Fluorinated Tetraphosphonate Cavitands
Abstract
:1. Introduction
2. Results and Discussion
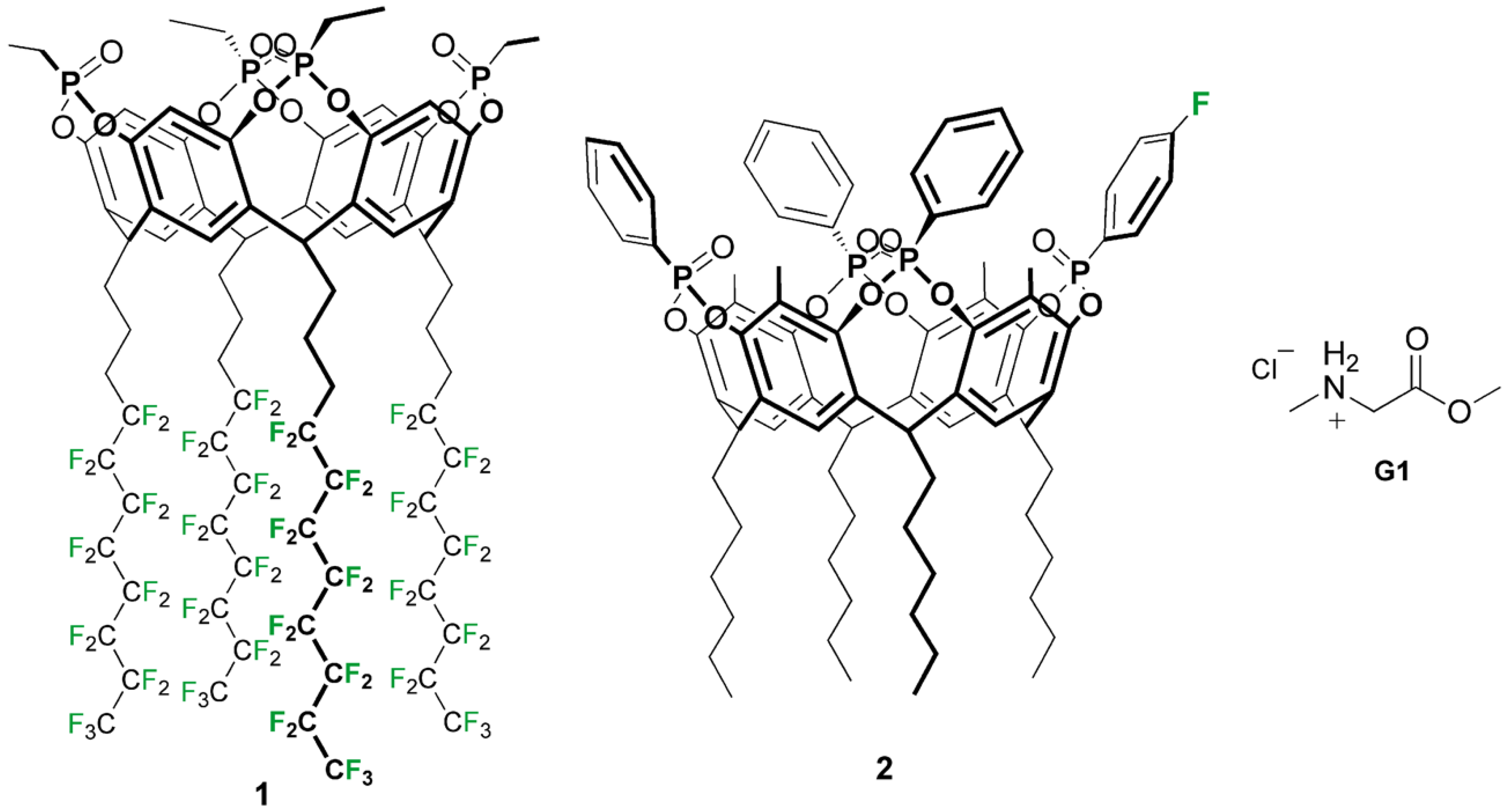
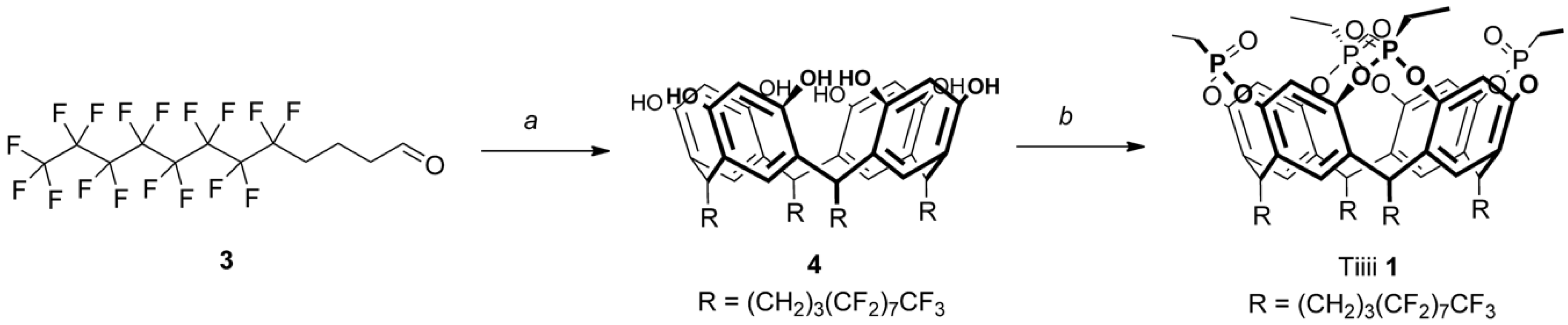
2.1. Synthesis of Cavitand 1
2.2. Synthesis of Cavitand 2
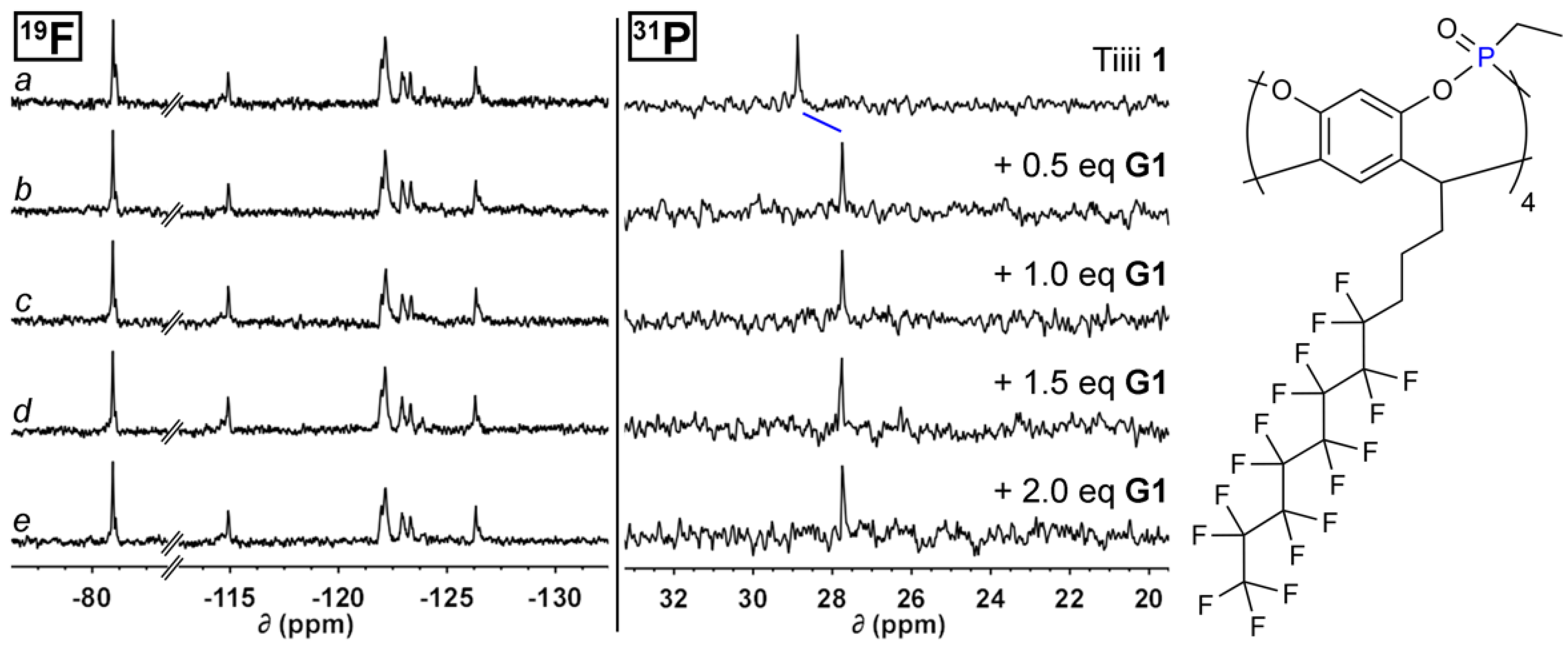
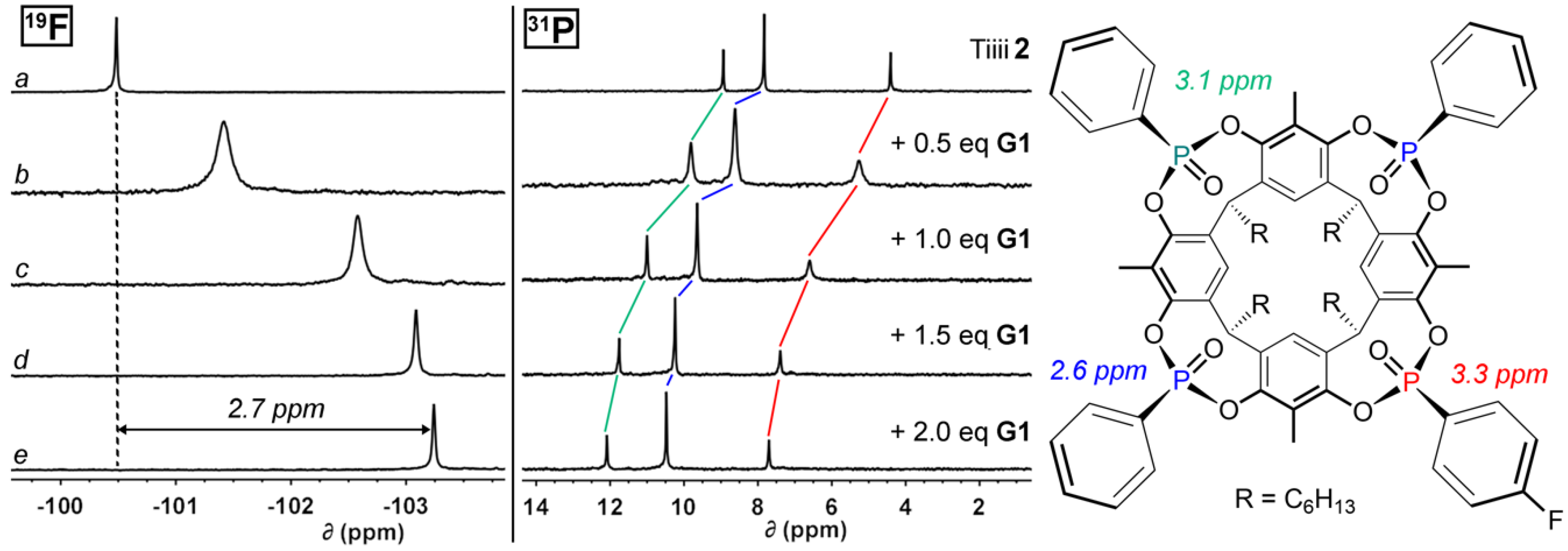
2.3. NMR Titration Experiments
3. Experimental
3.1. Materials and Methods
3.2. Syntheses
3.2.1. Synthesis of Resorcinarene [(CH2)3(CF2)7CF3, H] (4)
3.2.2. Synthesis of Tiiii [(CH2)3(CF2)7CF3, H, Et] (1)
3.2.3. Synthesis of Dichloro(4-fluorophenyl)phosphine (5)
3.2.4. Synthesis of Tiiii [C6H13, CH3, Ph] (7)
3.2.5. Synthesis of Cavitand 3POiii [C6H13, CH3, Ph] (8)
3.2.6. Synthesis of Tiiii [C6H13, CH3, 3Ph + 1PhFp] (2)
4. Conclusions
Supplementary Material
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pinalli, R.; Pedrini, A.; Dalcanale, E. Cavitands. In Comprehensive Supramolecular Chemistry II; Atwood, J.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 87–115. ISBN 9780128031995. [Google Scholar]
- Hooley, R.J.; Rebek, J. Chemistry and catalysis in functional cavitands. Chem. Biol. 2009, 16, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, N.; Brenner, E.; Sémeril, D.; Matt, D.; Harrowfield, J. The use of resorcinarene cavitands in metal-based catalysis. Eur. J. Org. Chem. 2017, 2017, 6100–6113. [Google Scholar] [CrossRef]
- Mirabaud, A.; Mulatier, J.-C.; Martinez, A.; Dutasta, J.-P.; Dufaud, V. Merging host-guest chemistry and organocatalysis for the chemical valorization of CO2. Catal. Today 2017, 281, 387–391. [Google Scholar] [CrossRef]
