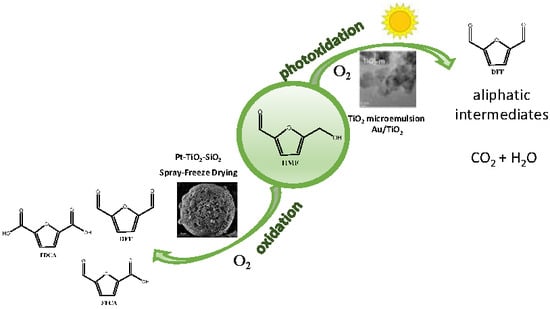
Selective Oxidation of HMF via Catalytic and Photocatalytic Processes Using Metal-Supported Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. HMF Oxidation Using Heterogeneous Catalysts
2.1.1. Catalyst Preparation and Characterisation
2.1.2. Catalytic Tests
2.2. Oxidation of HMF by a Photocatalytic Process
2.2.1. Catalyst Preparation and Characterisation
2.2.2. Photocatalytic Tests
3. Materials and Methods
3.1. Catalyst Preparation
3.1.1. Preparation of Pt/SiO2/TiO2 Catalysts by Colloidal Heterocoagulation and Spray-Freeze Drying
3.1.2. Titania Microemulsion Preparation (TiO2-m)
3.1.3. Synthesis of Au/TiO2 by the Deposition-Precipitation Method
3.2. Catalyst Characterisation
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albonetti, S.; Lolli, A.; Morandi, V.; Migliori, A.; Lucarelli, C.; Cavani, F. Conversion of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over Au-based catalysts: Optimization of active phase and metal–support interaction. Appl. Catal. B. Environ. 2015, 163, 520–530. [Google Scholar] [CrossRef]
- Davis, S.E.; Houk, L.R.; Tamargo, E.C.; Datye, A.K.; Davis, R.J. Oxidation of 5-hydroxymethylfurfural over supported Pt, Pd and Au catalysts. Catal. Today 2011, 160, 55–60. [Google Scholar] [CrossRef]
- Vuyyuru, K.R.; Strasser, P. Oxidation of biomass derived 5-hydroxymethylfurfural using heterogeneous and electrochemical catalysis. Catal. Today 2012, 195, 144–154. [Google Scholar] [CrossRef]
- Han, X.; Li, C.; Guo, Y.; Liu, X.; Zhang, Y.; Wang, Y. N-doped carbon supported Pt catalyst for base-free oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. Appl. Catal. A Gen. 2016, 526, 1–8. [Google Scholar] [CrossRef]
- Villa, A.; Schiavoni, M.; Campisi, S.; Veith, G.M.; Prati, L. Pd-modified Au on Carbon as an Effective and Durable Catalyst for the Direct Oxidation of HMF to 2,5-Furandicarboxylic Acid. ChemSusChem 2013, 6, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Grasset, F.L.; Katryniok, B.; Paul, S.; Nadello-Rataj, V.; Pera-Titus, M.; Clacens, J.M.; de Campo, F.; Dumeignil, F. Selective oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran over intercalated vanadium phosphate oxides. RSC Adv. 2013, 3, 9942–9948. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Yuan, Z.; Tang, D.; Ren, Y.; Lv, K.; Liu, B. Iron oxide encapsulated by ruthenium hydroxyapatite as heterogeneous catalyst for the synthesis of 2,5-diformylfuran. ChemSusChem 2014, 7, 3496–3504. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Molla, R.A.; Iqubal, M.A.; Islam, S.S.; Islam, S.M. Ruthenium Nanoparticles Supported on N-Containing Mesoporous Polymer Catalyzed Aerobic Oxidation Of Biomass-Derived 5-hydroxymethylfurfural (HMF) to 2,5-diformylfuran (DFF). Appl. Catal. A Gen. 2016, 520, 44–52. [Google Scholar] [CrossRef]
