Chromobacterium violaceum and Pseudomonas aeruginosa PAO1: Models for Evaluating Anti-Quorum Sensing Activity of Melaleuca alternifolia Essential Oil and Its Main Component Terpinen-4-ol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Essential Oil Composition
2.2. PCR Detection of the Sa442 and MecA Genes
2.3. Growth Inhibition Zones (mm) and MICs/MBCs Values (mg/mL) Determination
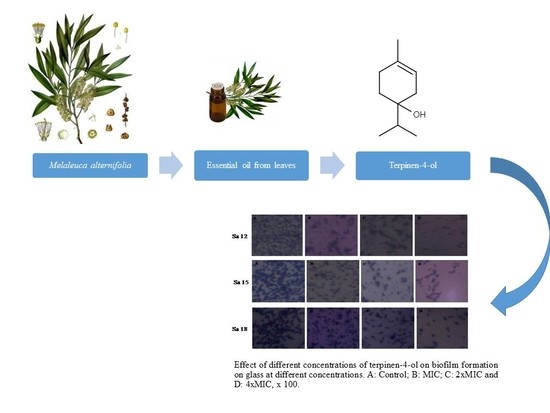
2.4. Biofilm Formation on Abiotic Materials
2.5. Anti-Adhesive Activity of M. alternifolia and Terpinen-4-ol on Polystyrene and Glass Surfaces
2.6. Anti-Biofilm Activity of M. alternifolia Essential Oil and Terpinen-4-ol on Polystyrene and Glass
2.7. Violacein Inhibition Assay in C. violaceum
2.8. Swarming Inhibition Assay
3. Materials and Methods
3.1. Microorganisms
3.2. PCR Detection of the Sa 442 Gene
3.2.1. Extraction of Bacterial DNA
3.2.2. PCR Detection of the Sa442 Gene
3.2.3. PCR Detection of the MecA Gene
3.3. Chemical Characterization of the Essential Oil
3.4. Antimicrobial Activities
3.4.1. Disk Diffusion Assay
3.4.2. Microdilution Method for the Determination of the MIC and MBC
3.5. Biofilm Production Assay by Staphylococcus Strains on Polystyrene, Glass and Stainless
3.6. Determination of Anti-Biofilm Anti-Adhesive Activities on Polystyrene and Glass
3.7. Violacein Inhibition Assay
3.8. Swarming Assay
4. Statistical Analysis
5. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Centre for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2013. Available online: https://www.cdc.gov/drugresistance/threat-report-2013/index.html (accessed on 16 October 2018).
- Hussain, A.I.; Anwar, F.; Nigam, P.S.; Sarker, S.D.; Moore, J.E.; Rao, J.R.; Mazumdar, A. Antibacterial activity of some Lamiaceae essential oils using resazurin as an indicator of cell growth. LWT–Food Sci. Technol. 2011, 44, 1199–1206. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [PubMed]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. The influence of natural products upon drug discovery. Nat. Prod. Rep. 2000, 17, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential Oils, A New Horizon in Combating Bacterial Antibiotic Resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Brady, A.; Loughlin, R.; Gilpin, D.; Kearney, P.; Tunney, M. In vitro activity of tea-tree oil against clinical skin isolates of meticillin-resistant and -sensitive Staphylococcus aureus and coagulase-negative staphylococci growing planktonically and as biofilms. J. Med. Microbiol. 2006, 55, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Ananda, B.S.; Kazmer, G.W.; Hinckley, L.; Andrew, S.M.; Venkitanarayanan, K. Antibacterial effect of plant derived antimicrobials on major bacterial mastitis pathogens in vitro. J. Dairy Sci. 2009, 92, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Jang, C.-H.; Cho, Y.B.; Choi, C.-H. Antibacterial Effect of Tea-tree Oil on Methicillin-resistant Staphylococcus aureus. Biofilm Formation of the Tympanostomy Tube: An In Vitro Study. In Vivo 2007, 21, 1027–1030. [Google Scholar] [PubMed]
- Kania, R.E.; Lamers, G.E.M.; Vonk, M.J.; Dorpmans, E.; Struik, J.; Tran Huy, P.; Hiemstra, P.; Bloemberg, G.V.; Grote, J.J. Characterization of Mucosal Biofilms on Human Adenoid Tissues. Laryngoscope 2008, 118, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Moser, C.; Wang, H.-Z.; Hoiby, N.; Song, Z.-J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.M.; Yin, W.F.; Ho, C.Y.; Mustafa, M.R.; Hadi, A.H.A.; Awang, K. Malabaricone C from Mysristacinnamomea exhibits anti-quorum sensing activity. J. Nat. Prod. 2011, 74, 2261–2264. [Google Scholar] [CrossRef] [PubMed]
