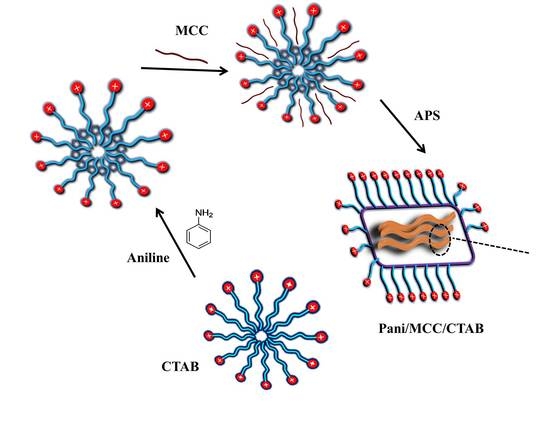
A Surfactant Directed Microcrystalline Cellulose/Polyaniline Composite with Enhanced Electrochemical Properties
Abstract
:1. Introduction
2. Results and Discussion
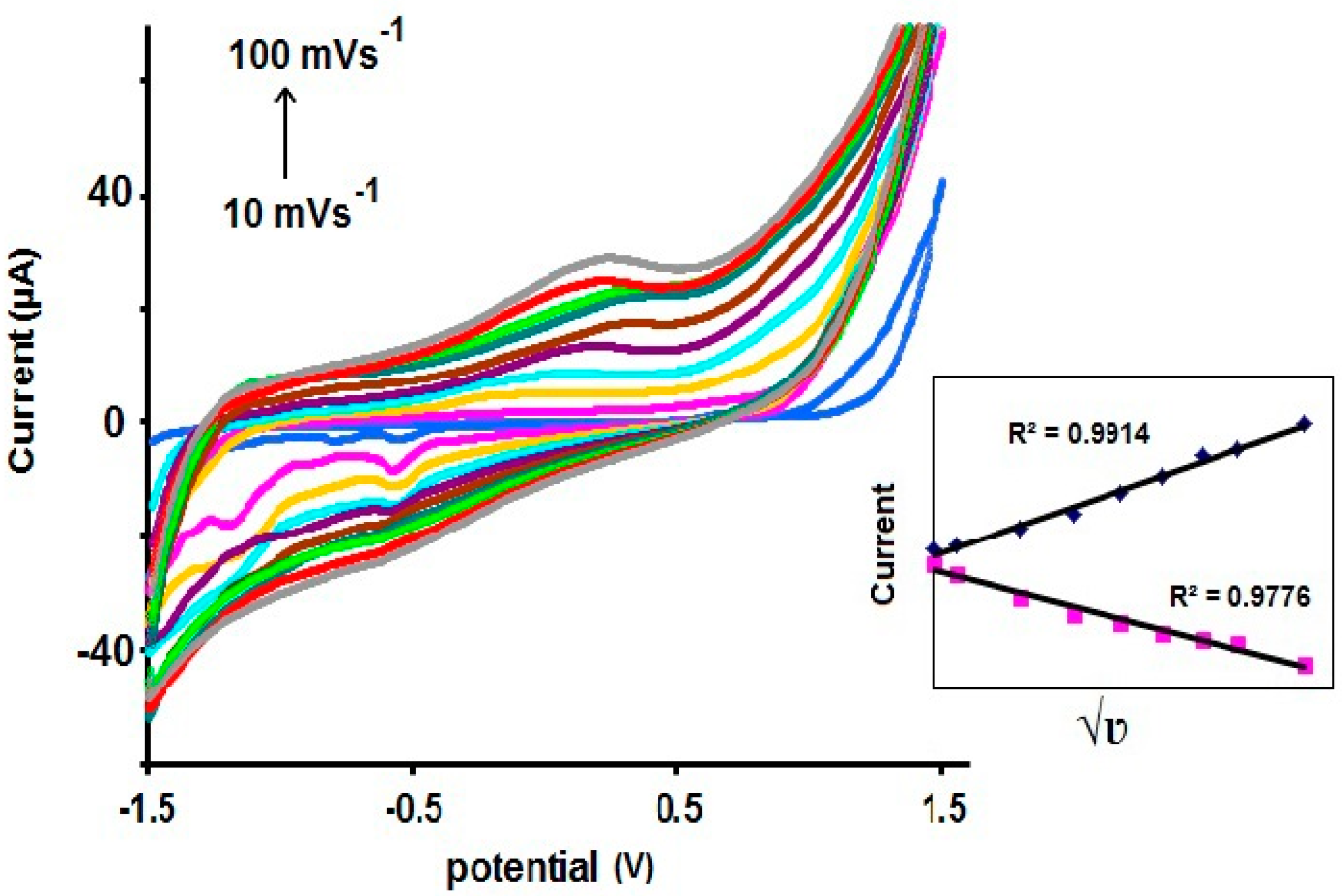
2.1. Electrochemical Properties
2.2. Chemical, Morphological and Structural Characterizations
2.2.1. Fourier Transform Infrared (FTIR) Spectroscopy
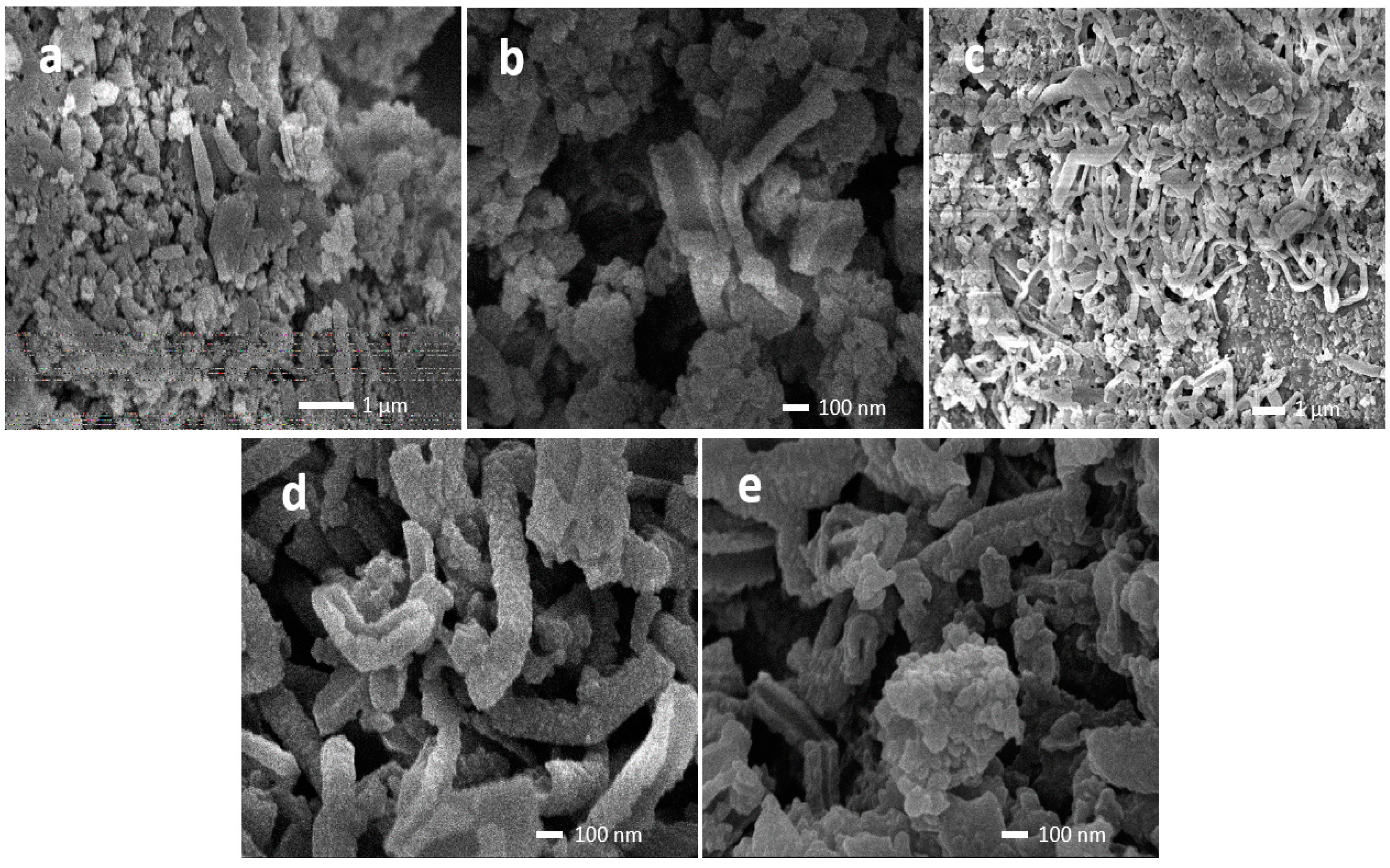
2.2.2. Morphology
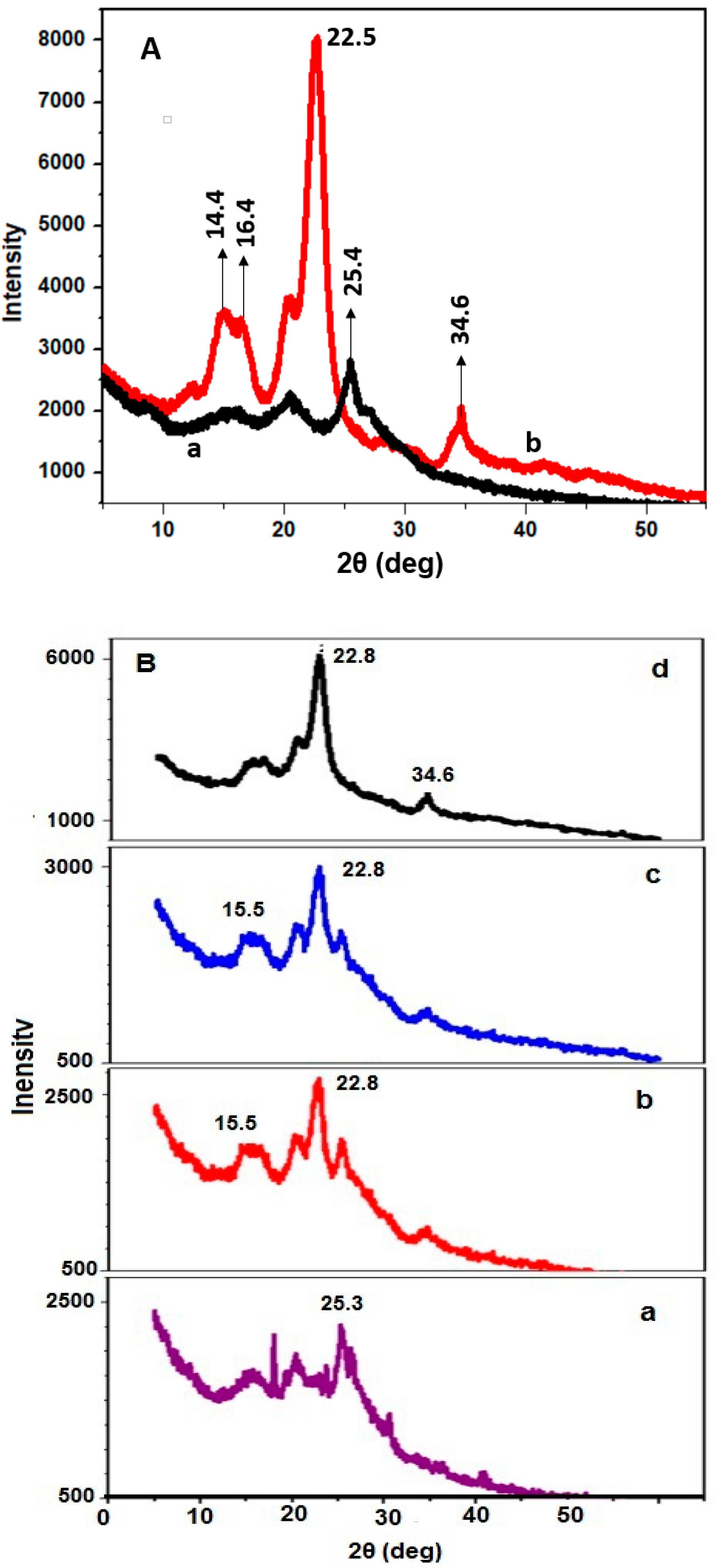
2.2.3. X-ray Diffraction Analysis
2.2.4. Thermogravimetry Analysis (TGA)
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Nanocomposite and Modified Electrode
3.3. Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nystrom, G.; Razaq, A.; Strømme, M.; Nyholm, L.; Mihranyan, A. Ultrafast All-Polymer Paper-Based Batteries. Nano Lett. 2009, 9, 3635–3639. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M., Jr. The Future Prospects of Microbial Cellulose in Biomedical Applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Yun, S.; Ounaies, Z. Discovery of Cellulose as a Smart Material. Macromolecules 2006, 39, 4202–4206. [Google Scholar] [CrossRef]
- Lee, A.D.H. Conductive Nanocrystalline Cellulose Polymer Composite Film as a Novel Mediator in Biosensor Applications. Master’s Thesis, University of Toronto, Toronto, Canada, ON, December 2011. [Google Scholar]
- Hoseini, S.H.; Entezami, A.A. Graft Copolymer of Polystyrene and Polypyrrole and Studies of Its Gas and Vapor Sensing Properties. Iran. Pol. J. 2005, 14, 101–110. [Google Scholar]
