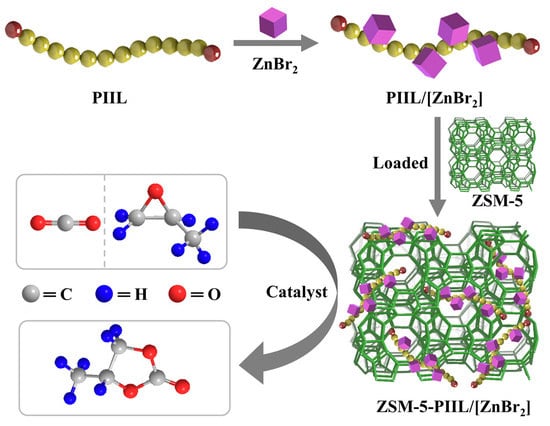
Immobilizing Polyether Imidazole Ionic Liquids on ZSM-5 Zeolite for the Catalytic Synthesis of Propylene Carbonate from Carbon Dioxide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.2. Scanning Electron Microscopy (SEM)
2.3. X-Ray Diffractometry (XRD)
2.4. Thermogravimetry (TG) and Derivative Thermogravimetry (DTG)
2.5. Effects of Different Catalysts on the Catalytic Performance
2.6. Effects of Reaction Conditions on the Catalytic Performance
2.7. Effect of the Catalyst Circulation on the Catalytic Performance
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Preparation of Immobilized Catalysts
3.3. Typical Procedure for the Synthesis of PC from PO and CO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rulev, Y.A.; Gugkaeva, Z.; Maleev, V.I.; North, M.; Belokon, Y.N. Robust bifunctional aluminium-salen catalysts for the preparation of cyclic carbonates from carbon dioxide and epoxides. Beilstein J. Org. Chem. 2015, 11, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Kember, M.R.; Buchard, A.; Williams, C.K. Catalysts for CO2/epoxide copolymerisation. Chem. Commun. 2011, 47, 141–163. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Wang, H.M. Recent advances in carbon dioxide capture, fixation, and activation by using N-heterocyclic carbenes. ChemSusChem 2014, 7, 962–998. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.M.; Liu, Y.; Guo, J.P.; Li, P.Z.; Huo, Z.Y.; Ma, P.T.; Niu, J.Y.; Wang, J.P. A multi-component polyoxometalate and its catalytic performance for CO2 cycloaddition reactions. Dalton Trans. 2015, 44, 10152–10155. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Na, W.; Wang, H.; Gao, W.G. Direct syntheses of Cu-Zn-Zr/SBA-15 mesoporous catalysts for CO2 hydrogenation to methanol. Chem. J. Chin. Univ. 2014, 35, 2616–2623. [Google Scholar]
- Agatemor, C.; Ibsen, K.N.; Tanner, E.E.L.; Mitragotri, S. Ionic liquids for addressing unmet needs in healthcare. Bioeng. Transl. Med. 2018, 3, 7–25. [Google Scholar] [PubMed]
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2009, 72, 1391–1398. [Google Scholar] [CrossRef]
- Sheldon, R. Catalytic reactions in ionic liquids. Chem. Commun. 2001, 23, 2399–2407. [Google Scholar] [CrossRef]
- Seddon, K.R. Ionic liquids for clean technology. J. Chem. Technol. Biotechnol. 1997, 68, 351–356. [Google Scholar] [CrossRef]
- Shi, F.; Gu, Y.L.; Zhang, Q.H.; Deng, Y.Q. Development of ionic liquids as green reaction media and catalysts. Catal. Surv. Asia 2004, 8, 179–186. [Google Scholar] [CrossRef]
- Peng, J.J.; Deng, Y.Q. Formation of propylene carbonate catalyzed by room temperature ionic liquids. Chin. J. Catal. 2001, 22, 598–600. [Google Scholar]
- Li, F.W.; Xiao, L.F.; Xia, C.G. Synthesis of cyclic carbonates catalyzed by ionic liquid mediated ZnBr2 catalytic system. Chem. J. Chin. Univ. 2005, 26, 343–345. [Google Scholar]
