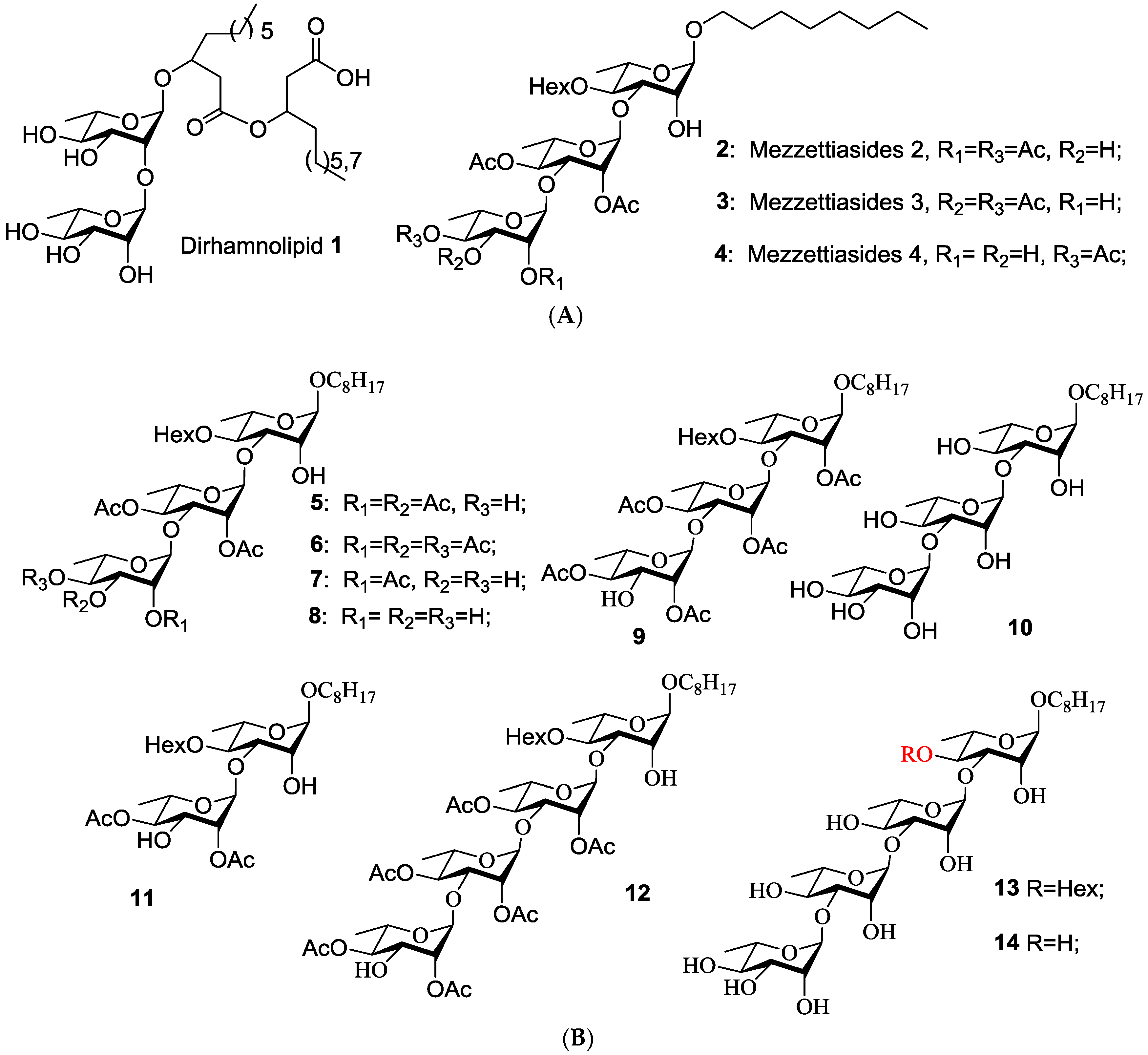
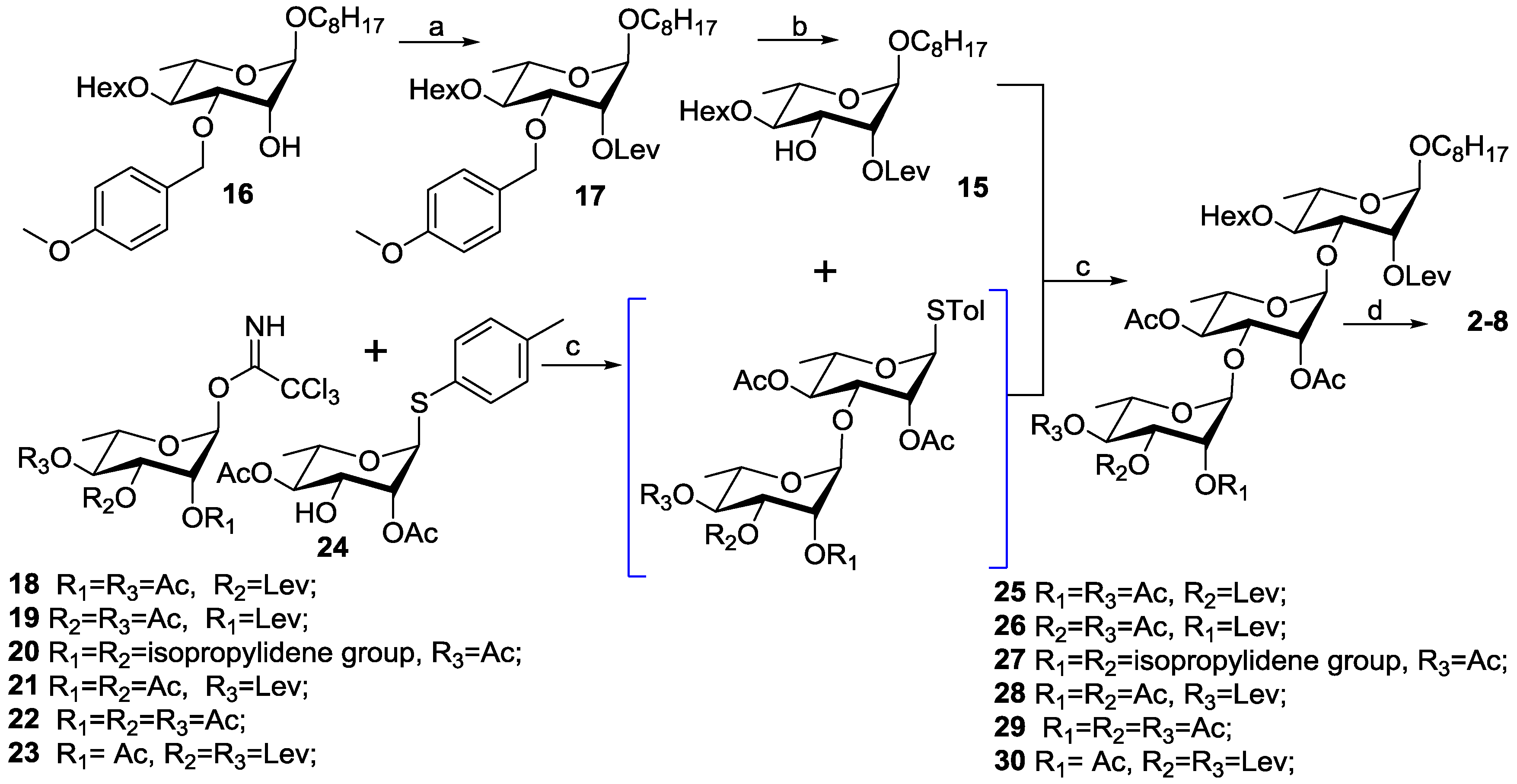
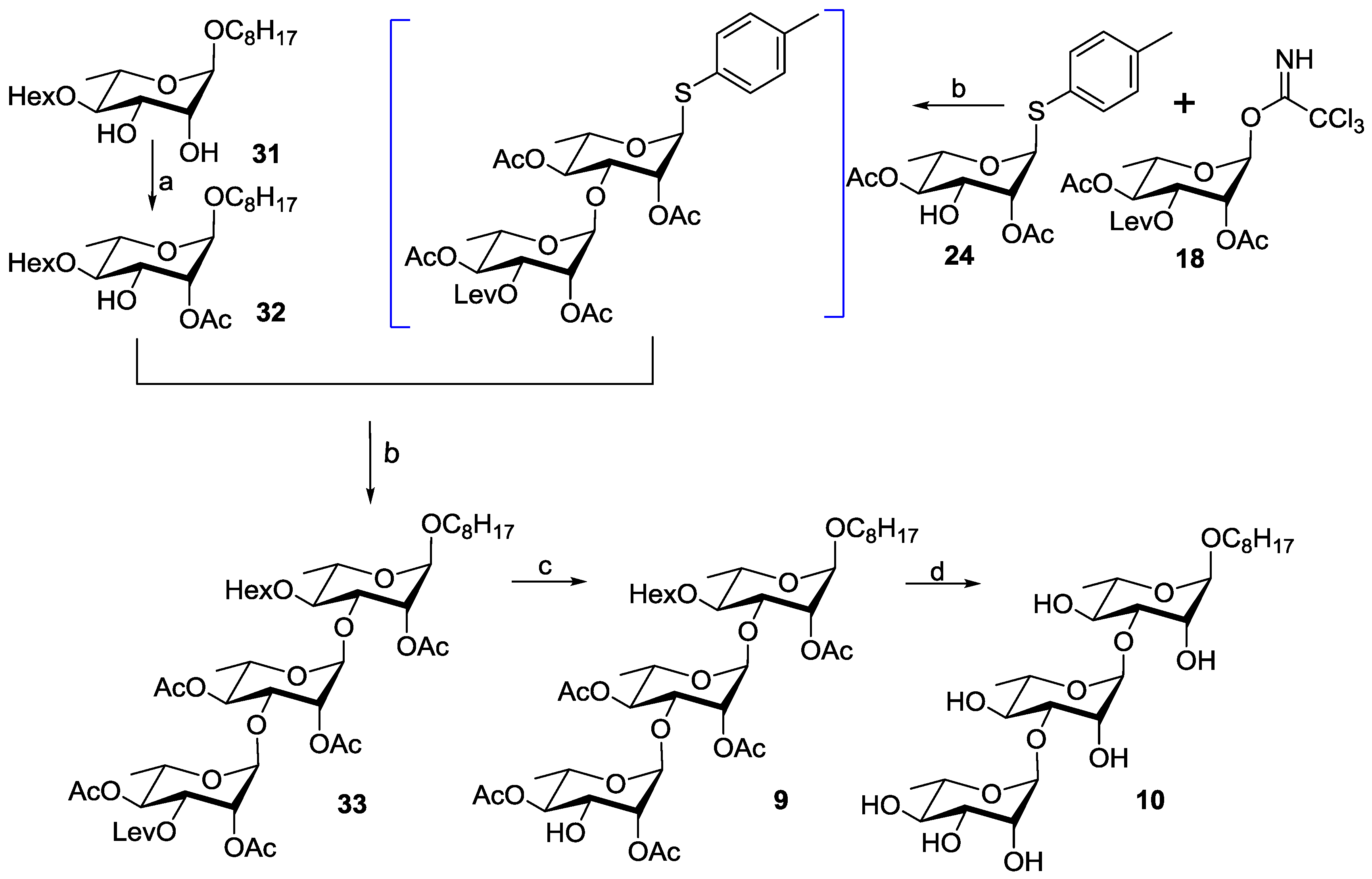
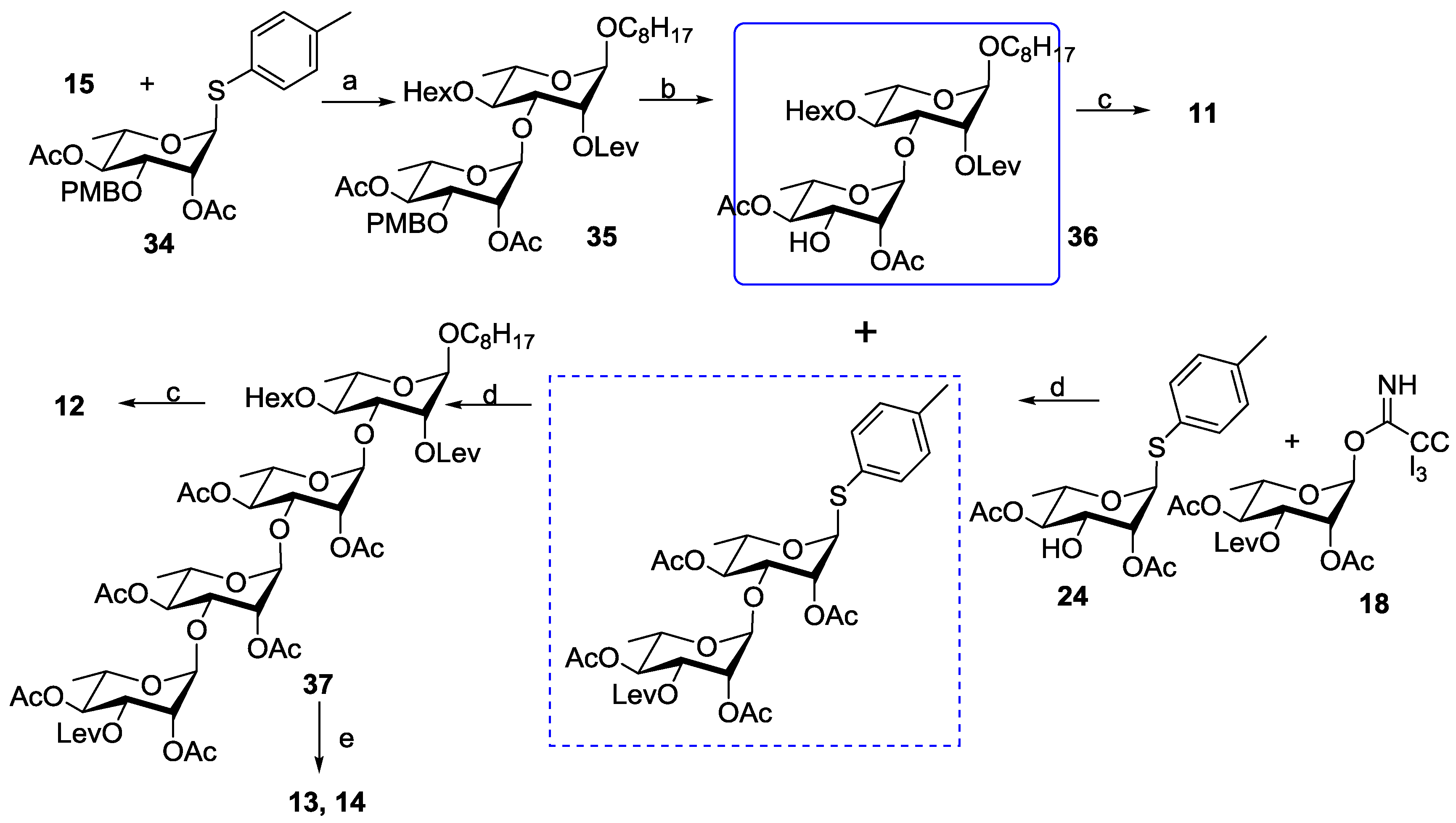
3.2. Chemical Synthesis
3.2.1. 1-Octyloxy-4-O-Hexanoyl-3-O-(P-Methoxybenzyl)-2-O-Levulinoyl-Α-l-Rhamno-Pyranoside (17)
To a solution of the known compound 16 (7.2 g, 14.8 mmol) in dry dichloromethane (120 mL), levulinic acid (12.2 g, 18.6 mmol), 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (4.2 g, 21.6 mmol), and 4-dimethylaminopyridine (0.36 g, 2.9 mmol) were added under argon. The mixture was stirred for 48 h, diluted with CH2Cl2 (200 mL), washed 1 mol·L−1 HCl (2 × 100 mL), satd aq NaHCO3 (2 × 100 mL), and brine (2 × 100 mL), dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (8:1, petroleum ether—EtOAc) to yield 17 (4.0 g, 92%). 1H-NMR (CDCl3): δ 7.18 (d, 2H, J = 8.6 Hz, Ar-H), 6.84 (d, 2H, J = 8.6 Hz, Ar-H), 4.83 (dd, 1H, J = 3.4, 1.9 Hz, H-2’), 4.99 (t, 1H, J = 9.8 Hz, H-4′), 4.72 (d, 1H, J = 1.6 Hz, H-1′), 