- Ma, H.; Zhou, B.; Li, H.S.; Li, Y.Q.; Ou, S.Y. Green Composite Films Composed of Nanocrystalline Cellulose and a Cellulose Matrix Regenerated from Functionalized Ionic Liquid Solution. CarbohydPolymer 2011, 84, 383–389. [Google Scholar] [CrossRef]
- Rawaida, L.R.; Abdi, M.M.; Tahir, P.M.; Moradbak, A.; Sulaiman, Y.; Lee, Y.H. Polyaniline-modified nanocellulose prepared from Semantan bamboo by chemical polymerization:preparation and characterization. RSC Adv. 2017, 7, 25191–25198. [Google Scholar]
- Dubitsky, Y.A.; Zhubanov, B.A. Polypyrrole-Poly (Vinyl Chloride) and Polypyrrole-Cellulose Acetate Conducting Composite Films by Opposite-Diffusion Polymerization. Synt. Met. 1993, 53, 303–307. [Google Scholar] [CrossRef]
- Hubbe, M.A. Method and Apparatus for Measuring an Electrical Property of Papermaking Furnish. U.S. Patent 5,936,151, 10 August 1999. [Google Scholar]
- Nystrom, G.; Mihranyan, A.; Razaq, A.; Lindstrom, T.; Nyholm, L.; Strømme, M.A. Nanocellulose Polypyrrole Composite Based on Microfibrillated Cellulose from Wood. J. Phys. Chem. B 2010, 114, 4178–4182. [Google Scholar] [CrossRef] [PubMed]
- Raba, J.; Mottola, H.A. Glucose Oxidase as an Analytical Reagent. Crit. Rev. Anal. Chem. 1995, 25, 1–42. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Wang, B.; Piao, G. Nanocomposite of polyaniline and cellulose nanocrystals prepared in lyotropic chiral nematic liquid crystals. J. Mater. 2013, 1–6. [Google Scholar] [CrossRef]
- Takeo, S.; Hiroki, O.; Shuhei, S.; Kenichi, O.; Hiroyuki, N. Emerging N-Type Redox-Active Radical Polymer for a Totally Organic Polymer-Based Rechargeable Battery. Adv. Mater. 2009, 21, 1627–1630. [Google Scholar]
