A Concise Review of the Conflicting Roles of Dopamine-1 versus Dopamine-2 Receptors in Wound Healing
Abstract
:1. Introduction
2. Influence on Angiogenesis and Dermal Wound Healing
3. Influence on Epidermis and Keratinocytes
4. Influence on Mesenchymal Stem Cells
5. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seiffert, K. Regulation of cutaneous immunity by catecholamines. In Neuroimmunology of the Skin: Basic Science to Clinical Practice; Granstein, R.D., Luger, T.A., Eds.; Springer: Berlin, Germany, 2008; pp. 65–74. [Google Scholar]
- Kim, L.R.; Whelpdale, K.; Zurowski, M.; Pomeranz, B. Sympathetic denervation impairs epidermal healing in cutaneous wounds. Wound Repair Regen. 1998, 6, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Sivamani, R.K.; Lam, S.T.; Isseroff, R.R. Beta adrenergic receptors in keratinocytes. Dermatol. Clin. 2007, 25, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012, 212, 1–98. [Google Scholar]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.M.; Gainetdinov, R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.; Arkinstall, S. Dopamine receptors. In The G-Protein Linked Receptor Fact Book; Academic Press: London, UK, 1994; pp. 97–110. [Google Scholar]
- Tammaro, A.; Cavallotti, C.; Gaspari, A.A.; Narcisi, A.; Parisella, F.R.; Cavallotti, C. Dopaminergic receptors in the human skin. J. Biol. Regul. Homeost. Agents 2012, 26, 789–795. [Google Scholar] [PubMed]
- Chakroborty, D.; Sarkar, C.; Lu, K.; Bhat, M.; Dasgupta, P.S.; Basu, S. Activation of Dopamine D1 Receptors in Dermal Fibroblasts Restores Vascular Endothelial Growth Factor-A Production by These Cells and Subsequent Angiogenesis in Diabetic Cutaneous Wound Tissues. Am. J. Pathol. 2016, 186, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Reimann, E.; Kingo, K.; Karelson, M.; Reemann, P.; Loite, U.; Keermann, M.; Abram, K.; Vasar, E.; Silm, H.; Koks, S. Expression profile of genes associated with the dopamine pathway in vitiligo skin biopsies and blood sera. Dermatology 2012, 224, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Fuziwara, S.; Suzuki, A.; Inoue, K.; Denda, M. Dopamine D2-like receptor agonists accelerate barrier repair and inhibit the epidermal hyperplasia induced by barrier disruption. J. Investig. Dermatol. 2005, 125, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Ganju, R.K.; Pompili, V.J.; Chakroborty, D. Enhanced peripheral dopamine impairs post-ischemic healing by suppressing angiotensin receptor type 1 expression in endothelial cells and inhibiting angiogenesis. Angiogenesis 2017, 20, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Ramchand, C.N.; Clark, A.E.; Ramchand, R.; Hemmings, G.P. Cultured human keratinocytes as a model for studying the dopamine metabolism in schizophrenia. Med. Hypotheses 1995, 44, 53–57. [Google Scholar] [CrossRef]