- Guo, L.Y.; Deng, L.L.; Jin, X.C.; Wu, H.; Yin, L.Z. Composite ionic liquids immobilized on MCM-22 as efficient catalysts for the cycloaddition reaction with CO2 and propylene oxide. Catal. Lett. 2017, 147, 2290–2297. [Google Scholar] [CrossRef]
- Wang, Y.H.; Li, W.; Luo, S.; Liu, S.X.; Ma, C.H.; Li, J. Research advances on the applications of immobilized ionic liquids functional materials. Acta Chim. Sin. 2018, 76, 85–94. [Google Scholar] [CrossRef]
- Dai, W.L.; Chen, L.; Yin, S.F.; Luo, S.L.; Au, C.T. 3-(2-Hydroxyl-Ethyl)-1-propylimidazolium bromide immobilized on SBA-15 as efficient catalyst for the synthesis of cyclic carbonates via the coupling of carbon dioxide with epoxides. Catal. Lett. 2010, 135, 295–304. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.Q.; Cheng, W.G.; Zhang, J.X.; Li, X.H.; Zhang, S.J.; She, Y.B. Chitosan functionalized ionic liquid as a recyclable biopolymer-supported catalyst for cycloaddition of CO2. Green Chem. 2012, 14, 654–660. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Tan, Z.T.; Liu, B.L.; Mao, D.S.; Xiong, C.R. Coconut shell activated carbon tethered ionic liquids for continuous cycloaddition of CO2 to epichlorohydrin in packed bed reactor. Catal. Commun. 2015, 68, 73–76. [Google Scholar] [CrossRef]
- Zhuo, C.W.; Qin, Y.S.; Wang, X.H.; Wang, F.S. Temperature-responsive catalyst for the coupling reaction of carbon dioxide and propylene oxide. Chin. J. Chem. 2018, 36, 299–305. [Google Scholar] [CrossRef]
- Della Monica, F.; Maity, B.; Pehl, T.; Buonerba, A.; De Nis, A.; Monari, M.; Grassi, A.; Rieger, B.; Cavallo, L.; Capacchione, C. [OSSO]-type iron(III) complexes for the low-pressure reaction of carbon dioxide with epoxides: Catalytic activity, reaction kinetics, and computational study. ACS Catal. 2018, 8, 6882–6893. [Google Scholar] [CrossRef]
- Bobbink, F.D.; Vasilyev, D.; Hulla, M.; Chamam, S.; Menoud, F.; Laurenczy, G.; Katsyuba, S.; Dyson, P.J. Intricacies of cation–anion combinations in imidazolium salt-catalyzed cycloaddition of CO2 into epoxides. ACS Catal. 2018, 8, 2589–2594. [Google Scholar] [CrossRef]
- Wang, G.J.; Wang, D.H.; Qiu, J.; Zhao, L.Q. Functional Polymer Materials, 1st ed.; East China University of Science and Technology Press: Shanghai, China, 2006; pp. 283–285. [Google Scholar]
- Sang, Y.; Liu, H.X.; He, S.C.; Li, H.S.; Jiao, Q.Z.; Wu, Q.; Sun, K.N. Catalytic performance of hierarchical H-ZSM-5/MCM-41 for methanol dehydration to dimethyl ether. J. Energy Chem. 2013, 22, 769–777. [Google Scholar] [CrossRef]
- Valkenberg, M.H.; deCastro, C.; Hölderich, W.F. Immobilisation of ionic liquids on solid supports. Green Chem. 2002, 4, 88–93. [Google Scholar] [CrossRef]
- Cheng, W.G.; Chen, X.; Sun, J.; Wang, J.Q.; Zhang, S.J. SBA-15 supported triazolium-based ionic liquids as highly efficient and recyclable catalysts for fixation of CO2 with epoxides. Catal. Today 2013, 200, 117–124. [Google Scholar] [CrossRef]
- Mao, D.S.; Guo, Q.S.; Meng, T. Effect of magnesium oxide modification on the catalytic performance of nanoscale HZSM-5 zeolite for the conversion of methanol to propylene. Acta Phys-Chim. Sin. 2010, 26, 2242–2248. [Google Scholar]