- Zhang, D.; Dumont, M.J. Advances in polymer precursor and bio-based polymers synthesised from 5-hydroxymthylfurfural. J. Pol. Sci. Part A Pol. Chem. 2017, 55, 1478–1492. [Google Scholar] [CrossRef]
- Burgess, S.K.; Kriegel, R.M.; Koros, W.J. Carbon dioxide sorption and transport in amorphous poly(ethylene furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- Burgess, S.K.; Karvan, O.; Johnson, J.R.; Kriegel, R.M.; Koros, W.J. Oxygen sorption and transport in amorphous poly(ethylene furanoate). Polymers 2014, 55, 4748–4756. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubaral, C.R.; Kriegel, R.J.; Koros, W.J. Chain mobility, thermal, mechanical properties of poly(ethylene furanoate) compared to poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Weinberger, S.; Canadell, J.; Quartinello, F.; Yeniad, B.; Arias, A.; Pellis, A.; Guebitz, G.M. Enzymatic degradation of poly(ethylene furanoate) powders and amorphous films. Catalysts 2017, 7, 318–328. [Google Scholar] [CrossRef]
- Pellis, A.; Haernvall, K.; Pichler, C.M.; Ghazaryan, G.; Breinbauer, R.; Guebitz, G.M. Enzymatic hydrolysis of poly(ethylene furanoate). J. Biotechnol. 2016, 235, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, J.A.; Motagamwala, A.H. Method to Produce Furandicarboxylic Acid (FDCA) from 5-Hydroxymethylfurfural (HMF). U.S. Patent 9617234, 11 April 2017. [Google Scholar]
- Sanborn, A. Process for Making 2,5-Furandicarboxylic Acid. U.S. Patent 9562028, 7 February 2017. [Google Scholar]
- Kambourakis, S.; Griffin, B.M.; Martin, K.V. Method for Synthesizing FDCA and Derivates Thereof. U.S. Patent 9506090, 29 November 2016. [Google Scholar]
- Sequeira, B.G.; De Menez, R.B.; Rabello, C.R.K.; Gomes, M., Jr. 2.5-Furandicarboxylic Acid Integrated Production Process. U.S. Patent 9199957, 1 December 2015. [Google Scholar]
- Van Harven, J.; Thiyagarajan, S.; Teruo Morita, A. Analyte Monitoring and Fluid Dispensing System. U.S. Patent 0119588, 8 September 2015. [Google Scholar]
- Shaikh, A.; Parker, K.R.; Janka, M.E.; Partin, L.R. Oxidative Purification Method for Producing Purified Dry Furan-2,5-Dicarboxylic Acid Assigned to Eastman Chemical Company. U.S. Patent 9156805, May 2014. [Google Scholar]
- Janka, M.; Lange, D.; Morrow, M.; Bowers, B.; Parker, K.; Shaikh, A.; Partin, L.; Jenkins, J.; Moody, P.; Shanks, T.; et al. Patents Assigned to Eastman Chemical Company. U.S. Patent 0011783, 14 September 2015. [Google Scholar]
- Furan Dicarboxylic Acid. Available online: http://www.ava-biochem.com/pages/en/products/fdca/applications.php (accessed on 9 May 2018).
- Dupont Industrial Bioscience. Available online: http://biosciences.dupont.com/news/dupont-industrial-biosciences-archer-daniels-midland-company-open-groundbreaking-biobased-pilot-fa/ (accessed on 9 May 2018).
- FDCA: Towards Sustainable Feedstock. Available online: https://www.synvina.com/products/fdca (accessed on 9 May 2018).