- Arreola, R.; Alvarez-Herrera, S.; Perez-Sanchez, G.; Becerril-Villanueva, E.; Cruz-Fuentes, C.; Flores-Gutierrez, E.O.; Garces-Alvarez, M.E.; de la Cruz-Aguilera, D.L.; Medina-Rivero, E.; Hurtado-Alvarado, G.; et al. Immunomodulatory Effects Mediated by Dopamine. J. Immunol. Res. 2016, 2016, 3160486. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Brachvogel, B.; Odorisio, T.; Koch, M. Regulation of angiogenesis: Wound healing as a model. Progress Histochem. Cytochem. 2007, 42, 115–170. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 2002, 20, 4368–4380. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. VEGF-A: A critical regulator of blood vessel growth. Eur. Cytokine Netw. 2009, 20, 158–163. [Google Scholar] [PubMed]
- Shome, S.; Rana, T.; Ganguly, S.; Basu, B.; Chaki Choudhury, S.; Sarkar, C.; Chakroborty, D.; Dasgupta, P.S.; Basu, S. Dopamine regulates angiogenesis in normal dermal wound tissues. PLoS ONE 2011, 6, e25215. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.L.; Myers, C.A.; Charboneau, A.; Young, D.M.; Boudreau, N. HoxD3 accelerates wound healing in diabetic mice. Am. J. Pathol. 2003, 163, 2421–2431. [Google Scholar] [CrossRef]
- Uyeno, L.A.; Newman-Keagle, J.A.; Cheung, I.; Hunt, T.K.; Young, D.M.; Boudreau, N. Hox D3 expression in normal and impaired wound healing. J. Surg. Res. 2001, 100, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, N.J.; Varner, J.A. The homeobox transcription factor HoxD3 promotes integrin alpha5beta1 expression and function during angiogenesis. J. Biol. Chem. 2004, 279, 4862–4868. [Google Scholar] [CrossRef] [PubMed]
- Ewing, A.G.; Bigelow, J.C.; Wightman, R.M. Direct in vivo monitoring of dopamine released from two striatal compartments in the rat. Science 1983, 221, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Nagy, J.A.; Pal, S.; Vasile, E.; Eckelhoefer, I.A.; Bliss, V.S.; Manseau, E.J.; Dasgupta, P.S.; Dvorak, H.F.; Mukhopadhyay, D. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat. Med. 2001, 7, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Chakroborty, D.; Mitra, R.B.; Banerjee, S.; Dasgupta, P.S.; Basu, S. Dopamine in vivo inhibits VEGF-induced phosphorylation of VEGFR-2, MAPK, and focal adhesion kinase in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1554–H1560. [Google Scholar] [CrossRef] [PubMed]