- Vidal, D.; Costas, M.; Lledó, A. A deep cavitand receptor functionalized with Fe(II) and Mn(II) aminopyridine complexes for bioinspired oxidation catalysis. ACS Catal. 2018, 8, 3667–3672. [Google Scholar] [CrossRef]
- Pinalli, R.; Dalcanale, E.; Ugozzoli, F.; Massera, C. Resorcinarene-based cavitands as building blocks for crystal engineering. CrystEngComm 2016, 18, 5788–5802. [Google Scholar] [CrossRef]
- Pochorovski, I.; Diederich, F. Development of redox-switchable resorcin [4] arene cavitands. Acc. Chem. Res. 2014, 47, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Ghang, Y.-J.; Perez, L.; Morgan, M.A.; Si, F.; Hamdy, O.M.; Beecher, C.N.; Larive, C.K.; Julian, R.R.; Zhong, W.; Cheng, Q.; et al. Anionic deep cavitands enable the adhesion of unmodified proteins at a membrane bilayer. Soft Matter 2014, 10, 9651–9656. [Google Scholar] [CrossRef] [PubMed]
- Bontempi, N.; Biavardi, E.; Bordiga, D.; Candiani, G.; Alessandri, I.; Bergese, P.; Dalcanale, E. Probing lysine mono-methylation in histone H3 tail peptides with an abiotic receptor coupled to a non-plasmonic resonator. Nanoscale 2017, 9, 8639–8646. [Google Scholar] [CrossRef] [PubMed]
- Clément, P.; Korom, S.; Struzzi, C.; Parra, E.J.; Bittencourt, C.; Ballester, P.; Llobet, E. Deep cavitand self-assembled on Au NPs-MWCNT as highly sensitive benzene sensing interface. Adv. Funct. Mater. 2015, 25, 4011–4020. [Google Scholar] [CrossRef]
- Tudisco, C.; Fragalà, M.E.; Giuffrida, A.E.; Bertani, F.; Pinalli, R.; Dalcanale, E.; Compagnini, G.; Condorelli, G.G. Hierarchical route for the fabrication of cavitand-modified nanostructured ZnO fibers for volatile organic compound detection. J. Phys. Chem. C 2016, 120, 12611–12617. [Google Scholar] [CrossRef]
