossRef]
- Dong, W.; Li, Q.; Scheiner, S. Comparative Strengths of Tetrel, Pnicogen, Chalcogen, and Halogen Bonds and Contributing Factors. Molecules 2018, 23, 1681. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zeng, Y.; Li, X.; Meng, L.; Zhang, X. Insight into the π-hole∙∙∙π-electrons tetrel bonds between F2ZO (Z = C, Si, Ge) and unsaturated hydrocarbons. Int. J. Quantum Chem. 2018, 118, e25521. [Google Scholar] [CrossRef]
- Zierkiewicz, W.; Michalczyk, M.; Scheiner, S. Comparison between Tetrel Bonded Complexes Stabilized by σ and π Hole Interactions. Molecules 2018, 23, 1416. [Google Scholar] [CrossRef] [PubMed]
- Zierkiewicz, W.; Michalczyk, M.; Scheiner, S. Implications of monomer deformation for tetrel and pnicogen bonds. Phys. Chem. Chem. Phys. 2018, 20, 8832–8841. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Hydrogen bonds, and s-hole and p-hole bonds–mechanisms protecting doublet and octet electron structures. Phys. Chem. Chem. Phys. 2017, 19, 29742–29759. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J.; Sokalski, W.A. Are Various σ-Hole Bonds Steered by the Same Mechanisms? ChemPhysChem. 2017, 18, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, Q.; Scheiner, S. Comparison of tetrel bonds in neutral and protonated complexes of pyridineTF3 and furanTF3 (T = C, Si, and Ge) with NH3. Phys. Chem. Chem. Phys. 2017, 19, 5550–5559. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Steric Crowding in Tetrel Bonds. J. Phys. Chem. A 2018, 122, 2550–2562. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Assembly of Effective Halide Receptors from Components. Comparing Hydrogen, Halogen, and Tetrel Bonds. J. Phys. Chem. A 2017, 121, 3606–3615. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-X.; Li, H.-B.; Cheng, J.-B.; Li, W.-Z.; Li, Q.-Z. Prominent enhancing effects of substituents on the strength of π···σ-hole tetrel bond. Int. J. Quantum Chem. 2017, 117, e25448. [Google Scholar] [CrossRef]
- Bauza, A.; Mooibroek, T.J.; Frontera, A. Tetrel Bonding Interactions. Chem. Rec. 2016, 16, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Southern, S.A.; Bryce, D.L. NMR Investigations of Noncovalent Carbon Tetrel Bonds. Computational Assessment and Initial Experimental Observation. J. Phys. Chem. A 2015, 119, 11891–11899. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Systematic Elucidation of Factors That Influence the Strength of Tetrel Bonds. J. Phys. Chem. A 2017, 121, 5561–5568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mani, D.; Arunan, E. The X–CY (X = O/F, Y = O/S/F/Cl/Br/N/P) ‘carbon bond’ and hydrophobic interactions. Phys. Chem. Chem. Phys. 2013, 15, 14377–14383. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, M.D.; Kiani, H.; Mohammadian-Sabet, F. Tuning of carbon bonds by substituent effects: An ab initio study. Mol. Phys. 2016, 114, 3658–3668. [Google Scholar] [CrossRef]
- Del Bene, J.E.; Alkorta, I.; Elguero, J. Anionic complexes of F− and Cl− with substituted methanes: Hydrogen, halogen, and tetrel bonds. Chem. Phys. Lett. 2016, 655, 115–119. [Google Scholar] [CrossRef]
- Liu, M.; Li, Q.; Cheng, J.; Li, W.; Li, H.-B. Tetrel bond of pseudohalide anions with XH3F (X = C, Si, Ge, and Sn) and its role in SN2 reaction. J. Chem. Phys. 2016, 145, 224310. [Google Scholar] [CrossRef] [PubMed]
- Marín-Luna, M.; Alkorta, I.; Elguero, J. Cooperativity in Tetrel Bonds. J. Phys. Chem. A 2016, 120, 648–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Fernández, C.; Montero-Campillo, M.M.; Alkorta, I.; Elguero, J. Weak interactions and cooperativity effects on disiloxane: A look at the building block of silicones. Mol. Phys. 2018, 116, 1539–1550. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Asadollahi, S.; Mousavian, P. Anionic tetrel bonds: An ab initio study. Chem. Phys. Lett. 2018, 691, 394–400. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Nurazar, R.; Mohammadian-Sabet, F. Cooperative effects between tetrel bond and other σ–hole bond interactions: A comparative investigation. Mol. Phys. 2015, 113, 3703–3711. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Q. Comparison for σ-hole and π-hole tetrel-bonded complexes involving cyanoacetaldehyde. Mol. Phys. 2018, 116, 222–230. [Google Scholar] [CrossRef]