- Dutta, D.; Sarma, T.K.; Chowdhury, D.; Chattopadhyay, A. A polyaniline-containing filter paper that acts as a sensor, acid, base, and endpoint indicator and also filters acids and bases. J. Colloid Interface Sci. 2005, 283, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, J.; Suresha, K.M.; Jaehwan, K. The preparation, characterization and actuation behavior of polyaniline and cellulose blended electro-active paper. Smart Mater. Struct. 2010, 19, 045011. [Google Scholar]
- Peng, B.L.; Dhar, N.; Liu, H.L.; Tam, K.C. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Can. J. Chem. Eng. 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Liu, D.Y.; Sui, G.X.; Bhattacharyya, D. Synthesis and characterisation of nanocellulose-based polyaniline conducting films. Compos. Sci. Technol. 2014, 99, 31–36. [Google Scholar] [CrossRef]
- Xia, L.; Wei, Z.; Wan, M. Conducting polymer nanostructures and their application in biosensors. J. Colloid Interface Sci. 2010, 341, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.B.; Silva, B.F.; Pimentel, S. Infrared Spectroscopy of Anionic, Cationic, and Zwitterionic Surfactants. Adv. Phys. Chem. 2012, 903272. [Google Scholar] [CrossRef]
- Dhyani, H.; Dhand, C.; Malhotra, B.D.; Sen, P. Polyaniline-CdS Quantum Dots Composite for Mediator Free Biosensing. Biosens. Bioelectron. 2011, 3, 112. [Google Scholar] [CrossRef]
- Ghosh, S.; Vishalakshi, B.; Kalpagam, V. Polyaniline in the conducting state in neutral medium. Synth. Met. 1992, 46, 349. [Google Scholar] [CrossRef]
- Upadhyay, J.; Kumar, A.; Gogoi, B.; Buragohain, A.K. Biocompatibility and antioxidant activity of polypyrrole nanotubes. Synth. Met. 2014, 189, 119–125. [Google Scholar] [CrossRef]