- Kievit, T.R.D.; Gillis, R.; Marx, S.; Brown, C.; Iglewski, B.H. Quorum-Sensing Genes in Pseudomonas aeruginosa Biofilms: Their Role and Expression Patterns. Appl. Environ. Microbiol. 2001, 67, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Jasmine Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 2015, 6, 26–41. [Google Scholar]
- Schonewille, E.; Nesse, L.L.; Hauck, R.; Windhorst, D.; Hafez, H.M.; Vestby, L.K. Biofilm building capacity of Salmonella enterica strains from the poultry farm environment. FEMS Immunol. Med. Microbiol. 2012, 65, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Homer, L.E.; Leach, D.N.; Lea, D.; Slade Lee, L.; Henry, R.J.; Baverstock, P.R. Natural variation in the essential oil content of Melaleuca alternifolia Cheel (Myrtaceae). Biochem. Syst. Ecol. 2000, 28, 367–382. [Google Scholar] [CrossRef]
- Brophy, J.; Davies, N.; Southwell, I.; Stiff, I.; Williams, L. Gas-Chromatographic Quality-Control for Oil of Melaleuca Terpinen-4-Ol Type (australian Tea Tree). J. Agric. Food Chem. 1989, 37, 1330–1335. [Google Scholar] [CrossRef]
- Mori, M.; Ikeda, N.; Kato, Y.; Minamino, M.; Watabe, K. Quality evaluation of essential oils. YakugakuZasshi 2002, 122, 253–261. [Google Scholar] [CrossRef]
- Shabir, G.A. Method development and validation for the GC–FID assay of p-cymene in tea tree oil formulation. J. Pharm. Biomed. Anal. 2005, 39, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.; Southwell, I. Monoterpenoid accumulation in Melaleuca alternifolia seedlings. Phytochemistry 2002, 59, 709–716. [Google Scholar] [CrossRef]
- Martineau, F.; Picard, F.J.; Roy, P.H.; Ouellette, M.; Bergeron, M.G. Species-Specific and Ubiquitous-DNA-Based Assays for Rapid Identification of Staphylococcus aureus. J. Clin. Microbiol. 1998, 36, 618–623. [Google Scholar] [PubMed]
- Ryffel, C.; Tesch, W.; Birch-Machin, I.; Reynolds, P.E.; Barberis-Maino, L.; Kayser, F.H.; Berger-Bächi, B. Sequence comparison of mecA genes isolated from methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. Gene 1990, 94, 137–138. [Google Scholar] [CrossRef]
- Ubukata, K.; Nonoguchi, R.; Song, M.D.; Matsuhashi, M.; Konno, M. Homology of mecA gene in methicillin-resistant Staphylococcus haemolyticus and Staphylococcus simulans to that of Staphylococcus aureus. Antimicrob. Agents Chemother. 1990, 34, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Susceptibility of transient and commensal skin flora to the essential oil of Melaleuca alternifolia (tea tree oil). Am. J. Infect. Control 1996, 24, 186–189. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. In-vitro activity of essential oils, in particular Melaleucalternifolia (tea tree) oil and tea tree oil products, against Candida spp. J. Antimicrob. Chemother. 1998, 42, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.D. Antiviral Activity of the Essential Oil of Melaleuca alternifolia (Maiden & Betche) Cheel (Tea Tree) Against Tobacco Mosaic Virus. J. Essent. Oil Res. 1995, 7, 641–644. [Google Scholar]
- Jandourek, A.; Vaishampayan, J.K.; Vazquez, J.A. Efficacy of melaleuca oral solution for the treatment of fluconazole refractory oral candidiasis in AIDS patients. AIDS 1998, 12, 1033–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caelli, M.; Porteous, J.; Carson, C.F.; Heller, R.; Riley, T.V. Tea tree oil as an alternative topical decolonization agent for methicillin-resistant Staphylococcus aureus. J. Hosp. Infect. 2000, 46, 236–237. [Google Scholar] [CrossRef]