- Wojcieszak, R.; Ferraz, C.P.; Sha, J.; Houda, S.; Rossi, L.M.; Paul, S. Advances in base-free oxidation of bio-base compounds on supported gold catalysts. Catalysts 2017, 7, 352–374. [Google Scholar] [CrossRef]
- Megias-Sayago, C.; Lolli, A.; Ivanova, S.; Albonetti, S.; Cavani, F.; Odriozola, J.A. Au/Al2O3–efficient catalyst for 5-hydroxymethylfurfural oxidation to 2,5-furandicarboxylic acid. Catal. Today 2018, in press. [Google Scholar] [CrossRef]
- Gao, T.; Gao, T.; Fang, W.; Cao, Q. Base-free aerobic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid in water by hydrotalcite-activated carbon composite supported gold catalyst. Mol. Catal. 2017, 439, 171–179. [Google Scholar] [CrossRef]
- Davis, S.E.; Zope, B.N.; Davis, R.J. On the mechanism of selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over supported Pt and Au catalysts. Green Chem. 2012, 14, 143–147. [Google Scholar] [CrossRef]
- Tarning, E.; Nielsen, I.S.; Egeblad, K.; Madsen, R.; Christensen, C.H. Chemicals from Renewables: Aerobic Oxidation of Furfural and Hydroxymethylfurfural over Gold Catalysts. ChemSusChem 2008, 1, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Gorbanev, Y.Y.; Klitgaard, S.K.; Woodley, J.M.; Christensen, H.; Riisager, A. Gold-Catalyzed Aerobic Oxidation of 5-Hydroxymethylfurfural in Water at Ambient Temperature. ChemSusChem 2009, 2, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Rathod, P.V.; Jadhav, V.H. Efficient Method for Synthesis of 2,5-Furandicarboxylic Acid from 5-Hydroxymethylfurfural and Fructose Using Pd/CC Catalyst under Aqueous Conditions. ACS Sust. Chem. Eng. 2018, 6, 5766–5771. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Wang, L.; Liang, T.; Wang, L.; Zhang, Y.; Zhang, J. Highly Porous Nitrogen- and Phosphorus-Codoped Graphene: An Outstanding Support for Pd Catalysts to Oxidize 5-Hydroxymethylfurfural into 2,5-Furandicarboxylic Acid. ACS Sust. Chem. Eng. 2017, 5, 11300–11306. [Google Scholar] [CrossRef]
- Shen, J.; Chen, H.; Chen, K.; Qin, Y.; Lu, X.; Ouyang, P.; Fu, J. Atomic Layer Deposition of a Pt-Skin Catalyst for Base-Free Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid. Ind. Eng. Chem. Res. 2018, 57, 2811–2818. [Google Scholar] [CrossRef]
- Han, X.; Geng, L.; Guo, Y.; Jia, R.; Liu, X.; Zhang, Y.; Wang, Y. Base-free aerobic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over a Pt/C–O–Mg. Green Chem. 2016, 18, 1597–1604. [Google Scholar] [CrossRef]
- Zhou, C.; Deng, W.; Wan, X.; Zhang, Q.; Yang, Y.; Wang, Y. Functionalized Carbon Nanotubes for Biomass Conversion: The Base-Free Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over Platinum Supported on a Carbon Nanotube Catalyst. ChemCatChem 2015, 7, 2853–2863. [Google Scholar] [CrossRef]
- Gong, W.; Zheng, K.; Ji, P. Platinum deposited on cerium coordination polymer for catalytic oxidation of hydroxymethylfurfural producing 2,5-furandicarboxylic acid. RSC Adv. 2017, 7, 34776–34782. [Google Scholar] [CrossRef] [Green Version]
- Rass, H.A.; Essayem, N.; Besson, M. Selective Aerobic Oxidation of 5-HMF into 2,5-Furandicarboxylic Acid with Pt Catalysts Supported on TiO2- and ZrO2-Based Supports. ChemSusChem 2015, 8, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Pasini, T.; Piccinini, M.; Blosi, M.; Bonelli, R.; Albonetti, S.; Dimitratos, N.; Lopez-Sanchez, J.A.; Sankar, M.; He, Q.; Kiely, C.J.; et al. Selective oxidation of 5-hydroxymethyl-2-furfuralusing supported gold–copper nanoparticles. Green Chem. 2011, 13, 2091–2099. [Google Scholar] [CrossRef]
- Albonetti, S.; Pasini, T.; Lolli, A.; Blosi, M.; Piccinini, M.; Dimitratos, N.; Lopez-Sanchez, J.A.; Morgan, D.J.; Carley, A.F.; Hutchings, G.J.; et al. Selective oxidation of 5-hydroxymethyl-2-furfural over TiO2-supported gold-copper catalysts prepared from preformed nanoparticles: Effect of Au/Cu ratio. Catal. Today 2012, 195, 120–126. [Google Scholar] [CrossRef]