4.54 (d, 1H, J = 11.6 Hz, Ar-CH2-1), 4.32 (d, 1H, J = 11.6 Hz, Ar-CH2-2), 3.80 (dd, 1H, J = 9.6, 3.2 Hz, H-3′), 3.74–3.76 (m, 1H, H-5′), 3.60–3.65 (m, 1H, H-1-1), 3.36–3.41 (m, 1H, H-1-2), 2.63–2.75 (m, 4H, 2 × COCH2), 2.24–2.27 (m, 2H, COCH2), 2.17 (s, 3H, COCH3), 1.56–1.62 (m, 4H, 2 × CH2), 1.30–1.33 (m, 14H, 7 × CH2), 1.18 (d, 3H, J = 6.2 Hz, H-6′), 0.90 (t, 3H, J = 7.1 Hz, CH3), 0.89 (t, 3H, J = 7.1 Hz, CH3); 13C-NMR (CDCl3): δ 206.4, 172.7, 171.9, 159.2, 130.1, 129.3 (two), 113.5 (two), 97.6 (Rha-C-1), 74.4. 70.8, 68.0, 66.4, 55.2, 38.1, 34.4, 31.8, 31.3, 29.8, 29.4, 29.3, 29.2, 28.3; 26.0, 24.5, 22.7, 22.3, 17.5, 14.1, 13.8; HRESIMS calcd for C33H52O9Na [M + Na]+ 615.3509; found, 615.3520.
3.2.2. 1-Octyloxy-4-O-hexanoyl-2-O-levulinoyl-α-l-rhamnopyranoside (15)
To a stirred mixture of 17 (3.6 g, 6.0 mmol) in CH2Cl2 (54 mL) and H2O (6 mL), 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ, 2.1 g, 9.0 mmol) was added at room temperature. The reaction was stirred for 12 h until