- Nandini, S.; Nalini, S.; Reddy, M.B.M.; Suresh, G.S.; Melo, J.S.; Niranjana, P.; Shanmugam, S. Synthesis of one-dimensional gold nanostructures and the electrochemical application of the nanohybrid containing functionalized graphene oxide for cholesterol biosensing. Bioelectrochemistry 2016, 110, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ansari, A.A.; Rafi, M.; Alandis, N.M. Optical and electrical conducting properties of Polyaniline/Tin oxide nanocomposite. ARAB J. Chem. 2013, 6, 341–345. [Google Scholar] [CrossRef]
- Khairkar, S.R.; Raut, A.R. Synthesis of Chitosan-graft-Polyaniline-Based Composites. AJMSE 2014, 4, 62–67. [Google Scholar] [CrossRef]
- Martone, P.T.; Estevez, J.M.; Lu, F.; Ruel, K.; Denny, M.W.; Somerville, C.; Ralph, J. Discovery of lignin in seaweed reveals convergent evolution of cell wall architecture. Curr. Biol. 2009, 19, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Barik, A.; Solanki, P.R.; Kaushik, A. Polyanilinecarboxymethyl cellulose nanocomposite for cholesterol detection. J. Nanosci. Nanotechnol. 2010, 10, 6479–6488. [Google Scholar] [CrossRef] [PubMed]
- Luong, N.D.; Korhonen, J.T.; Soininen, A.J.; Ruokolainen, J.; Johansson, L.S.; Seppälä, J. Processable polyaniline suspensions through in situ polymerization onto nanocellulose. Eur. Polym. J. 2013, 49, 335–344. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, Q.; Liang, B.; Qin, Z. Preparation and Capacitive Properties of Polyaniline/Nanocellulose Composites with Core-Shell Structure. In Proceedings of the International Conference on Advances in Energy, Environment and Chemical Engineering, Changsha, China, 26–27 September 2015. [Google Scholar]