- Harper, R.A.; Flaxman, B.A. Effect of pharmacological agents on human keratinocyte mitosis in vitro. II. Inhibition by catecholamines. J. Cell. Physiol. 1975, 86 Pt 1, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Green, H.N.; Ghadially, F.N. Relation of shock, carbohydrate utilization and cortisone to mitotic activity in the epidermis of the adult male mouse. Br. Med. J. 1951, 1, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Bullough, W.S. Mitotic and functional homeostasis: A speculative review. Cancer Res. 1965, 25, 1683–1727. [Google Scholar] [PubMed]
- Aoyagi, T.; Kamigaki, K.; Iizuka, H.; Miura, Y. The effects of db-cAMP and related compounds on the outgrowing epidermis in vitro. J. Dermatol. 1981, 8, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Denda, M.; Sato, J.; Tsuchiya, T.; Elias, P.M.; Feingold, K.R. Low humidity stimulates epidermal DNA synthesis and amplifies the hyperproliferative response to barrier disruption: Implication for seasonal exacerbations of inflammatory dermatoses. J. Investig. Dermatol. 1998, 111, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Denda, M.; Sato, J.; Masuda, Y.; Tsuchiya, T.; Koyama, J.; Kuramoto, M.; Elias, P.M.; Feingold, K.R. Exposure to a dry environment enhances epidermal permeability barrier function. J. Investig. Dermatol. 1998, 111, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Pullar, C.E.; Isseroff, R.R. Cyclic AMP mediates keratinocyte directional migration in an electric field. J. Cell Sci. 2005, 118 Pt 9, 2023–2034. [Google Scholar] [CrossRef] [PubMed]
- Le Poole, I.C.; van den Wijngaard, R.M.; Smit, N.P.; Oosting, J.; Westerhof, W.; Pavel, S. Catechol-O-methyltransferase in vitiligo. Arch. Dermatol. Res. 1994, 286, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Parrado, A.C.; Canellada, A.; Gentile, T.; Rey-Roldan, E.B. Dopamine agonists upregulate IL-6 and IL-8 production in human keratinocytes. Neuroimmunomodulation 2012, 19, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Abe, R.; Fujita, Y.; Ando, S.; Inokuma, D.; Shimizu, H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J. Immunol. 2008, 180, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Karp, J.M.; Leng Teo, G.S. Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell 2009, 4, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Cherng, W.J.; Yang, N.I.; Kuo, L.T.; Hsu, C.M.; Yeh, H.I.; Lan, Y.J.; Yeh, C.H.; Stanford, W.L. Late-outgrowth endothelial cells attenuate intimal hyperplasia contributed by mesenchymal stem cells after vascular injury. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Hocking, A.M.; Gibran, N.S. Mesenchymal stem cells: Paracrine signaling and differentiation during cutaneous wound repair. Exp. Cell Res. 2010, 316, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Paus, R.; Tiede, S.; Day, P.; Bayat, A. Exploring the role of stem cells in cutaneous wound healing. Exp. Dermatol. 