- Guo, L.Y.; Ma, X.Y.; Li, C.Y.; Deng, L.L.; Bai, S.Y. Preparation and catalytic properties of chloride 1-carboxyl polyether-3-mtthyl imidazole ionic liquid. Acta Pet. Sin. (Pet. Process Sect.) 2017, 33, 342–348. [Google Scholar]
- Guo, L.Y.; Ma, X.Y.; Wang, L.Y.; Deng, L.L.; Jin, X.C. Preparation and catalytic properties of chloride 1-amino polyether-3-methyl imidazole ionic liquid. Chem. Ind. Eng. Prog. 2017, 36, 581–587. [Google Scholar]
- Guo, L.Y.; Zhang, B.; Li, C.Y.; Ma, X.Y.; Chang, X.T.; Gao, X. Synthesis of polyether ionic liquids and its influence on properties of phenolic resin. Plastics. 2015, 44, 57–59. [Google Scholar]
- Yuan, S.B.; She, L.Q.; Liu, X.Y.; Li, X.W.; Pang, L.; Huang, H.Z.; Zhou, Y. Characterization and aromazization activity of gallium modified HZSM-5 catalysts. Chin. J. Catal. 1988, 1, 25–31. [Google Scholar]
- Leng, Y.; Lu, D.; Jiang, P.P.; Zhang, C.J.; Zhao, J.W.; Zhang, W.J. Highly cross-linked cationic polymer microspheres as an efficient catalyst for facile CO2 fixation. Catal. Commun. 2016, 74, 99–103. [Google Scholar] [CrossRef]
- Saptal, V.B.; Bhanage, B.M. Bifunctional ionic liquids for the multitask fixation of carbon dioxide into valuable chemicals. ChemCatChem 2016, 8, 244–250. [Google Scholar] [CrossRef]
- Han, L.; Li, H.Q.; Choi, S.J.; Park, M.S.; Lee, S.M.; Kim, Y.J.; Park, D.W. Ionic liquids grafted on carbon nanotubes as highly efficient heterogeneous catalysts for the synthesis of cyclic carbonates. Appl. Catal. A 2012, 429, 67–72. [Google Scholar] [CrossRef]
- Fujita, S.; Nishiura, M.; Arai, M. Synthesis of styrene carbonate from carbon dioxide and styrene oxide with various zinc halide-based ionic liquids. Catal. Lett. 2010, 135, 263–268. [Google Scholar] [CrossRef]
- Kim, M.I.; Choi, S.J.; Kim, D.W.; Park, D.W. Catalytic performance of zinc containing ionic liquids immobilized on silica for the synthesis of cyclic carbonates. J. Ind. Eng. Chem. 2014, 20, 3102–3107. [Google Scholar] [CrossRef]
- Takahashi, T.; Watahiki, T.; Kitazume, S.; Yasuda, H.; Sakakura, T. Synergistic hybrid catalyst for cyclic carbonate synthesis: Remarkable acceleration caused by immobilization of homogeneous catalyst on silica. Chem. Commun. 2006, 37, 1664–1666. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cheng, W.G.; Fan, W.; Wang, Y.H.; Meng, Z.Y.; Zhang, S.J. Reusable and efficient polymer-supported task-specific ionic liquid catalyst for cycloaddition of epoxide with CO2. Catal. Today 2009, 148, 361–367. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Adam, F. A facile and efficient synthesis of styrene carbonate via cycloaddition of CO2 to styrene oxide over ordered mesoporous MCM-41-Imi/Br catalyst. Appl. Catal. B 2013, 136, 150–159. [Google Scholar] [CrossRef]
Sample Availability: Samples of the PIILs and the immobilized catalysts are available from the authors. |