- Ede, S.R.; Nithiyanantham, U.; Kundu, S. DNA-encapsulated chain and wire-like β-MnO2 organosol for oxidative polymerization of pyrrole to polypyrrole. Phys. Chem. Chem. Phys. 2015, 17, 5474–5484. [Google Scholar] [CrossRef] [PubMed]
- Murugan, A.V.; Muraliganth, T.; Manthiram, A. Rapid, facile microwave-solvothermal synthesis of graphene nanosheets and their polyaniline nanocomposites for energy strorage. Chem. Mater. 2009, 21, 5004–5006. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Song, W.; Liu, Z. Controllable Synthesis of Conducting Polypyrrole Nanostructures. J. Phys. Chem. 2006, 110, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Ayad, M.; El-Hefnawy, G.; Zaghlol, S. Facile synthesis of polyaniline nanoparticles; its adsorption behavior. Chem. Eng. J. 2013, 217, 460–465. [Google Scholar] [CrossRef]
- Kondawar, S.B.; Deshpande, M.D.; Agrawal, S.P. Transport Properties of Conductive Polyaniline Nanocomposites Based on Carbon Nanotubes. J. Compos. Mater. 2012, 2, 32–36. [Google Scholar] [CrossRef]
- Terinte, N.; Ibbett, R.; Schuster, K.C. Overview on native cellulose and microcrystalline cellulose I structure studied by X-ray diffraction (WAXD): Comparison between measurement techniques. Lenzinger Berichte 2011, 89, 118–131. [Google Scholar]
- Joelma, M.; Cardoso, R.; Fogliato, M.; Lima, S.; Lenz, D.M. Polyaniline Synthesized with Functionalized Sulfonic Acids for Blends Manufacture. Mater. Res. 2007, 10, 425–429. [Google Scholar]