2009, 18, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Shome, S.; Dasgupta, P.S.; Basu, S. Dopamine regulates mobilization of mesenchymal stem cells during wound angiogenesis. PLoS ONE 2012, 7, e31682. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Nissen, N.N.; Polverini, P.J.; Koch, A.E.; Volin, M.V.; Gamelli, R.L.; DiPietro, L.A. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am. J. Pathol. 1998, 152, 1445–1452. [Google Scholar] [PubMed]
- Lee, S.H.; Lee, Y.J.; Song, C.H.; Ahn, Y.K.; Han, H.J. Role of FAK phosphorylation in hypoxia-induced hMSCS migration: Involvement of VEGF as well as MAPKS and eNOS pathways. Am. J. Physiol. Cell Physiol. 2010, 298, C847–C856. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Kosugi, T.; Haneda, M.; Rivard, C.J.; Long, D.A. Abnormal angiogenesis in diabetic nephropathy. Diabetes 2009, 58, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Galiano, R.D.; Tepper, O.M.; Pelo, C.R.; Bhatt, K.A.; Callaghan, M.; Bastidas, N.; Bunting, S.; Steinmetz, H.G.; Gurtner, G.C. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am. J. Pathol. 2004, 164, 1935–1947. [Google Scholar] [CrossRef]
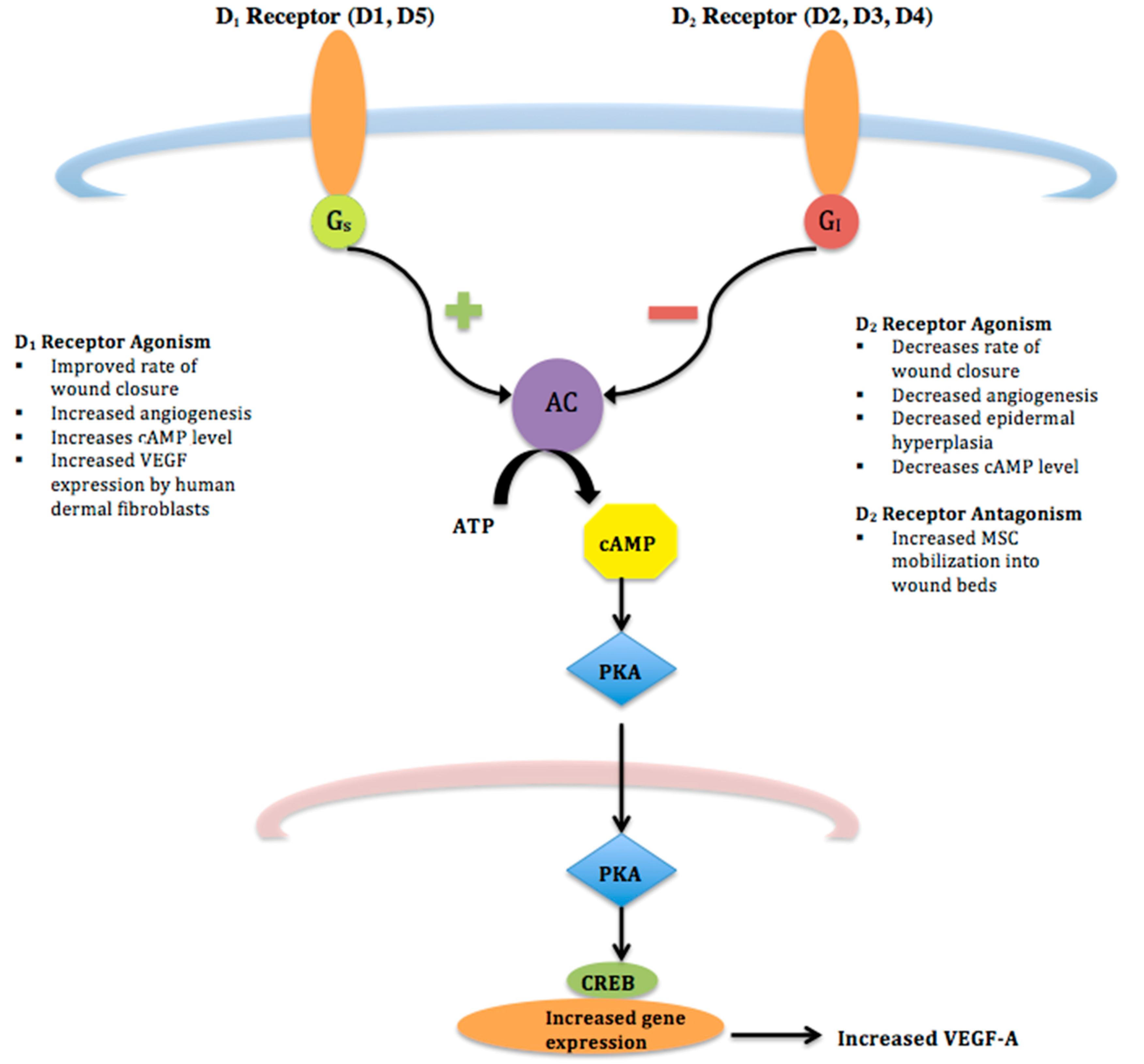
| Agonism of D1 Receptors | Agonism of D2 Receptors | Antagonism of D2 Receptors | |
|---|---|---|---|
| Wound Closure | Increases [9] | Decreases | Increases [20] |
| Angiogenesis | Increases [9] | Decreases [11] | Increases [20] |
| Epidermal hyperplasia | - | Decreases [11] | |
| MSC Mobilization into wound beds | Increases [11] | ||
| VEGF-A Expression | Increases [9] | Decreases [25,26] | |
| Cyclic AMP level | Increases [7] | Decreases [11] | |
| Keratinocyte mitosis | Exogenous DA inhibits keratinocyte mitosis in vitro [27] | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaughn, A.R.; Davis, M.J.; Sivamani, R.K.; Isseroff, R.R. A Concise Review of the Conflicting Roles of Dopamine-1 versus Dopamine-2 Receptors in Wound Healing. Molecules 2018, 23, 50. https://doi.org/10.3390/molecules23010050
Vaughn AR, Davis MJ, Sivamani RK, Isseroff RR. A Concise Review of the Conflicting Roles of Dopamine-1 versus Dopamine-2 Receptors in Wound Healing. Molecules. 2018; 23(1):50. https://doi.org/10.3390/molecules23010050
Chicago/Turabian StyleVaughn, Alexandra R., Michael James Davis, Raja K. Sivamani, and Roslyn Rivkah Isseroff. 2018. "A Concise Review of the Conflicting Roles of Dopamine-1 versus Dopamine-2 Receptors in Wound Healing" Molecules 23, no. 1: 50. https://doi.org/10.3390/molecules23010050