the reaction was complete as judged by TLC. The reaction mixture was poured into saturated aqueous NaHCO3 (50 mL) and extracted with CH2Cl2 (2 × 150 mL). The combined organic phase was washed with satd aq NaHCO3 (2 × 150 mL), and brine (2 × 150 mL), dried over Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (5:1, petroleum ether–EtOAc) to provide 15 (2.7 g, 94%). 1H-NMR (CDCl3): δ 5.13 (dd, 1H, J = 3.4, 1.8 Hz, H-2′), 4.85 (t-like, 1H, J = 9.7 Hz, H-4′), 4.72 (d, 1H, J = 1.4 Hz, H-1′), 4.00 (d, 1H, J = 9.2 Hz, H-3′), 3.78–3.83 (m, 1H, H-5′), 3.66 (dt, 1H, J = 9.5, 6.6 Hz, H-1-1), 3.42 (dt, 1H, J = 9.6, 6.5 Hz, H-1-2), 2.81 (t, 2H, J = 6.4 Hz, COCH2CH2CO), 2.67 (t, 2H, J = 6.4 Hz, COCH2CH2CO), 2.37 (td, 2H, J = 7.2, 3.7 Hz, COCH2), 2.21 (s, 3H, CH3CO), 1.63–1.68 (m, 2H, CH2), 1.55–1.61 (m, 2H, H-2), 1.29–1.34 (m, 14H, 7 × CH2), 1.20 (d, 3H, J = 6.4 Hz, H-6′), 0.90 (t, 3H, J = 7.0 Hz, CH3), 0.88 (t, 3H, J = 7.1 Hz, CH3); 13C-NMR (CDCl3): δ 207.1, 174.1, 172.2, 97.2 (Rha-C-1′), 