- Blosi, M.; Ortelli, S.; Lolli, A.; Andreoli, S.; Benito, P.; Albonetti, S. Bimetallic Nanoparticles as Efficient Catalysts: Facile and Green Microwave Synthesis. Materials 2016, 9, 550–575. [Google Scholar] [CrossRef] [PubMed]
- Lolli, A.; Albonetti, S.; Utili, L.; Amadori, R.; Ospitali, F.; Lucarelli, C.; Cavani, F. Insights into the reaction mechanism for 5-hydroxymethylfurfural oxidation to FDCA on bimetallic Pd–Au nanoparticles. Appl. Catal. A Gen. 2015, 504, 408–419. [Google Scholar] [CrossRef]
- Wan, X.; Zhou, C.; Chen, J.; Deng, W.; Zhang, Q.; Yang, Y.; Wang, Y. Base-Free Aerobic Oxidation of 5-Hydroxymethyl-furfural to 2,5-Furandicarboxylic Acid in Water Catalyzed by Functionalized Carbon Nanotube-Supported Au–Pd Alloy Nanoparticles. ACS Catal. 2014, 4, 2175–2185. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, W.; Li, S.; Xie, J.; Li, J.; Zhou, Y.; Wang, J. Hydrophilic mesoporous poly(ionic liquid)-supported Au–Pd alloy nanoparticles towards aerobic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid under mild conditions. Green Chem 2017, 19, 3820–3830. [Google Scholar] [CrossRef]
- Antonyraj, C.A.; Huynh, N.T.T.; Park, A.-K.; Shin, S.; Kim, Y.J.; Lee, K.Y.; Cho, L.K. Basic anion-exchange resin (AER)-supported Au-Pd alloy nanoparticles for the oxidation of 5-hydroxymethyl-2-furfural (HMF) into 2,5-furan dicarboxylic acid (FDCA). Appl. Catal. A Gen. 2017, 547, 230–236. [Google Scholar] [CrossRef]
- Gao, Z.; Xie, R.; Fan, G.; Yang, L.; Li, F. Highly Efficient and Stable Bimetallic AuPd over La-Doped Ca–Mg–Al Layered Double Hydroxide for Base-Free Aerobic Oxidation of 5-Hydroxymethylfurfural in Water. ACS Sustain. Chem. Eng. 2017, 5, 5852–5861. [Google Scholar] [CrossRef]
- Lolli, A.; Amadori, R.; Lucarelli, C.; Cutrufello, M.G.; Rombi, E.; Cavani, F.; Albonetti, S. Hard-template preparation of Au/CeO2 mesostructured catalysts and their activity for the selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. Microporous Mesoporous Mater. 2016, 226, 466–475. [Google Scholar] [CrossRef]
- Siyo, B.; Schneider, M.; Radnik, J.; Pohl, M.M.; Langer, P.; Steinfeldt, N. Influence of support on the aerobic oxidation of HMF into FDCA over preformed Pd nanoparticle based materials. Appl. Catal. A Gen. 2014, 478, 107–116. [Google Scholar] [CrossRef]
- Casanova, O.; Iborra, S.; Corma, A. Biomass into Chemicals: Aerobic Oxidation of 5-Hydroxymethyl-2-furfural into 2,5-Furandicarboxylic Acid with Gold Nanoparticle Catalysts. ChemSusChem 2009, 2, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Gorbanev, Y.Y.; Kegnaes, S.; Riisager, A. Effect of Support in Heterogeneous Ruthenium Catalysts Used for the Selective Aerobic Oxidation of HMF in Water. Top. Catal. 2011, 54, 1318–1324. [Google Scholar] [CrossRef]
- Yokota, T.; Takahata, Y.; Katsuyama, T.; Matsuda, Y. A new technique for preparing ceramics for catalyst support exhibiting high porosity and high heat resistance. Catal. Today 2001, 69, 11–15. [Google Scholar] [CrossRef]
- Gutierrez-Gonzalez, C.F.; Agoouram, S.; Torrecillas, R.; Moya, J.S. Lopez-Esteban, S. Ceramic/metal nanocomposites by lyophilization: Processing and HRTEM study. Mater. Res. Bull. 2012, 47, 285–289. [Google Scholar] [CrossRef] [Green Version]
- Abdolreza, S.; Mojtaba, G. Producion of Nanoparticle Assemblies by Electro-Spraying and Freeze-Drying of Colloids: A New Method to Resolve Handling Problem of Nanoparticles. Iran J. Chem. Chem. Eng. 2008, 27, 69–79. [Google Scholar]
- Eslamian, M.; Shekarriz, M. Recent advances in nanoparticle preparation by spray and micro-emulsion methods. Recent Pat. Nanotechnol. 2009, 3, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef] [PubMed]
- Ortelli, S.; Costa, A.L. Nanoencapsulation techniques as a “safer by (molecular) design” tool. Nano-Struct. Nano-Objects 2018, 13, 155–162. [Google Scholar] [CrossRef]
- IUPAC Gold Book. Available online: https://goldbook.iupac.org/ (accessed on 10 May 2018).
