Measurements, Thermodynamic Modeling, and a Hydrogen Bonding Study on the Solubilities of Metoprolol Succinate in Organic Solvents
Abstract
:1. Introduction
2. Results
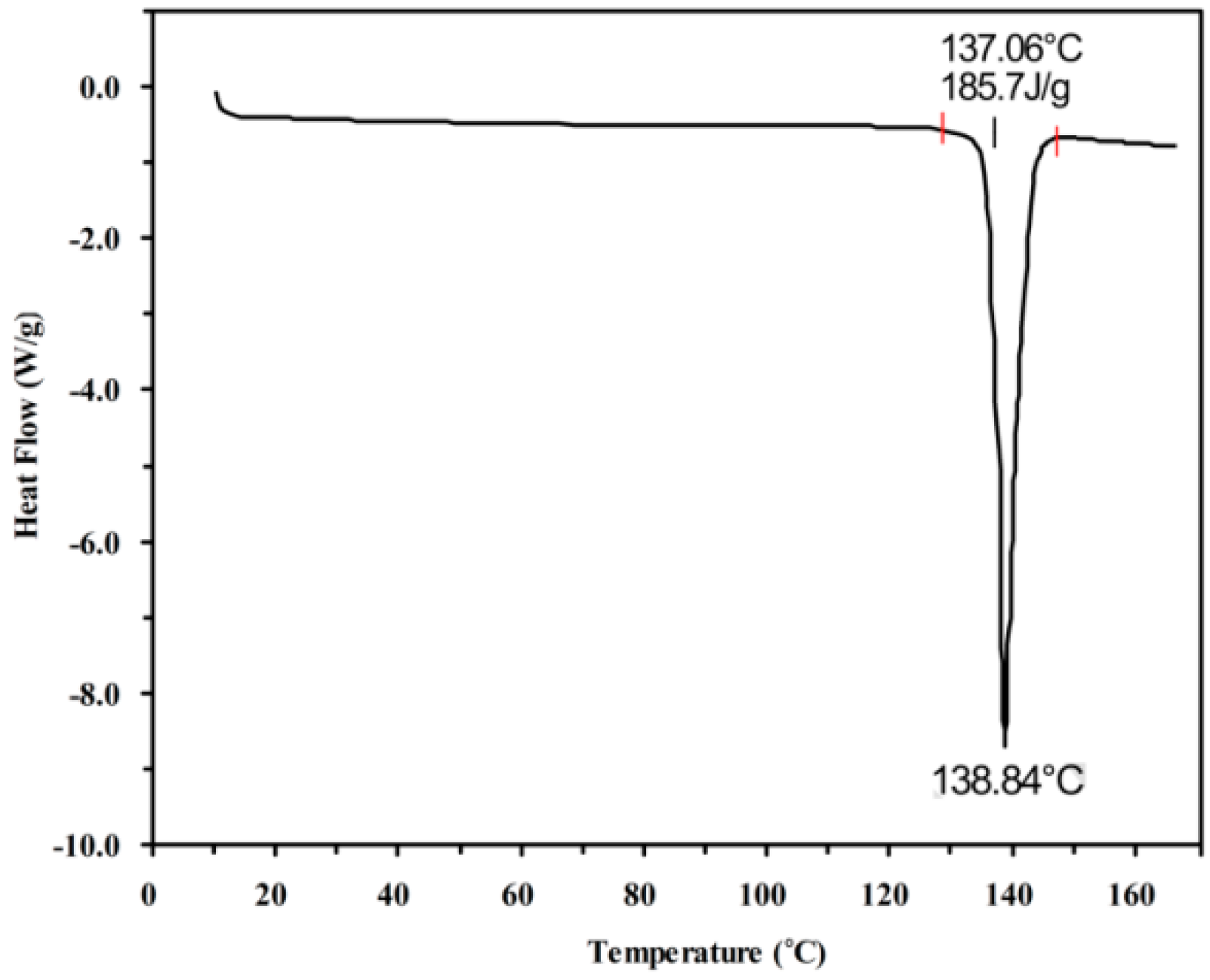
2.1. Melting Point and Enthalpy of Fusion
2.2. Solubilities
2.3. Thermodynamic Functions of Dissolution
2.4. Phase Equilibrium Models and Correlations
2.4.1. Modified Apelblat Equation
2.4.2. Wilson Model
2.4.3. NRTL Model
2.4.4. Correlation Results
2.5. QSPR and DFT Studies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. HPLC Analysis
4.3. Solubility Measurements
4.4. Thermal Analysis
4.5. Theoretical Calculations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Falkner, B.; Francos, G.; Kushner, H. Metoprolol succinate, a selective beta-adrenergic blocker, has no effect on insulin sensitivity. J. Clin. Hypertens. 2006, 8, 336–343. [Google Scholar] [CrossRef]
- Frishman, W.H.; Hainer, J.W.; Sugg, J. A factorial study of combination hypertension treatment with metoprolol succinate extended release and felodipine extended, release: Results of the metoprolol succinate-felodipine antihypertension combination trial (M-FACT). Am. J. Hypertens. 2006, 19, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.B.; More, K.R.; Gupta, L.; Jha, M.S.; Magar, L. Identification, synthesis, isolation and characterization of new impurity in metoprolol tartrate tablets. J. Pharm. Biomed. Anal. 2016, 117, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.S.; Chen, Y.; Yuan, L. Synthesis of related substances E and J of metoprolol. Chin. J. Med. Chem. 2013, 23, 393–396. [Google Scholar]
- Chen, J.; Sarma, B.; Evans, J.M.B.; Myerson, A.S. Pharmaceutical crystallization. Cryst. Growth Des. 2011, 11, 887–895. [Google Scholar] [CrossRef]
- Paoli, P.; Rossi, P.; Macedi, E.; Ienco, A.; Chelazzi, L.; Bartolucci, G.L.; Bruni, B. Similar but Different: The Case of Metoprolol Tartrate and Succinate Salts. Cryst. Growth Des. 2016, 16, 789–799. [Google Scholar] [CrossRef]