- Carson, C.F.; Mee, B.J.; Riley, T.V. Mechanism of Action of Melaleuca alternifolia (Tea Tree) Oil on Staphylococcus aureus Determined by Time-Kill, Lysis, Leakage, and Salt Tolerance Assays and Electron Microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Vergara, A.; Normanno, G.; Di Ciccio, P.; Pedonese, F.; Nuvoloni, R.; Parisi, A.; Santagada, G.; Colagiorgi, A.; Zanardi, E.; Ghidini, S.; et al. Biofilm Formation and Its Relationship with the Molecular Characteristics of Food-Related Methicillin-Resistant Staphylococcus aureus (MRSA). J. Food Sci. 2017, 82, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.J.; Turner, R.J.; Marques, L.L.R.; Ceri, H. Biofilms: A new understanding of these microbial communities is driving a revolution that may transform the science of microbiology. Am. Sci. 2005, 93, 508–515. [Google Scholar] [CrossRef]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yao, X.; Zhu, Z.; Tang, T.; Dai, K.; Sadovskaya, I.; Flahaut, S.; Jabbouri, S. Effect of berberine on Staphylococcus epidermidis biofilm formation. Int. J. Antimicrob. Agents 2009, 34, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Kwieciński, J.; Eick, S.; Wójcik, K. Effects of tea tree (Melaleuca alternifolia) oil on Staphylococcus aureus in biofilms and stationary growth phase. Int. J. Antimicrob. Agents 2009, 33, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Fazli, M.; Almblad, H.; Rybtke, M.L.; Givskov, M.; Eberl, L.; Tolker-Nielsen, T. Regulation of biofilm formation in Pseudomonas and Burkholderia species. Environ. Microbiol. 2014, 16, 1961–1981. [Google Scholar] [CrossRef] [PubMed]
- Bai, A.J.; Vittal, R.R. Quorum Sensing Inhibitory and Anti-Biofilm Activity of Essential Oils and Their in vivo Efficacy in Food Systems. Food Biotechnol. 2014, 28, 269–292. [Google Scholar] [CrossRef]
- Luís, Â.; Duarte, A.; Gominho, J.; Domingues, F.; Duarte, A.P. Chemical composition, antioxidant, antibacterial and anti-quorum sensing activities of Eucalyptus globulus and Eucalyptus radiata essential oils. Ind. Crops Prod. 2016, 79, 274–282. [Google Scholar] [CrossRef]
- Persson, T.; Hansen, T.H.; Rasmussen, T.B.; Skindersø, M.E.; Givskov, M.; Nielsen, J. Rational design and synthesis of new quorum-sensing inhibitors derived from acylated homoserine lactones and natural products from garlic. Org. Biomol. Chem. 2005, 3, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.A.; Zahin, M.; Hasan, S.; Husain, F.M.; Ahmad, I. Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett. Appl. Microbiol. 2009, 49, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.V.; Moreira, M.R.; Ponce, A. Antiquorum Sensing and Antimicrobial Activity of Natural Agents with Potential Use in Food. J. Food Saf. 2012, 32, 379–387. [Google Scholar] [CrossRef]
- Murdoch, F.; Sammons, R.; Chapple, I. Isolation and characterization of subgingival staphylococci from periodontitis patients and controls. Oral Dis. 2004, 10, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Geha, D.J.; Uhl, J.R.; Gustaferro, C.A.; Persing, D.H. Multiplex PCR for identification of methicillin-resistant staphylococci in the clinical laboratory. J. Clin. Microbiol. 1994, 32, 1768–1772. [Google Scholar] [PubMed]
- Goodner, K.L. Practical retention index models of OV-101, DB-1, DB-5, and DB-Wax for flavor and fragrance compounds. LWT–Food Sci. Technol. 2008, 41, 951–958. [Google Scholar] [CrossRef]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavour and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicone and Carbowax 20M phases. J. Chromatogr. 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Wiley Registry of Mass Spectral Data, with NIST Spectral Data CD Rom, 7th ed.; John Wiley & Sons: New York, NY, USA, 1998.