- Früh, A.E.; Artoni, F.; Brighenti, R.; Dalcanale, E. Strain field self-diagnostic poly(dimethylsiloxane) elastomers. Chem. Mater. 2017, 29, 7450–7457. [Google Scholar] [CrossRef]
- Pinalli, R.; Dalcanale, E. Supramolecular sensing with phosphonate cavitands. Acc. Chem. Res. 2013, 46, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Trzciński, J.W.; Pinalli, R.; Riboni, N.; Pedrini, A.; Bianchi, F.; Zampolli, S.; Elmi, I.; Massera, C.; Ugozzoli, F.; Dalcanale, E. In search of the ultimate benzene sensor: The EtQxBox solution. ACS Sens. 2017, 2, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.X.; Hallac, R.R.; Chiguru, S.; Mason, R.P. New frontiers and developing applications in 19F NMR. Prog. Nucl. Magn. Reson. Spectrosc. 2013, 70, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Swager, T.M. Detection and differentiation of neutral organic compounds by 19F NMR with a tungsten calix [4] arene imido complex. J. Am. Chem. Soc. 2013, 135, 18770–18773. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Markopoulos, G.; Swager, T.M. 19F NMR fingerprints: Identification of neutral organic compounds in a molecular container. J. Am. Chem. Soc. 2014, 136, 10683–10690. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Swager, T.M. Simultaneous chirality sensing of multiple amines by 19F NMR. J. Am. Chem. Soc. 2015, 137, 3221–3224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, L.; Swager, T.M. Simultaneous identification of neutral and anionic species in complex mixtures without separation. Angew. Chem. Int. Ed. 2016, 55, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, L.; Carril, M.; Padro, D.; Mancin, F. Multimodal 19F NMR dopamine detection and imaging with a nanoparticle-based displacement assay. Chem. Eur. J. 2018, 24, 13036–13042. [Google Scholar] [CrossRef] [PubMed]