- Boutonnet, M.; Kizling, J.; Stenius, P.; Maire, G. The preparation of monodisperse colloidal metal particles from microemulsions. Colloids Surf. 1982, 5, 209–225. [Google Scholar] [CrossRef]
- Eriksson, S.; Nylén, U.; Rojas, S.; Boutonnet, M. Preparation of catalysts from microemulsions and their applications in heterogeneous catalysis. Appl. Catal. Gen. 2004, 265, 207–219. [Google Scholar] [CrossRef]
- Stubenrauch, C. Microemulsions: Background, New Concepts, Applications, Perspectives; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Hyde, E.D.E.R.; Seyfaee, A.; Neville, F.; Moreno-Atanasio, R. Colloidal Silica Particle Synthesis and Future Industrial Manufacturing Pathways: A Review. Ind. Eng. Chem. Res. 2016, 55, 8891–8913. [Google Scholar] [CrossRef]
- Hsueh, H.-Y.; Yao, C.-T.; Ho, R.-M. Well-ordered nanohybrids and nanoporous materials from gyroid block copolymer templates. Chem. Soc. Rev. 2015, 44, 1974–2018. [Google Scholar] [CrossRef] [PubMed]
- Buceta, D.; Pineiro, Y.; Vazquez-Vazquez, C.; Rivas, J.; Arturo Lopez-Quintela, M. Metallic Clusters: Theoretical Background, Properties and Synthesis in Microemulsions. Catalysts 2014, 4, 356–374. [Google Scholar] [CrossRef] [Green Version]
- Jones, B.H.; Lodge, T.P. Nanocasting nanoporous inorganic and organic materials from polymeric bicontinuous microemulsion templates. Polym. J. 2012, 44, 131–146. [Google Scholar] [CrossRef] [Green Version]
- Boutonnet, M.; Logdberg, S.; Svensson, E.E. Recent developments in the application of nanoparticles prepared from w/o microemulsions in heterogeneous catalysis. Curr. Opin. Colloid Interface Sci. 2008, 13, 270–286. [Google Scholar] [CrossRef]
- De Rogatis, L.; Cargnello, M.; Gombac, V.; Lorenzut, B.; Montini, T.; Fornasiero, P. Embedded Phases: A Way to Active and Stable Catalysts. Chemsuschem 2010, 3, 24–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basile, F.; Mafessanti, R.; Fasolini, A.; Fornasari, G.; Lombardi, E.; Vaccari, A. Effect of synthetic method on CeZr support and catalytic activity of related Rh catalyst in the oxidative reforming reaction. J. Eur. Ceram. Soc. 2018. [Google Scholar] [CrossRef]
- Yurdakal, S.; Sina Tek, B.; Alagöz, O.; Augugliaro, V.; Loddo, V.; Palmisano, G.; Palmisano, L. Hotocatalytic Selective Oxidation of 5-(Hydroxymethyl)-2-furaldehyde to 2,5-Furandicarbaldehyde in Water by Using Anatase, Rutile, and Brookite TiO2 Nanoparticles. ACS Sustain. Chem. Eng. 2013, 1, 456–461. [Google Scholar] [CrossRef] [Green Version]
- Krivtsov, I.; Ilkaeva, M.; Salas-Colera, E.; Amghouz, Z.; García, J.R.; Díaz, E.; Ordóñez, S.; Villar-Rodil, S. Consequences of Nitrogen Doping and Oxygen Enrichment on Titanium Local Order and Photocatalytic Performance of TiO2 Anatase. J. Phys. Chem. C 2017, 121, 6770–6780. [Google Scholar] [CrossRef]