- Snoussi, M.; Hajlaoui, H.; Noumi, E.; Usai, D.; Sechi, L.A.; Zanetti, S.; Bakhrouf, A. In-vitro anti-Vibrio spp. activity and chemical composition of some Tunisian aromatic plants. World. J. Microbiol. Biotechnol. 2008, 24, 3071–3076. [Google Scholar] [CrossRef]
- Mack, D.; Bartscht, K.; Fischer, C.; Rohde, H.; de Grahl, C.; Dobinsky, S.; Horstkotte, M.A.; Kiel, K.; Knobloch, J.K.-M. Genetic and biochemical analysis of Staphylococcus epidermidis biofilm accumulation. In Methods in Enzymology; Doyle, R.J., Ed.; Academic Press: Cambridge, MA, USA, 2001; Volume 336, pp. 215–239. [Google Scholar]
- Rachid, S.; Ohlsen, K.; Wallner, U.; Hacker, J.; Hecker, M.; Ziebuhr, W. Alternative Transcription Factor ςB Is Involved in Regulation of Biofilm Expression in a Staphylococcus aureus Mucosal Isolate. J. Bacteriol. 2000, 182, 6824–6826. [Google Scholar] [CrossRef] [PubMed]
- da Silva Meira, Q.G.; de Medeiros Barbosa, I.; Alves Aguiar Athayde, A.J.; de Siqueira-Júnior, J.P.; de Souza, E.L. Influence of temperature and surface kind on biofilm formation by Staphylococcus aureus from food-contact surfaces and sensitivity to sanitizers. Food Control 2012, 25, 469–475. [Google Scholar] [CrossRef]
- Packiavathy, I.A.S.V.; Agilandeswari, P.; Musthafa, K.S.; Karutha Pandian, S.; Veera Ravi, A. Antibiofilm and quorum sensing inhibitory potential of Cuminumcyminum and its secondary metabolite methyl eugenol against Gram negative bacterial pathogens. Food Res. Int. 2012, 45, 85–92. [Google Scholar] [CrossRef]
Sample Availability: Samples of TTO and Terpinen-4-ol are available from the authors. |




| N. | Ki a | Ki b | % c | Identification d | |
|---|---|---|---|---|---|
| 1 | α-Thujene | 916 | 930 | 0.9 | 1,2 |
| 2 | α-Pinene | 921 | 1032 | 2.7 | 1,2,3 |
| 3 | β-Pinene | 980 | 979 | 0.7 | 1,2,3 |
| 4 | Myrcene | 985 | 990 | 0.8 | 1,2 |
| 5 | α-Terpinene | 1010 | 1189 | 7.7 | 1,2,3 |
| 6 | p-Cymene | 1018 | 1269 | 4.7 | 1,2,3 |
| 7 | 1,8-Cineole | 1024 | 1213 | 5.2 | 1,2,3 |
| 8 | γ-Terpinene | 1054 | 1256 | 19.5 | 1,2,3 |
| 9 | α-Terpinolene | 1083 | 1265 | 3.1 | 1,2 |
| 10 | α-Terpineol | 1180 | 1188 | 3.3 | 1,2,3 |
| 11 | Terpinen-4-ol | 1173 | 1611 | 40.4 | 1,2,3 |
| 12 | Isoledene | 1382 | 1367 | 1.2 | 1,2 |
| 13 | Aromadendrene | 1442 | 1628 | 0.5 | 1,2 |
| 14 | allo-Aromadendrene | 1458 | 1661 | 1.5 | 1,2,3 |
| 15 | δ-Cadinene | 1523 | 1773 | 1.5 | 1,2 |
| Total | 93.8 |
| Strains | M. alternifolia | Terpinen-4-ol | Cefoxitin (5 µg/mL) | ||||