- Pinalli, R.; Pedrini, A.; Dalcanale, E. Biochemical sensing with macrocyclic receptors. Chem. Soc. Rev. 2018, 47, 7006–7026. [Google Scholar] [CrossRef] [PubMed]
- Yücesan, G.; Zorlu, Y.; Stricker, M.; Beckmann, J. Metal-organic solids derived from arylphosphonic acids. Coord. Chem. Rev. 2018, 369, 105–122. [Google Scholar] [CrossRef]
- Schütrumpf, A.; Duthie, A.; Lork, E.; Yücesan, G.; Beckmann, J. Synthesis of some Di- and Tetraphosphonic acids by suzuki cross-coupling. Z. Anorg. Allg. Chem. 2018, 644, 1134–1142. [Google Scholar] [CrossRef]
- Sreekumar, A.; Poisson, L.M.; Rajendiran, T.M.; Khan, A.P.; Cao, Q.; Yu, J.; Laxman, B.; Mehra, R.; Lonigro, R.J.; Li, Y.; et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009, 457, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Janjic, J.M.; Ahrens, E.T. Fluorine-containing nanoemulsions for MRI cell tracking. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 492–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, S.; Kiuchi, T.; Pan, N. A “teflon-footed” resorcinarene: A hexameric capsule in fluorous solvents and fluorophobic effects on molecular encapsulation. Angew. Chem. Int. Ed. 2007, 46, 6442–6445. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; O’Neal, K.; Osipov, M.; Ngwendson, J.N.; Geib, S.J.; Weber, S.G.; Curran, D.P. Synthesis, characterization, and applications of fluorous resorcin [4] arenes. New J. Chem. 2010, 34, 2732–2734. [Google Scholar] [CrossRef]