- Augugliaro, V.; Bellardita, M.; Loddo, V.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Overview on oxidation mechanisms of organic compounds by TiO2 in heterogeneous photocatalysis. J. Photochem. Photobiol. C Photochem. 2012, 13, 224–245. [Google Scholar] [CrossRef] [Green Version]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous Photocatalytic Degradation of Organic Contaminants over Titanium Dioxide: A Review of Fundamentals, Progress and Problems. J. Photochem. Photobiol. C Photochem. Reviews. 2008, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Carbajo, J.; Tolosana-Moranchel, A.; Casas, J.A.; Faraldos, M.; Bahamonde, A. Analysis of photoefficiency in TiO2 aqueous suspensions: Effect of titania hydrodynamic particle size and catalyst loading on their optical properties. Appl. Catal. B. Environ. 2018, 221, 1–8. [Google Scholar] [CrossRef]
- Zhou, B.; Song, J.; Zhang, Z.; Jiang, Z.; Zhang, P.; Han, B. Highly selective photocatalytic oxidation of biomass-derived chemicals to carboxyl compounds over Au/TiO2. Green Chem. 2017, 19, 1075–1081. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Q.; Guo, C.; Wu, Y.; Wu, T. Photocatalytic Selective Oxidation of 5-Hydroxymethylfurfural to 2,5-Diformylfuran over Nb2O5 under Visible Light. ACS Sustain. Chem. Eng. 2017, 5, 3517–3523. [Google Scholar] [CrossRef]
- Krivtsov, I.; García-López, E.I.; Marcì, G.; Palmisano, L.; Amghouz, Z.; García, J.R.; Ordóñez, S.; Díaz, E. Elective Photocatalytic Oxidation of 5-Hydroxymethyl-2-Furfural to 2,5-Furandicarboxyaldehyde in Aqueous Suspension of g-C3N4. Appl. Catal. B Environ. 2017, 204, 430–439. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, P.; Zhang, Z.; Yang, C.; Zhang, B.; Deng, K.; Bottle, S.; Zhu, H. Selective Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid Using O2 and a Photocatalyst of Co-thioporphyrazine Bonded to g-C3N4. J. Am. Chem. Soc. 2017, 139, 14775–14782. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; He, Y.; Zhang, H.; Feng, Z.; Wu, Y.; Wu, T. Photocatalytic selective oxidation of biomass-derived 5-hydroxymethylfurfural to 2,5-diformylfuran on metal-free g-C3N4 under visible light irradiation. Mol. Catal. 2017, 436, 10–18. [Google Scholar] [CrossRef]
- Ilkaeva, M.; Krivtsov, I.; García-lópez, E.I.; Marcì, G.; Khainakova, O.; García, J.R.; Palmisano, L. Selective photocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxaldehyde by polymeric carbon nitride-hydrogen peroxide adduct. J. Catal. 2018, 359, 212–222. [Google Scholar] [CrossRef]
- Andersson, M.; Kiselev, A.; Österlund, L.; Palmqvist, A.E.C. Microemulsion-Mediated Room-Temperature Synthesis of High-Surface-Area Rutile and Its Photocatalytic Performance. J. Phys. Chem. C 2007, 111, 6789–6797. [Google Scholar] [CrossRef]
- Lan, J.; Lin, J.; Chen, Z.; Yin, G. Transformation of 5-Hydroxymethylfurfural (HMF) to Maleic Anhydride by Aerobic Oxidation with Heteropolyacid Catalysts. ACS Catal. 2015, 5, 2035–2041. [Google Scholar] [CrossRef]
- Li, S.; Su, K.; Li, Z.; Cheng, B. Selective oxidation of 5-hydroxymethylfurfural with H2O2 catalyzed by a molybdenum complex. Green Chem. 2016, 18, 2122–2128. [Google Scholar] [CrossRef]