74.4, 72.9, 68.7, 68.1, 66.0, 38.2, 34.3, 31.8, 31.2, 29.4, 29.3, 29.2, 28.2, 26.0, 24.6, 22.6, 22.3, 17.4, 14.1, 13.9; HRESIMS calcd for C25H44O8Na [M + Na]+ 495.2934; found, 495.2947.
3.2.3. General Procedure for the Preparation of 25–30
To a solution of compound 24 (354 mg, 1.0 mmol), 18–23 (637 mg for 18, 19, 21; 508 mg for 20; 566 mg for 22; 711 mg for 23, 1.3 mmol, respectively) and 4 Å molecular sieves in dry CH2Cl2 (20 mL) were added TMSOTf (22 μL, 0.10 mmol) at −78 °C under argon. After stirring at −78 °C for 0.5 h and then at 0 °C for 0.5 h, to the reaction mixture was added 15 (378 mg, 0.80 mmol) in dried CH2Cl2 (2 mL) under argon. After stirring at 0 °C for 0.5 h, N-iodosuccinimide (289 mg, 1.30 mmol) and silver trifluoromethanesulfonate (46 mg, 0.16 mmol) were added. The reaction was stirred for an additional 4 h while warming to room temperature. After the reaction was complete as judged by TLC, the reaction mixture was filtered and concentrated under reduced pressure. Then