- Rocaboy, C.; Bauer, W.; Gladysz, J.A. Convenient syntheses of a family of easily recoverable fluorous primary, secondary, and tertiary aliphatic amines NH3-x[(CH2)m(CF2)7CF3]x (m = 3–5; x = 1–3)—fine tuning of basicities and fluorous phase affinities. Eur. J. Org. Chem. 2000, 2000, 2621–2628. [Google Scholar] [CrossRef]
- Alvey, L.J.; Meier, R.; Soos, T.; Bernatis, P.; Gladysz, J.A. Syntheses and carbonyliridium complexes of unsymmetrically substituted fluorous trialkylphosphanes: Precision tuning of electronic properties, including insulation of the perfluoroalkyl groups. Eur. J. Inorg. Chem. 2000, 2000, 1975–1983. [Google Scholar] [CrossRef]
- Grabiak, R.C.; Miles, J.A.; Schwenzer, G.M. Synthesis of phosphonic dichlorides and correlation of their P-31 chemical shifts. Phosphorus Sulfur Relat. Elem. 1980, 9, 197–202. [Google Scholar] [CrossRef]
- Cherbuliez, E.; Rabinowitz, G.; Weber, J. Recherches sur la formation et la transformation des esters XL. Sur la phosphonylation d’alcools divers par l’oxyde p-fluorophénylphosphonique. Helv. Chim. Acta 1962, 45, 2665–2669. [Google Scholar] [CrossRef]
- Melegari, M.; Massera, C.; Pinalli, R.; Yebeutchou, R.M.; Dalcanale, E. Supramolecular sensing of short chain alcohols with mixed-bridged thio-phosphonate cavitands. Sens. Actuators B Chem. 2013, 179, 74–80. [Google Scholar] [CrossRef]
- Menozzi, D.; Biavardi, E.; Massera, C.; Schmidtchen, F.-P.; Cornia, A.; Dalcanale, E. Thermodynamics of host–guest interactions between methylpyridinium salts and phosphonate cavitands. Supramol. Chem. 2010, 22, 768–775. [Google Scholar] [CrossRef]
- Misztal, K.; Tudisco, C.; Sartori, A.; Malicka, J.M.; Castelli, R.; Condorelli, G.G.; Dalcanale, E. Hierarchical self-assembly of luminescent EuIII complexes on silicon. Eur. J. Inorg. Chem. 2014, 2014, 2687–2694. [Google Scholar] [CrossRef]