- Chen, C.-T.; Nguyen, C.V.; Wang, Z.-Y.; Bando, Y.; Yamauchi, Y.; Saleh Bazziz, M.T.; Fatehmella, A.; Aslam Farooq, W.; Yoshikawa, T.; Masuda, T.; et al. Hydrogen Peroxide Assisted Selective Oxidation of 5-Hydroxymethylfurfural in Water under Mild Conditions. ChemCatChem 2018, 10, 361–365. [Google Scholar] [CrossRef]
- Yurdakal, S.; Tek, B.S.; Degirmenci, C.; Palmisano, G. Selective photocatalytic oxidation of aromatic alcohols in solar-irradiated aqueous suspensions of Pt, Au, Pd and Ag loaded TiO2 catalysts. Catal. Today 2017, 281, 53–59. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Li, W.; Yang, Q.; Hou, Q.; Wei, L.; Liu, L.; Huang, F.; Ju, M. Enhancement of photocatalytic performance with the use of noble-metal-decorated TiO2 nanocrystals as highly active catalysts for aerobic oxidation under visible-light irradiation. Appl. Catal. B Environ. 2017, 210, 352–367. [Google Scholar] [CrossRef]
- Wang, J.; Ando, R.A.; Camargo, P.H.C. Controlling the Selectivity of the Surface Plasmon Resonance Mediated Oxidation of p-Aminothiophenol on Au Nanoparticles by Charge Transfer from UV-excited TiO2. Angew. Chem. Int. Ed. 2015, 54, 6909–6912. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the TiO2 and Au/TiO2 catalysts are available from the authors. |



















| Sample Name | TiO2:SiO2 (w/w%) | Pt (wt %) | Surface Area (m2/g) |
|---|---|---|---|
| SiO2 | - | - | 210 |
| TiO2 (P25) | - | - | 46 |
| Ti | - | - | 59 |
| TiSi | 1:0.5 | - | 100 |
| TiPt | - | 0.5 | 41 |
| TiSiPt | 1:0.5 | 0.5 | 91 |
| Sample | Crystallite Size/nm | λ/nm | SBET/m2/g | |
|---|---|---|---|---|
| TiO2 Crystallites | Au Crystallites | |||
| TiO2-c | 17 | - | 371 | 82 |
| TiO2-m | 8.3 | - | 413 | 132 |
| Au/TiO2-c | 17 | <3 | 380 | 89 |
| Au/TiO2-m | 8.3 | - * | 429 | 130 |
| Catalyst | O2 Content (vol %) | HMF Conv. (%) | DFF Yield (%) | FFCA Yield (%) | CO2 Yield (%) | Others Yield (%) |
|---|---|---|---|---|---|---|
| TiO2-m | 11 | 10 | 2 | - | 3 | 5 |
| 21 | 12 | 2 | - | 2 | 8 | |
| 100 | 22 | 5 | - | 8 | 9 | |
| Au-TiO2-m | 11 | 12 | 2 | - | 2 | 8 |
| 21 | 17 | 3 | 1 | 5 | 8 | |
| 100 | 15 | 3 | 1 | 8 | 3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lolli, A.; Maslova, V.; Bonincontro, D.; Basile, F.; Ortelli, S.; Albonetti, S. Selective Oxidation of HMF via Catalytic and Photocatalytic Processes Using Metal-Supported Catalysts. Molecules 2018, 23, 2792. https://doi.org/10.3390/molecules23112792
Lolli A, Maslova V, Bonincontro D, Basile F, Ortelli S, Albonetti S. Selective Oxidation of HMF via Catalytic and Photocatalytic Processes Using Metal-Supported Catalysts. Molecules. 2018; 23(11):2792. https://doi.org/10.3390/molecules23112792
Chicago/Turabian StyleLolli, Alice, Valeriia Maslova, Danilo Bonincontro, Francesco Basile, Simona Ortelli, and Stefania Albonetti. 2018. "Selective Oxidation of HMF via Catalytic and Photocatalytic Processes Using Metal-Supported Catalysts" Molecules 23, no. 11: 2792. https://doi.org/10.3390/molecules23112792