the residue was diluted with CH2Cl2 (100 mL), and washed with aqueous Na2S2O3 (50 mL), satd aq NaHCO3 (50 mL), and brine (2 × 50 mL). The organic layer was dried over Na2SO4, and concentrated in vacuo. The filtrate was concentrated and purified by silica gel column chromatography (1:2, EtOAc- petroleum ether) to furnish 25–30 as a white amorphous solid, respectively.
1-Octyloxy-2,4-di-O-acetyl-3-O-levulinoylα-l-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-4-O-hexanoyl-2-O-levulinoyl-α-l-rhamno-pyranoside (25). 86% for two steps; 1H-NMR (CDCl3): δ 5.29 (dd, 1H, J = 3.6, 1.7 Hz, H-2′′′), 5.27 (dd, 1H, J = 10.1, 3.5 Hz, H-3′′′), 5.19 (d, 1H, J = 2.3 Hz, H-1′), 5.10 (dd, 1H, J = 3.5, 1.7 Hz, H-2′′), 5.05 (t, 1H, J = 10.1 Hz, H-4′′′), 5.00 (t, 1H, J = 9.7 Hz, H-4′′), 4.90 (t, 1H, J = 10.0 Hz, H-4′), 4.85 (d, 1H, J = 1.4 Hz, H-1′′), 4.67 (dd, 1H, J = 4.3, 2.4 Hz, H-2′), 4.61 (d, 1H, J = 1.4 Hz, H-1′′′), 4.20–4.25 (m, 1H, H-5′′′), 4.07 (dd, 1H, J = 9.9, 3.6 Hz, H-3′′), 3.98 (dd, 1H, J = 9.8, 4.3 Hz, H-3′), 3.76–3.79 (m, 1H, H-5′′), 3.60–3.64 (m, 1H, H-5′), 3.43–3.48 (m, 1H, H-1-1), 3.35–3.39 (m, 1H, H-1-2), 2.77–2.87 (m, 2H, CH2CO), 2.73–2.76 (m, 1H, CH2CO-1), 2.60–2.65 (m, 2H, CH2CO), 2.52–2.57 (m, 2H, CH2CO), 2.40–2.45 (m, 1H, CH2CO-2), 2.31 (td, 2H, J = 7.1, 1.3 Hz, COCH2), 2.19, 2.17, 2.14, 2.12, 2.11, 1.70 (each s, each 3H, each CH3CO), 1.54–1.65 (m, 4H, 2 × CH2), 1.26–1.34 (m, 14H, 7 × CH2), 1.20 (d, 3H, J = 6.2 Hz, H-6′), 1.19 (d, 3H, J = 6.2 Hz, H-6), 1.16 (d, 3H, J = 6.2 Hz, H-6′′′), 0.92 (t, 3H, J = 7.0 Hz, CH3), 0.90 (t, 3H, J = 7.0 Hz, CH3); 13C-NMR (CDCl3): δ 206.3, 206.1, 172.3, 172.2, 171.2, 170.4, 170.2, 170.0, 99.8, 97.4, 97.2, 78.8, 77.8, 72.1, 71.3, 71.1, 70.5, 70.2, 69.5, 69.3, 69.1, 68.1, 67.2, 66.4, 37.7, 37.6, 34.3, 31.8, 31.3, 29.9, 29.7, 29.4, 29.3, 29.2, 28.0, 27.8, 26.1, 24.6, 22.6, 22.4, 22.3, 21.0, 20.8, 20.7, 17.6, 17.5, 16.7, 14.1, 13.9; HRESIMS calcd for C50H78O22Na [M + Na]+ 1053.4882; found, 1053.4885.