| Catalyst | Conversion Rate (%) | Selectivity (%) | Yield (%) | Purity (%) | |
|---|---|---|---|---|---|
| 1 | HO-[PECH-MIM]Cl | 90.4 | 96.4 | 87.1 | 99.1 |
| 2 | HOOC-[PECH-MIM]Cl | 95.1 | 96.8 | 92.1 | 99.4 |
| 3 | H2N-[PECH-MIM]Cl | 93.3 | 95.3 | 88.9 | 98.9 |
| 4 | ZnBr2 | 40.8 | 68.2 | 27.8 | 95.2 |
| 5 | HO-[PECH-MIM]Cl/[ZnBr2] | 100 | 97.1 | 97.1 | 99.5 |
| 6 | HOOC-[PECH-MIM]Cl/[ZnBr2] | 100 | 98.9 | 98.9 | 99.7 |
| 7 | H2N-[PECH-MIM]Cl/[ZnBr2] | 100 | 97.6 | 97.6 | 99.4 |
| 8 | ZSM-5 | 9.4 | 41.2 | 3.87 | 96.4 |
| 9 | ZSM-5-HO-[PECH-MIM]Cl/[ZnBr2] | 95.8 | 96.5 | 92.4 | 98.3 |
| 10 | ZSM-5-HOOC-[PECH-MIM]Cl/[ZnBr2] | 96.3 | 96.8 | 93.2 | 99.2 |
| 11 | ZSM-5-H2N-[PECH-MIM]Cl/[ZnBr2] | 96.1 | 96.4 | 92.6 | 99.0 |
| Factor | Conversion Rate (%) | Selectivity (%) | |||
|---|---|---|---|---|---|
| Pressure (MPa) | Temperature (°C) | Time (h) | |||
| 1 | 2.0 | 110 | 0.75 | 82.3 | 92.4 |
| 2 | 2.0 | 120 | 1.0 | 90.6 | 90.1 |
| 3 | 2.0 | 130 | 1.25 | 95.4 | 85.2 |
| 4 | 2.5 | 110 | 1.0 | 95.9 | 98.2 |
| 5 | 2.5 | 120 | 1.25 | 97.6 | 97.5 |
| 6 | 2.5 | 130 | 0.75 | 98.3 | 97.4 |
| 7 | 3.0 | 110 | 1.25 | 92.1 | 94.6 |
| 8 | 3.0 | 120 | 0.75 | 97.9 | 90.3 |
| 9 | 3.0 | 130 | 1.0 | 99.5 | 84.2 |
| K1j | 268.3/267.7 | 270.3/285.2 | 278.5/280.1 | — | — |
| K2j | 291.8/293.1 | 286.1/277.9 | 286.0/272.5 | — | — |
| K3j | 289.5/269.1 | 293.2/266.8 | 285.1/277.3 | — | — |
| R | 23.5/25.4 | 22.9/18.4 | 7.5/7.6 | — | — |
| Catalyst | N (wt%) | C (wt%) | H (wt%) | PIILgrafted (mmol/g) | Standard Error (mmol/g) |
|---|---|---|---|---|---|
| ZSM-5-HOOC-[PECH-MIM]Cl/[ZnBr2] 1 | 3.149 | 7.744 | 2.000 | 1.121 | 1.780 × 10−3 |
| ZSM-5-HOOC-[PECH-MIM]Cl/[ZnBr2] 2 | 2.275 | 7.131 | 1.694 | 0.810 | 1.472 × 10−3 |
| ZSM-5-HOOC-[PECH-MIM]Cl/[ZnBr2] 3 | 2.256 | 7.059 | 1.681 | 0.803 | 1.633 × 10−3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Jin, X.; Wang, X.; Yin, L.; Wang, Y.; Yang, Y.-W. Immobilizing Polyether Imidazole Ionic Liquids on ZSM-5 Zeolite for the Catalytic Synthesis of Propylene Carbonate from Carbon Dioxide. Molecules 2018, 23, 2710. https://doi.org/10.3390/molecules23102710
Guo L, Jin X, Wang X, Yin L, Wang Y, Yang Y-W. Immobilizing Polyether Imidazole Ionic Liquids on ZSM-5 Zeolite for the Catalytic Synthesis of Propylene Carbonate from Carbon Dioxide. Molecules. 2018; 23(10):2710. https://doi.org/10.3390/molecules23102710
Chicago/Turabian StyleGuo, Liying, Xianchao Jin, Xin Wang, Longzhu Yin, Yirong Wang, and Ying-Wei Yang. 2018. "Immobilizing Polyether Imidazole Ionic Liquids on ZSM-5 Zeolite for the Catalytic Synthesis of Propylene Carbonate from Carbon Dioxide" Molecules 23, no. 10: 2710. https://doi.org/10.3390/molecules23102710
APA StyleGuo, L., Jin, X., Wang, X., Yin, L., Wang, Y., & Yang, Y.-W. (2018). Immobilizing Polyether Imidazole Ionic Liquids on ZSM-5 Zeolite for the Catalytic Synthesis of Propylene Carbonate from Carbon Dioxide. Molecules, 23(10), 2710. https://doi.org/10.3390/molecules23102710