- Zhang, X.; Zhu, J.; Haldolaarachchige, N.; Ryu, J.; Young, D.P.; Wei, S.; Guo, Z. Synthetic process engineered polyaniline nanostructures with tunable morphology and physical properties. Polymer 2012, 53, 2109–2120. [Google Scholar] [CrossRef]
- Casado, U.M.; Aranguren, M.I.; Marcovich, N.E. Preparation and characterization of conductive nanostructured particles based on polyaniline and cellulose nanofibers. Ultrason. Sonochem. 2014, 21, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Zhao, Z.; Chen, H.; Niu, G.; Shi, H. Heterogeneous preparation of cellulose–polyaniline conductive composites with cellulose activated by acids and its electrical properties. Carbohydr. Polym. 2009, 75, 660–664. [Google Scholar] [CrossRef]
- Goworek, J.; Kierys, A.; Gac, W.; Borówka, A.; Kusak, R. Thermal degradation of CTAB in as-synthesized MCM-41. J. Therm. Anal. Calorim. 2009, 96, 375–382. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdi, M.M.; Md Tahir, P.; Liyana, R.; Javahershenas, R. A Surfactant Directed Microcrystalline Cellulose/Polyaniline Composite with Enhanced Electrochemical Properties. Molecules 2018, 23, 2470. https://doi.org/10.3390/molecules23102470
Abdi MM, Md Tahir P, Liyana R, Javahershenas R. A Surfactant Directed Microcrystalline Cellulose/Polyaniline Composite with Enhanced Electrochemical Properties. Molecules. 2018; 23(10):2470. https://doi.org/10.3390/molecules23102470
Chicago/Turabian StyleAbdi, Mahnaz M., Paridah Md Tahir, Rawaida Liyana, and Ramin Javahershenas. 2018. "A Surfactant Directed Microcrystalline Cellulose/Polyaniline Composite with Enhanced Electrochemical Properties" Molecules 23, no. 10: 2470. https://doi.org/10.3390/molecules23102470