Synthesis and Structural Identification of a Biaryl Ether-Linked Zearalenone Dimer
Abstract
:1. Introduction
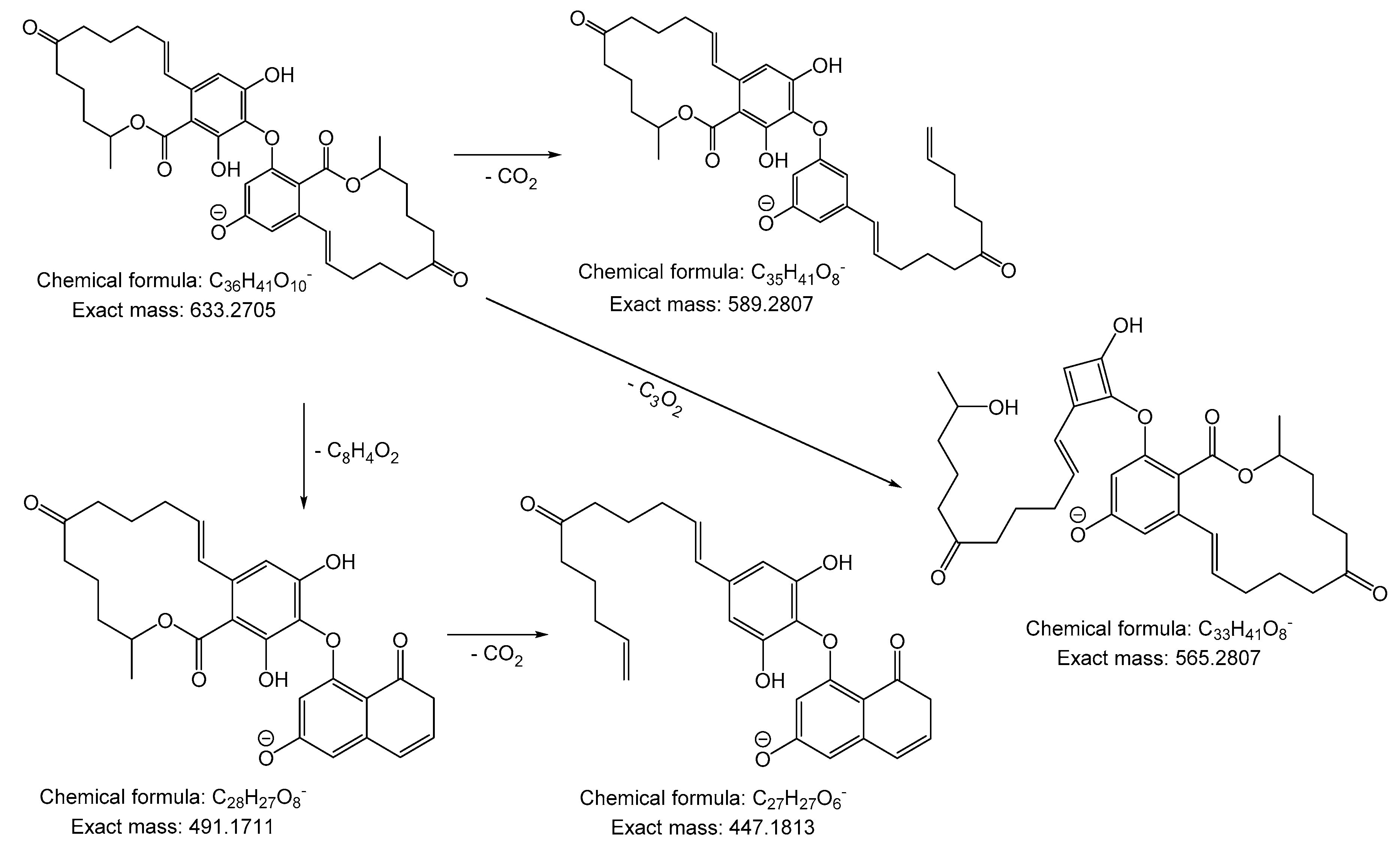
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and General Experimental Procedures
3.2. Electrochemical and Chemical Production of ZEN Dimer
3.3. Purification of ZEN Dimer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caldwell, R.W.; Tuite, J.; Stob, M.; Baldwin, R. Zearalenone production by fusarium species. Appl. Microbiol. 1970, 20, 31–34. [Google Scholar] [PubMed]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Antonissen, G. Emerging fusarium and alternaria mycotoxins: Occurrence, toxicity and toxicokinetics. Toxins 2017, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Placinta, C.M.; D’Mello, J.P.F.; Macdonald, A.M.C. A review of worldwide contamination of cereal grains and animal feed with fusarium mycotoxins. Anim. Feed Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cottrill, B.; Cravedi, J.P.; Di Domenico, A.; Doerge, D.; Dogliotti, E.; Edler, L.; et al. Scientific opinion on the risks for public health related to the presence of zearalenone in food efsa panel on contaminants in the food chain. EFSA J. 2011, 9, 2197. [Google Scholar]
- Hetmanski, M.T.; Scudamore, K.A. Detection of zearalenone in cereal extracts using high-performance liquid chromatography with post-column derivatization. J. Chromatogr. 1991, 588, 47–52. [Google Scholar] [CrossRef]
- Koppen, R.; Riedel, J.; Proske, M.; Drzymala, S.; Rasenko, T.; Durmaz, V.; Weber, M.; Koch, M. Photochemical trans-/cis-isomerization and quantitation of zearalenone in edible oils. J. Agric. Food Chem. 2012, 60, 11733–11740. [Google Scholar] [CrossRef] [PubMed]