- Fan, J.P.; Cao, Y.H.; Zhang, X.H.; Jiang, D.Q.; Yu, J.X. Determination and Modeling of the Solubilities of Oleanolic Acid and Ursolic Acid in Ethanol + Sodium Hydroxide + Water Mixed Solvents from T = 283.2 to 323.2 K. J. Chem. Eng. Data 2017, 62, 3991–3997. [Google Scholar] [CrossRef]
- Mabhoot, A.; Jouyban, A. Solubility of sodium phenytoin in ethanol +water mixtures at various temperatures. Chem. Eng. Commun. 2016, 203, 1009–1012. [Google Scholar] [CrossRef]
- Shakeel, F.; Alajmi, M.F.; Haq, N.; Siddiqui, N.A.; Alam, P.; Al-Rehaily, A.J. Solubility and thermodynamic function of a bioactive compound bergenin in various pharmaceutically acceptable neat solvents at different temperatures. J. Chem. Thermodyn. 2016, 101, 19–24. [Google Scholar] [CrossRef]
- Siddique, S.; Bose, A.; Khanam, J. Modulation of drug (metoprolol succinate) release by inclusion of hydrophobic polymer in hydrophilic matrix. Drug Dev. Ind. Pharm. 2011, 37, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Polli, J.E.; Rekhi, G.S.; Augsburger, L.L.; Shah, V.P. Methods to compare dissolution profiles and a rationale for wide dissolution specifications for metoprolol tartrate tablets. J. Pharm. Sci. 1997, 86, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Du, C.; Zhao, H. Solubility, solution thermodynamics and preferential solvation for 4-bromopyrazole in mixed solvents of (methanol/ethanol + water) from T = (283.15 to 318.15) K. J. Chem. Thermodyn. 2017, 112, 146–154. [Google Scholar] [CrossRef]
- Deosarkar, S.D.; Kalyankar, T.M. Structural properties of aqueous metoprolol succinate solutions. Density, viscosity, and refractive index at 311 K. Russ. J. Phys. Chem. A 2013, 87, 1060–1062. [Google Scholar]
- Ha, E.S.; Kuk, D.H.; Ha, D.H.; Sim, W.Y.; Baek, I.H.; Kim, J.S.; Kim, M.S. Determination and correlation of solubility of sarpogrelate hydrochloride in eight solvents at different temperatures. J. Mol. Liq. 2017, 237, 141–145. [Google Scholar] [CrossRef]
- Yao, G.B.; Yao, Q.C.; Xia, Z.X.; Li, Z.H. Solubility determination and thermodynamic modelling of 3,5-dimethylpyrazole in nine organic solvents from T = (283.15 to 313.15) K and mixing properties of solutions. J. Chem. Thermodyn. 2017, 110, 99–109. [Google Scholar] [CrossRef]