|---|---|---|---|---|---|---|---|
| DZI * (mm ± SD) | MIC | MBC | DZI (mm ± SD) | MIC | MBC | ||
| ATCC 6538 | 26 ± 0 | 0.048 | 50 | 23.5 ± 0.7 | 0.048 | 50 | 26 |
| ATCC 43300 | 23.33 ± 1.89 | 0.048 | 50 | 22 ± 2 | 0.048 | 25 | 26 |
| Sa1 | 19.33 ± 0.58 | 0.78 | 25 | 6 ± 0 | 0.78 | 6.25 | 21 |
| Sa3 | 19.67 ± 0.58 | 3.125 | >50 | 25 ± 0 | 0.048 | 25 | 21 |
| Sa4 | 37.5 ± 0.7 | 3.125 | >50 | 20.67 ± 0.58 | 0.048 | 25 | 14 |
| Sa16 | 18.67 ± 0.58 | 0.048 | >50 | 20 ± 0 | 0.048 | 25 | 21 |
| Sa17 | 20.5 ± 1 | 0.048 | >50 | 24 ± 1 | 0.048 | 25 | 21 |
| Sa18 | 27 ± 1 | 0.048 | >50 | 25 ± 1 | 0.048 | 25 | 21 |
| Sa21 | 13 ± 1 | 0.048 | 50 | 29.67 ± 0.58 | 0.048 | 6.25 | 14 |
| Sa26 | 19.33 ± 0.58 | 0.048 | >50 | 23.33 ± 0.58 | 0.048 | 50 | 21 |
| Sa5 | 20 ± 1 | 0.048 | >50 | 29.67 ± 0.58 | 0.048 | 25 | 21 |
| Sa6 | 21 ± 1 | 0.048 | >50 | 6 ± 0 | 0.048 | 25 | 17 |
| Sa7 | 19.67 ± 0.58 | 0.048 | >50 | 24.33 ± 2.08 | 0.048 | 25 | 21 |
| Sa13 | 28.5 ± 0.7 | 0.048 | >50 | 28.33 ± 0.58 | 0.048 | 25 | 18 |
| Sa24 | 25.5 ± 0.7 | 0.048 | >50 | 30.33 ± 0.58 | 0.048 | 25 | 21 |
| Sa25 | 17.67 ± 0.58 | 0.048 | >50 | 20 ± 1 | 0.048 | 25 | 19 |
| Sa28 | 19.33 ± 0.58 | 0.048 | >50 | 21 ± 1 | 0.097 | 25 | 21 |
| Sa29 | 20 ± 0 | 0.048 | 50 | 22 ± 0 | 0.048 | 25 | 21 |
| Sa30 | 19.33 ± 0.58 | 0.048 | >50 | 20 ± 0 | 0.048 | 25 | 14 |
| Sa31 | 13.5 ± 0.7 | 0.048 | 50 | 26 ± 1 | 0.048 | 50 | 8 |
| Sa32 | 16.5 ± 0.7 | 0.048 | >50 | 18.5 ± 0.7 | 0.048 | 50 | 20 |
| Sa9 | 27 ± 1 | 0.048 | >50 | 29.33 ± 0.58 | 0.048 | 25 | 14 |
| Sa27 | 19.67 ± 0.58 | 0.048 | >50 | 21.33 ± 0.58 | 0.048 | 50 | 16 |
| Sa2 | 18 ± 0 | 0.78 | >50 | 29.67 ± 0.58 | 0.048 | 25 | 21 |
| Sa8 | 26.67 ± 0.58 | 0.048 | >50 | 29.33 ± 0.58 | 0.048 | 25 | 20 |
| Sa10 | 27 ± 1 | 0.048 | >50 | 30 ± 0 | 0.048 | 25 | 6 |
| Sa12 | 18.5 ± 0.7 | 0.048 | >50 | 26 ± 0 | 0.048 | 25 | 21 |
| Sa15 | 28 ± 0 | 0.048 | >50 | 25 ± 0 | 0.048 | 25 | 6 |
| Sa19 | 25 ± 0 | 0.048 | 50 | 20.67 ± 0.58 | 1.52 | 25 | 19 |
| Sa23 | 26.5 ± 0.7 | 0.048 | 50 | 21 ± 0 | 0.048 | 25 | 21 |
| Strains | Origin | Biofilm on Polystyrene | Biofilm on Glass | ||
|---|---|---|---|---|---|
| OD570 ± SD | Biofilm Potency | OD570 ± SD | Biofilm Potency | ||
| ATCC 6538 | Type strain | 2.90 ± 0.05 | High producer | 2.23 ± 0.5 | High producer |
| ATCC 43300 | Type strain | 0.71 ± 0.15 | Low producer | 2.98 ± 0.4 | High producer |
| Sa1 | Blood culture | 0.19 ± 0.01 | Low producer | 1.42 ± 0.55 | High producer |