- Drzymala, S.S.; Binder, J.; Brodehl, A.; Penkert, M.; Rosowski, M.; Garbe, L.-A.; Koch, M. Estrogenicity of novel phase i and phase ii metabolites of zearalenone and cis-zearalenone. Toxicon 2015, 105, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Pfohlleszkowicz, A.; Chekirghedira, L.; Bacha, H. Genotoxicity of zearalenone, an estrogenic mycotoxin—DNA adduct formation in female mouse-tissues. Carcinogenesis 1995, 16, 2315–2320. [Google Scholar] [CrossRef]
- Binder, S.B.; Schwartz-Zimmermann, H.E.; Varga, E.; Bichl, G.; Michlmayr, H.; Adam, G.; Berthiller, F. Metabolism of zearalenone and its major modified forms in pigs. Toxins 2017, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Borzekowski, A.; Drewitz, T.; Keller, J.; Pfeifer, D.; Kunte, H.-J.; Koch, M.; Rohn, S.; Maul, R. Biosynthesis and characterization of zearalenone-14-sulfate, zearalenone-14-glucoside and zearalenone-16-glucoside using common fungal strains. Toxins 2018, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Brodehl, A.; Moller, A.; Kunte, H.J.; Koch, M.; Maul, R. Biotransformation of the mycotoxin zearalenone by fungi of the genera rhizopus and aspergillus. FEMS Microbiol. Lett. 2014, 359, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, K.H.; Pettersson, H. Metabolism of zearalenone in rat liver. Acta Pharmacol. Toxicol. 1978, 43, 285–290. [Google Scholar] [CrossRef]
- Bravin, F.; Duca, R.C.; Balaguer, P.; Delaforge, M. In vitro cytochrome p450 formation of a mono-hydroxylated metabolite of zearalenone exhibiting estrogenic activities: Possible occurrence of this metabolite in vivo. Int. J. Mol. Sci. 2009, 10, 1824–1837. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, A.A.; Pfeiffer, E.; Rapp, A.; Metzler, M. Hydroxylation of the mycotoxin zearalenone at aliphatic positions: Novel mammalian metabolites. Mycotoxin Res. 2012, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Haase, H.; Koch, M. Hydroxylation and dimerization of zearalenone: Comparison of chemical, enzymatic and electrochemical oxidation methods. World Mycotoxin J. 2017, 10, 297–307. [Google Scholar] [CrossRef]
- Wezeman, T.; Bräse, S.; Masters, K.-S. Xanthone dimers: A compound family which is both common and privileged. Nat. Prod. Rep. 2015, 32, 6–28. [Google Scholar] [CrossRef] [PubMed]
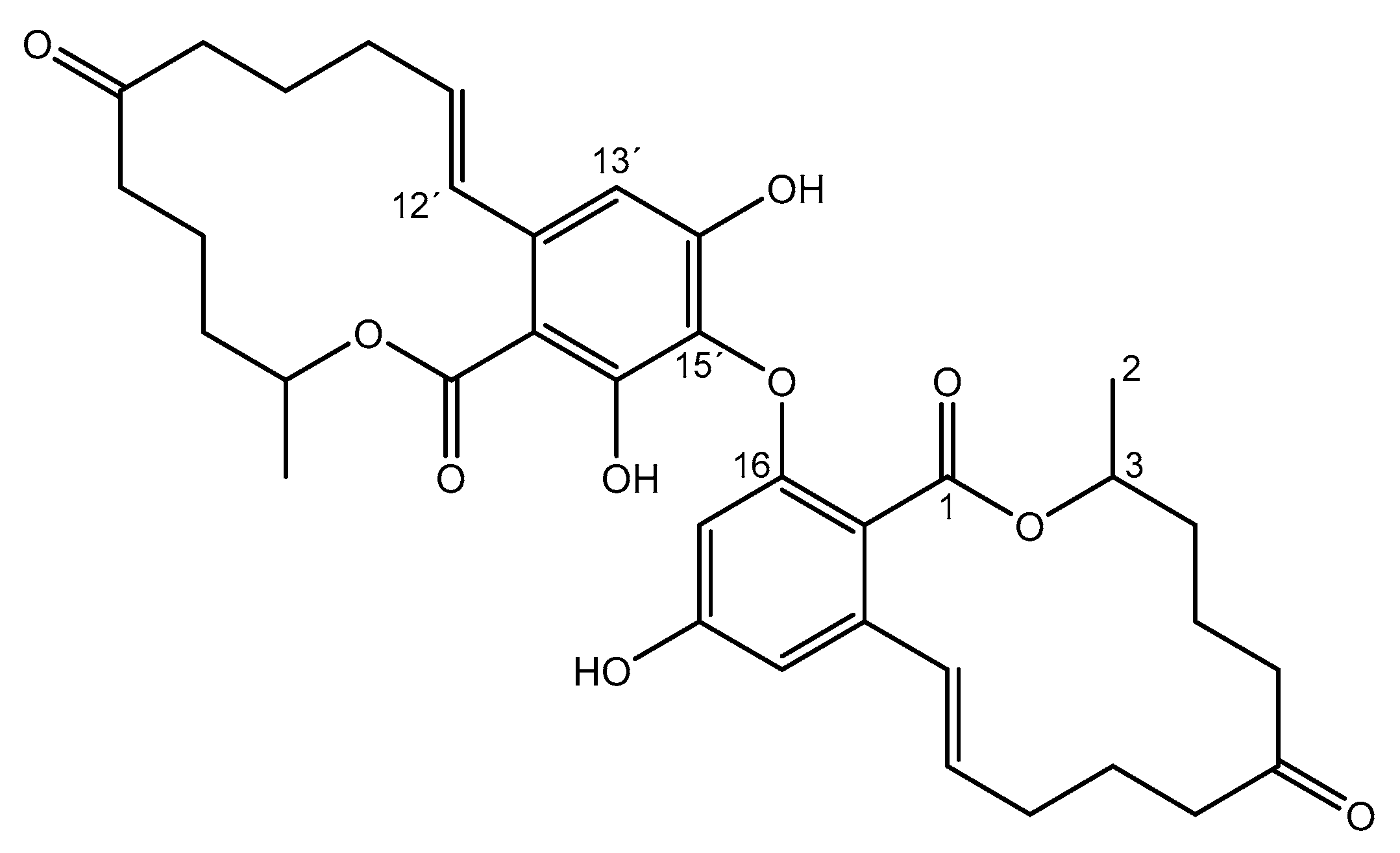
| Position | 1H (ppm) | 13C (ppm) |
|---|---|---|
| 1, 1′ | - | 213.8, 214.1 |
| 2 | 1.39 (d, J = 6.4 Hz, 3H) | 35.9 |
| 3 | 5.20–5.36 (m, 1H) | 73.7 |
| 10, 10′ | - | 32.4, 32.4 |
| 11 | 6.03 (ddd, J = 4.4, 9.6, 14.6 Hz, 1H) | 134.4 |
| 12 | 6.38–6.41 (m, 1H) | 130.2 |
| 13 | 6.58 (d, J = 2.1 Hz, 1H) | 106.7 |
| 14 | - | 161.0 |
| 15 | 5.77 (d, J = 2.1 Hz, 1H) | 100.8 |
| 2′ | 1.34 (d, J = 6.2 Hz, 3H) | 35.9 |
| 3′ | 5.06–5.16 (m, 1H) | 74.3 |
| 11′ | 5.71 (ddd, J = 4.0, 9.8, 15.6 Hz, 1H) | 137.2 |
| 12′ | 6.34–6.37 (m, 1H) | 126.4 |
| 13′ | 6.42 (s, 1H) | 104.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, J.; Hantschke, L.; Haase, H.; Koch, M. Synthesis and Structural Identification of a Biaryl Ether-Linked Zearalenone Dimer. Molecules 2018, 23, 2624. https://doi.org/10.3390/molecules23102624
Keller J, Hantschke L, Haase H, Koch M. Synthesis and Structural Identification of a Biaryl Ether-Linked Zearalenone Dimer. Molecules. 2018; 23(10):2624. https://doi.org/10.3390/molecules23102624
Chicago/Turabian StyleKeller, Julia, Luisa Hantschke, Hajo Haase, and Matthias Koch. 2018. "Synthesis and Structural Identification of a Biaryl Ether-Linked Zearalenone Dimer" Molecules 23, no. 10: 2624. https://doi.org/10.3390/molecules23102624