- Mylangam, C.K.; Beeravelli, S.; Medikonda, J.; Pidaparthi, J.S.; Kolapalli, V.R. Badam gum: A natural polymer in mucoadhesive drug delivery. design, optimization, and biopharmaceutical evaluation of badam gum-based metoprolol succinate buccoadhesive tablets. Drug Deliv. 2014, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khoubnasabjafari, M.; Shayanfar, A.; Martinez, F.; Acree, W.E.; Jouyban, A. Generally trained models to predict solubility of drugs in carbitol + water mixtures at various temperatures. J. Mol. Liq. 2016, 219, 435–438. [Google Scholar] [CrossRef]
- Shu, M.; Zhu, L.; Yuan, M.; Wang, L.Y.; Wang, Y.F.; Yang, L.B.; Sha, Z.L.; Zeng, M. Solubility and Solution Thermodynamic Properties of 4-(4-Hydroxyphenyl)-2-butanone (Raspberry Ketone) in Different Pure Solvents. J. Solut. Chem. 2017, 46, 1995–2013. [Google Scholar] [CrossRef]
- Mottahedin, P.; Asl, A.H.; Lotfollahi, M.N. Experimental and modeling investigation on the solubility of β-carotene in pure and ethanol-modified subcritical water. J. Mol. Liq. 2017, 237, 257–265. [Google Scholar] [CrossRef]
- Fang, S.; Zuo, X.B.; Xu, X.J.; Ren, D.H. Density, Viscosity and Excess Molar Volume of Binary Mixtures of Tri-n-octylamine + Diluents (n-Heptane, n-Octane, n-Nonane and n-Decane) at Various Temperatures. J. Chem. Thermodyn. 2014, 68, 281–287. [Google Scholar] [CrossRef]
- Shakeel, F.; Salem-Bekhit, M.M.; Haq, N.; Siddiqui, N.A. Solubility and thermodynamics of ferulic acid in different neat solvents: measurement, correlation and molecular interactions. J. Mol. Liq. 2017, 236, 144–150. [Google Scholar] [CrossRef]
- Wilson, G.M. Vapor–liquid equilibrium XI: A new expression for the excess free energy of mixing. J. Am. Chem. Soc. 1964, 86, 127–130. [Google Scholar] [CrossRef]
- Renon, H.; Prausnitz, J.M. Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 1968, 14, 135–144. [Google Scholar] [CrossRef]
- Chen, W.; Su, B.G.; Xing, H.B.; Yang, Y.W.; Ren, Q.L. Solubilities of cholesterol and desmosterol in binary solvent mixtures of n-hexane + ethanol. Fluid Phase Equilib. 2009, 287, 1–6. [Google Scholar] [CrossRef]