| Sa3 | Blood culture | 0.13 ± 0.02 | Low producer | 0.76 ± 0.05 | Low producer |
| Sa4 | Blood culture | 0.59 ± 0.05 | Low producer | 0.9 ± 0.22 | Low producer |
| Sa16 | Blood culture | 0.22 ± 0.03 | Low producer | 0.66 ± 0.04 | Low producer |
| Sa17 | Blood culture | 0.17 ± 0.03 | Low producer | 1.39 ± 0.07 | High producer |
| Sa18 | Blood culture | 2.62 ± 0.39 | High producer | 2.76 ± 0.15 | High producer |
| Sa21 | Blood culture | 0.24 ± 0.06 | Low producer | 1.46 ± 0.2 | High producer |
| Sa26 | Blood culture | 0.21 ± 0.01 | Low producer | 1.58 ± 0.2 | High producer |
| Sa5 | Superficial pus | 0.46 ± 0.05 | Low producer | 1.01 ± 0.2 | High producer |
| Sa6 | Superficial pus | 0.26 ± 0.03 | Low producer | 2.72 ± 0.24 | High producer |
| Sa7 | Superficial pus | 0.22 ± 0.02 | Low producer | 1.84 ± 0.13 | High producer |
| Sa13 | Superficial pus | 0.15 ± 0.02 | Low producer | 3.1 ± 0.4 | High producer |
| Sa24 | Superficial pus | 0.62 ± 0.08 | Low producer | 1.64 ± 0.23 | High producer |
| Sa25 | Superficial pus | 0.12 ± 0.01 | Low producer | 0.17 ± 0.05 | Low producer |
| Sa28 | Superficial pus | 0.14 ± 0.02 | Low producer | 0.53 ± .0.02 | Low producer |
| Sa29 | Superficial pus | 0.14 ± 0.03 | Low producer | 2.46 ± 0.07 | High producer |
| Sa30 | Superficial pus | 1.00 ± 0.08 | High producer | 3.25 ± 0.05 | High producer |
| Sa31 | Superficial pus | 0.18 ± 0.04 | Low producer | 2.97 ± 0.07 | High producer |
| Sa32 | Superficial pus | 0.16 ± 0.07 | Low producer | 3.27 ± 0.07 | High producer |
| Sa9 | Deep pus | 0.15 ± 0.01 | Low producer | 1.58 ± 0.05 | High producer |
| Sa27 | Deep pus | 0.14 ± 0.03 | Low producer | 1.1 ± 0.04 | High producer |
| Sa2 | Various | 0.12 ± 0.02 | Low producer | 0.28 ± 0.03 | Low producer |
| Sa8 | Various | 0.42 ± 0.13 | Low producer | 2.58 ± 0.04 | High producer |
| Sa10 | Various | 0.80 ± 0.30 | Low producer | 1.91 ± 0.09 | High producer |
| Sa12 | Various | 2.96 ± 0.11 | High producer | 3.38 ± 0.12 | High producer |
| Sa15 | Various | 1.77 ± 0.60 | High producer | 2.22 ± 0.04 | High producer |
| Sa19 | Various | 0.15 ± 0.01 | Low producer | 0.51 ± 0.03 | Low producer |
| Sa23 | Various | 0.13 ± 0.01 | Low producer | 0.15 ± 0.02 | Low producer |
| Strains | Biofilm on Polystyrene | Biofilm on Glass | |||
|---|---|---|---|---|---|
| M. alternifolia | Terpinen-4-ol | M. alternifolia | Terpinen-4-ol | ||