- Ghara, M.; Pan, S.; Kumar, A.; Merino, G.; Chattaraj, P.K. Structure, stability, and nature of bonding in carbon monoxide bound EX3+ complexes (E = group 14 element; X = H, F, Cl, Br, I). J. Comput. Chem. 2016, 37, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Comparison of halide receptors based on H, halogen, chalcogen, pnicogen, and tetrel bonds. Faraday Discuss. 2017, 203, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.Y.; Bruice, T.C. Comparison of the dynamics for ground-state and transition-state structures in the active site of catechol O-methyltransferase. J. Am. Chem. Soc. 2000, 122, 7165–7171. [Google Scholar] [CrossRef]
- Lotta, T.; Vidgren, J.; Tilgmann, C.; Ulmanen, I.; Melen, K.; Julkunen, I.; Taskinen, J. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: A revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 1995, 34, 4202–4210. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.M.; Tipton, K.F. Nitrocatechol derivatives as inhibitors of catechol-O-methyltransferase. Biochem. Soc. Trans. 1996, 24, 64S. [Google Scholar] [CrossRef] [PubMed]





| Enzyme | PDB Code | Resolution (Å) | Ligand | Electron Donor (X) | R(C∙∙∙X) Length (Å) A | θ(S–C∙∙∙X) Angle (°) A |
|---|---|---|---|---|---|---|
| ASH1L | 4YNM | 2.19 | H2O | O | 2.99 (B) | 163 (B) |
| Bud23 | 4QTU | 2.12 | Ethylene glycol | O | 3.04 (B) | 173 (B) |
| COMT | 2CL5 | 1.6 | BIA 8-176 B | O | 2.70 (A), 2.69 (B) | 173 (A), 172 (B) |
| COMT | 3S68 | 1.85 | Tolcapone C | O | 2.50 | 166 |
| COMT | 4XUC | 1.8 | Compound 18 D | O | 2.64 | 175 |
| COMT | 4XUD | 2.4 | Compound 32 E | O | 2.73 | 166 |
| COMT | 5LSA | 1.5 | 3,5-Dinitrocatechol | O | 2.71 | 173 |
| DhpI | 3OU6 | 2.3 | Sulfate | O | 3.00 (A), 3.09 (B), 2.97 (C) | 175 (A), 175 (B), 176 (C) |
| G9A | 5VSC | 1.4 | H2O | O | 3.14 (A), 3.17 (B) | 166 (A), 168 (B) |
| GLP | 5TTG | 1.66 | H2O | O | 3.15 (A), 3.24 (B) | 168 (A), 169 (B) |
| MMSET | 5LSU | 2.14 | H2O | O | 3.13 (B) | 160 (B) |
| PrmA | 2NXE | 1.75 | H2O | O | 3.08 (B) | 171 (B) |
| PRMT5 | 5EML | 2.39 | H2O | O | 3.09 (A) | 163 (A) |
| RsmF | 3M6V | 1.82 | H2O | O | 3.20 (A), 3.23 (B) | 164 (A), 162 (B) |
| SMYD2 | 3S7B | 2.42 | AZ505 F | O | 2.77 | 169 |
| SMYD2 | 3TG4 | 2.0 | Glycerol | O | 3.23 | 176 |
| SMYD2 | 5ARG | 1.99 | SGC Probe BAY-598 G | Cl | 3.43 | 175 |
| SMYD3 | 3QWP | 1.53 | Glycerol | O | 3.01 | 163 |
| SMYD3 | 5CCL | 1.5 | Oxindole compound H | O | 2.89 | 164 |
| SMYD3 | 5CCM | 2.3 | EPZ030456 I | O | 2.78 | 168 |
| Structure | PDB | X | R(C∙∙∙X) (Å) | θ(S–C∙∙∙X) (°) | Eint | ET | EH |
|---|---|---|---|---|---|---|---|
| SMYD2 | 5ARG | Cl | 3.431 | 175.0 | −5.2 | 0.63 | 0.10 |
| SMYD3 | 5CCL | O | 2.885 | 164.3 | −9.0 | 0.62 | 0.38 |
| G9A | 5VSC | O | 3.145 | 165.6 | −7.0 | 0.46 | 0.16 |
| COMT | 5LSA | O- | 2.712 | 172.7 | −65.7 | 1.33 | 0.16 |
| Structure | PDB | ∆σC | ∆σH | ∆νstr | ∆νbend |
|---|---|---|---|---|---|
| SMYD2 | 5ARG | −1.94 | −0.14 | 0.8 | −11.5 |
| SMYD3 | 5CCL | −2.27 | −0.28 | 5.7 | −38.3 |
| G9A | 5VSC | −2.36 | −0.29 | 3.9 | −22.9 |
| COMT | 5LSA | −6.29 | −0.95 | 4.8 | −56.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trievel, R.C.; Scheiner, S. Crystallographic and Computational Characterization of Methyl Tetrel Bonding in S-Adenosylmethionine-Dependent Methyltransferases. Molecules 2018, 23, 2965. https://doi.org/10.3390/molecules23112965
Trievel RC, Scheiner S. Crystallographic and Computational Characterization of Methyl Tetrel Bonding in S-Adenosylmethionine-Dependent Methyltransferases. Molecules. 2018; 23(11):2965. https://doi.org/10.3390/molecules23112965
Chicago/Turabian StyleTrievel, Raymond C., and Steve Scheiner. 2018. "Crystallographic and Computational Characterization of Methyl Tetrel Bonding in S-Adenosylmethionine-Dependent Methyltransferases" Molecules 23, no. 11: 2965. https://doi.org/10.3390/molecules23112965