- Fang, S.; Fu, Y.Y.; Wang, Q.; Zhang, G.Y. Mixing properties of tris(2-ethylhexyl) phosphate with alkanes at different temperatures and data treatment using several correlation equations based on Eyring’s absolute reaction theory. J. Mol. Liq. 2010, 154, 111–116. [Google Scholar] [CrossRef]
- Žilnik, L.F.; Jazbinšek, A.; Hvala, A.; Vrečer, F.; Klamt, A. Solubility of sodium diclofenac in different solvents. Fluid Phase Equilib. 2007, 261, 140–145. [Google Scholar] [CrossRef]
- Grant, D.J.W.; Mehdizadeh, M.; Chow, A.H.-L.; Fairbrother, J.E. Non-linear van’t Hoff solubility-temperature plots and their pharmaceutical interpretation. Int. J. Pharm. 1984, 18, 25–38. [Google Scholar] [CrossRef]
- Zhu, P.P.; Chen, Y.X.; Fang, J.; Wang, Z.L.; Xie, C.; Hou, B.H.; Chen, W.; Xu, F.X. Solubility and solution thermodynamics of thymol in six pure organic solvents. J. Chem. Thermodyn. 2016, 92, 198–206. [Google Scholar] [CrossRef]
- Xie, Y.; Shi, H.W.; Du, C.B.; Cong, Y.; Zhao, H.K. Modeling of solubility of 1,5-dinitro-naphthalen in eight organic solvents from T = (273.15 to 313.15) K and dissolution properties. J. Mol. Liq. 2016, 221, 1054–1062. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, F.B.; Zhang, G.L.; Ma, J.C.; Zhao, L. Solubility of sebacic acid in binary water plus ethanol solvent mixtures. J. Chem. Eng. Data 2008, 53, 838–840. [Google Scholar] [CrossRef]
- Li, J.; Hao, H.X.; Guo, N.N.; Wang, N.; Hao, Y.H.; Luan, Y.N.; Chen, K.; Huang, X. Solubility and thermodynamic properties of maltol in different pure solvents. J. Mol. Liq. 2017, 243, 313–323. [Google Scholar] [CrossRef]
- Liu, J.P.; Wilding, W.V.; Giles, N.F.; Rowley, R.L. A quantitative structure property relation correlation of the dielectric constant for organic chemicals. J. Chem. Eng. Data 2010, 55, 41–45. [Google Scholar] [CrossRef]
- Liang, X.R.; Chen, X.Y.; Chen, F.Y.; Su, W.K. Solubility of avermectin B1a in some pure and mixed solvents from (278.2 to 318.2) K. J. Chem. Eng. Data 2010, 55, 2340–2342. [Google Scholar] [CrossRef]