| MIC | 7.63 ± 0.73 | 74.62 ± 1.9 | 42.35 ± 9.89 | 28.58 ± 1.78 | |
| Sa12 | 2 × MIC | 21.15 ± 1.91 | 78.03 ± 2.26 | 54.63 ± 1.51 | 68.37 ± 1.06 |
| 4 × MIC | 26.48 ± 2.52 | 83.27 ± 2.51 | 66.56 ± 1.69 | 75.45 ± 2.43 | |
| MIC | 31.67 ± 2.21 | 83.95 ± 4.44 | 4.5 ± 2.47 | 56.43 ± 9.26 | |
| Sa15 | 2 × MIC | 44.57 ± 1.81 | 89.26 ± 3.37 | 20.45 ± 6.08 | 72.75 ± 0.92 |
| 4 × MIC | 59.05 ± 2.83 | 91.24 ± 5.81 | 40.85 ± 1.61 | 73.79 ± 9.47 | |
| MIC | 4.92 ± 6.75 | 83.35 ± 7.11 | 23.42 ± 2.19 | 56.91 ± 2.26 | |
| Sa18 | 2 × MIC | 19.59 ± 3.26 | 87.39 ± 4.37 | 28.51 ± 1.42 | 57.35 ± 2.34 |
| 4 × MIC | 27.74 ± 1.76 | 91.03 ± 5.05 | 49.09 ± 1.55 | 62.76 ± 1.26 | |
| Concentration | % of Violacein Inhibition | |
|---|---|---|
| M. alternifolia | Terpinen-4-ol | |
| MIC | 69.3 ± 2 | 34.74 ± 1.6 |
| MIC/2 | 58.98 ± 1.7 | 18.04 ± 0.1 |
| MIC/4 | 45.74 ± 1.3 | 17.87 ± 2 |
| MIC/8 | 37.15 ± 1.8 | 1.47 ± 1 |
| MIC/16 | 31.34 ± 2.3 | 1.32 ± 1.3 |
| MIC/32 | 23.55 ± 1.7 | 1.02 ± 0.9 |
| Component | Concentrations | ||
|---|---|---|---|
| 50 µg/mL | 75 µg/mL | 100 µg/mL | |
| TTO oil | 16.67 ± 0 | 25 ± 1.17 | 33.33 ± 1.09 |
| Terpinen-4-ol | 25 ± 0 | 25 ± 0 | 25 ± 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noumi, E.; Merghni, A.; M. Alreshidi, M.; Haddad, O.; Akmadar, G.; De Martino, L.; Mastouri, M.; Ceylan, O.; Snoussi, M.; Al-sieni, A.; et al. Chromobacterium violaceum and Pseudomonas aeruginosa PAO1: Models for Evaluating Anti-Quorum Sensing Activity of Melaleuca alternifolia Essential Oil and Its Main Component Terpinen-4-ol. Molecules 2018, 23, 2672. https://doi.org/10.3390/molecules23102672
Noumi E, Merghni A, M. Alreshidi M, Haddad O, Akmadar G, De Martino L, Mastouri M, Ceylan O, Snoussi M, Al-sieni A, et al. Chromobacterium violaceum and Pseudomonas aeruginosa PAO1: Models for Evaluating Anti-Quorum Sensing Activity of Melaleuca alternifolia Essential Oil and Its Main Component Terpinen-4-ol. Molecules. 2018; 23(10):2672. https://doi.org/10.3390/molecules23102672
Chicago/Turabian StyleNoumi, Emira, Abderrahmen Merghni, Mousa M. Alreshidi, Ons Haddad, Gültekin Akmadar, Laura De Martino, Maha Mastouri, Ozgur Ceylan, Mejdi Snoussi, Abdulbasit Al-sieni, and et al. 2018. "Chromobacterium violaceum and Pseudomonas aeruginosa PAO1: Models for Evaluating Anti-Quorum Sensing Activity of Melaleuca alternifolia Essential Oil and Its Main Component Terpinen-4-ol" Molecules 23, no. 10: 2672. https://doi.org/10.3390/molecules23102672