- Mettra, B.; Bretonnière, Y.; Mulatier, J.C.; Bibal, B.; Tinant, B.; Aronica, C.; Dutasta, J.P. Design of differently P-substituted 4iPO fluorescent tetraphosphonate cavitands. Supramol. Chem. 2013, 25, 672–681. [Google Scholar] [CrossRef]
- Biavardi, E.; Tudisco, C.; Maffei, F.; Motta, A.; Massera, C.; Condorelli, G.G.; Dalcanale, E. Exclusive recognition of sarcosine in water and urine by a cavitand-functionalized silicon surface. Proc. Natl. Acad. Sci. USA 2012, 109, 2263–2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinalli, R.; Brancatelli, G.; Pedrini, A.; Menozzi, D.; Hernández, D.; Ballester, P.; Geremia, S.; Dalcanale, E. The origin of selectivity in the complexation of N-methyl amino acids by tetraphosphonate cavitands. J. Am. Chem. Soc. 2016, 138, 8569–8580. [Google Scholar] [CrossRef] [PubMed]
- Tunstad, L.M.; Tucker, J.A.; Dalcanale, E.; Weiser, J.; Bryant, J.A.; Sherman, J.C.; Hegelson, R.C.; Knobler, C.B.; Cram, D.J. Host-guest complexation. 48. octol building blocks for cavitands and carcerands. J. Org. Chem. 1989, 54, 1305–1312. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrini, A.; Bertani, F.; Dalcanale, E. Fluorinated Tetraphosphonate Cavitands. Molecules 2018, 23, 2670. https://doi.org/10.3390/molecules23102670
Pedrini A, Bertani F, Dalcanale E. Fluorinated Tetraphosphonate Cavitands. Molecules. 2018; 23(10):2670. https://doi.org/10.3390/molecules23102670
Chicago/Turabian StylePedrini, Alessandro, Federico Bertani, and Enrico Dalcanale. 2018. "Fluorinated Tetraphosphonate Cavitands" Molecules 23, no. 10: 2670. https://doi.org/10.3390/molecules23102670