- Liang, X.R.; Si, C.X.; Chen, F.Y.; Su, W.K.; Yu, X.N. Solubility of tiamulin hydrogen fumarate in acetone, acetonitrile, ethyl acetate, ethyl formate, and butyl acetate from (288.2 to 318.2) K. J. Chem. Eng. Data 2009, 54, 1126–1128. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. 3. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Fox. Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
Sample Availability: Samples of Metoprolol Succinate are not available from the authors. |

| T/K | Methanol | Ethanol | n-Butanol | n-Propanol | Isopropanol | Ethyl Acetate | Acetone |
|---|---|---|---|---|---|---|---|
| 288.2 | 2.845 ± 0.068 | 0.435 ± 0.010 | 0.177 ± 0.006 | 0.165 ± 0.006 | 0.074±0.002 | 0.019 ± 0.002 | 0.061 ± 0.004 |
| 293.2 | 3.548 ± 0.096 | 0.559 ± 0.012 | 0.259 ± 0.001 | 0.258 ± 0.008 | 0.109 ± 0.002 | 0.028 ± 0.005 | 0.089 ± 0.002 |
| 298.2 | 4.741 ± 0.107 | 0.822 ± 0.015 | 0.377 ± 0.019 | 0.373 ± 0.006 | 0.160 ± 0.001 | 0.040 ± 0.003 | 0.130 ± 0.002 |
| 303.2 | 6.424 ± 0.228 | 1.047 ± 0.009 | 0.536 ± 0.024 | 0.548 ± 0.005 | 0.219 ± 0.003 | 0.058 ± 0.004 | 0.173 ± 0.004 |
| 308.2 | 8.745 ± 0.091 | 1.416 ± 0.050 | 0.788 ± 0.065 | 0.831 ± 0.019 | 0.316 ± 0.011 | 0.084 ± 0.006 | 0.234 ± 0.008 |
| 313.2 | 12.547 ± 0.012 | 2.175 ± 0.084 | 1.111 ± 0.085 | 1.240 ± 0.027 | 0.465 ± 0.027 | 0.118 ± 0.009 | 0.299 ± 0.003 |
| 318.2 | 16.631 ± 0.112 | 3.172 ± 0.098 | 1.567 ± 0.086 | 1.795 ± 0.012 | 0.659 ± 0.053 | 0.165 ± 0.008 | 0.425 ± 0.015 |
| Solvent | ΔHsol,apparent/(kJ·mol−1) | ΔGsol,apparent/(kJ·mol−1) | ΔSsol,apparent/(J·kJ−1·mol−1) |
|---|---|---|---|
| Methanol | 45.87 | 12.63 | 109.74 |
| Ethanol | 50.11 | 17.14 | 108.87 |
| n-Butanol | 55.50 | 18.96 | 120.63 |
| n-Propanol | 60.48 | 18.88 | 137.34 |
| Isopropanol | 55.26 | 21.17 | 112.54 |
| Ethyl acetate | 54.94 | 24.59 | 100.20 |
| Acetone | 48.06 | 21.90 | 86.37 |
| Solvent | Methanol | Ethanol | n-Butanol | n-Propanol | Isopropanol | Ethyl Acetate | Acetone |
|---|---|---|---|---|---|---|---|
| Modified Apelblat equation | |||||||
| A | −632.7 | −120.6 | −133.5 | −144.8 | −238.9 | −134.8 | −117.7 |
| B | 23603.6 | −1.76 | −2.05 | −2.31 | 4736.27 | −2.08 | −1.70 |
| C | 96.21 | 19.92 | 22.05 | 24.03 | 37.61 | 21.88 | 19.08 |
| Wilson model | |||||||
| a12 | −17.95 | −13.26 | −15.27 | −10.30 | −23.11 | −20.08 | −24.62 |
| b12 | 8001.6 | 2711.6 | 5797.5 | 2208.8 | 5882.2 | 6752.4 | 8799.2 |
| a21 | −35.33 | 0.41 | −2.35 | 0.39 | 1.33 | −2.00 | −0.76 |
| b21 | 9900.8 | 602.7 | 949.3 | 552.1 | 256.3 | 750.8 | 0.4 |
| NRTL model | |||||||
| a12 | 0.0687 | 0.7800 | 345.90 | 387.52 | −0.0685 | 1.8082 | 0.1989 |
| b12 | −938.5 | −1045.6 | −1426.6 | −1202.9 | −736.6 | −1213.6 | −903.2 |
| a21 | 5.41 | 4.16 | 21.02 | 19.00 | 13.10 | 6.59 | 10.73 |
| b21 | −110.5 | −173.3 | −7899.9 | −7300.7 | −2779.1 | −972.6 | −1293.4 |
| Solvent | ARD% | ||
|---|---|---|---|
| Modified Apelblat | NRTL Model | Wilson Model | |
| Methanol | 1.50 | 1.59 | 0.85 |
| ethanol | 5.29 | 4.17 | 2.38 |
| n-Butanol | 0.47 | 0.92 | 0.44 |
| n-Propanol | 1.16 | 1.98 | 1.27 |
| Isopropanol | 1.28 | 1.29 | 1.17 |
| Ethyl acetate | 1.06 | 2.41 | 0.64 |
| Acetone | 2.73 | 4.71 | 2.34 |
| Average | 1.93 | 2.44 | 1.30 |
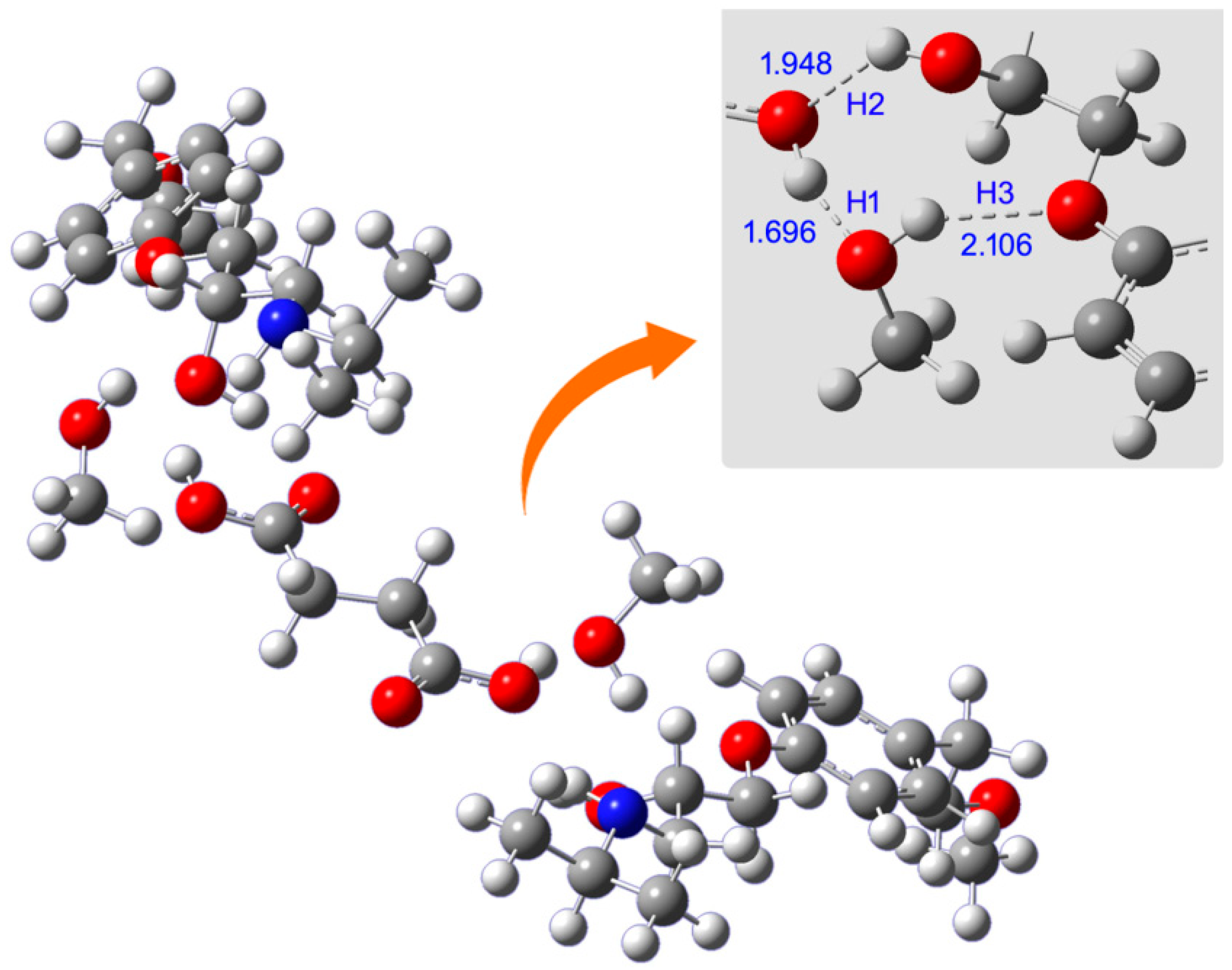
| Solvent | Bond Distance/Å | ||
|---|---|---|---|
| H1 | H2 | H3 | |
| Methanol | 1.696 | 1.948 | 2.106 |
| Ethanol | 3.851 | 1.975 | 2.023 |
| n-Butanol | 3.870 | 1.998 | 2.051 |
| n-Propanol | 3.877 | 1.997 | 2.048 |
| Isopropanol | 3.865 | 1.960 | 2.256 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Liang, X.; Lei, H. Measurements, Thermodynamic Modeling, and a Hydrogen Bonding Study on the Solubilities of Metoprolol Succinate in Organic Solvents. Molecules 2018, 23, 2469. https://doi.org/10.3390/molecules23102469
Shen J, Liang X, Lei H. Measurements, Thermodynamic Modeling, and a Hydrogen Bonding Study on the Solubilities of Metoprolol Succinate in Organic Solvents. Molecules. 2018; 23(10):2469. https://doi.org/10.3390/molecules23102469
Chicago/Turabian StyleShen, Jian, Xianrui Liang, and Hao Lei. 2018. "Measurements, Thermodynamic Modeling, and a Hydrogen Bonding Study on the Solubilities of Metoprolol Succinate in Organic Solvents" Molecules 23, no. 10: 2469. https://doi.org/10.3390/molecules23102469