1-Octyloxy-3,4-di-O-acetyl-2-O-levulinoyl-α-l-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-4-O-hexanoyl-2-O-levulinoyl-α-l-rhamno-pyranoside (26). 88% for two steps; 1H-NMR (CDCl3): δ 5.10–5.14 (m, 3H, H-3′′′, H-2′′, H-2′′′), 5.06 (t, 1H, J = 10.1 Hz, H-4′′′), 5.04 (t, 1H, J = 10.0 Hz, H-4′′), 5.00 (t, 1H, J = 9.7 Hz, H-4′), 4.98 (dd, 1H, J = 3.4, 1.6 Hz, H-2′), 4.96 (d, 1H, J = 1.3 Hz, H-1′′′), 4.84 (d, 1H, J = 1.4 Hz, H-1′′), 4.66 (d, 1H, J = 1.5 Hz, H-1′), 4.11 (dd, 1H, J = 10.0, 3.4 Hz, H-3′′), 4.06 (dd, 1H, J = 9.7, 3.4 Hz, H-3′), 3.74–3.84 (m, 3H, H-5′, H-5′′, H-5′′′), 3.61–3.65 (m, 1H, H-1-1), 3.37–3.41 (m, 1H, H-1-2), 2.69–2.80 (m, 6H, 3 × COCH2), 2.66 (t, 2H, J = 6.5 Hz, COCH2), 2.42–2.48 (m, 1H, COCH2-1), 2.31–2.37 (m, 1H, COCH2-2), 2.22, 2.20, 2.17, 2.13, 2.05, 1.98 (each s, each 3H, each COCH3), 1.54–1.66 (m, 4H, 2 × CH2), 1.29–1.33 (m, 14H, 7 × CH2), 1.19 (d, 3H, J = 6.2 Hz, H-6′), 1.18 (d, 3H, J = 6.2 Hz, H-6′′), 1.17 (d, 3H, J = 6.2 Hz, H-6′′′), 0.90 (t, 3H, J = 7.2 Hz, CH3), 0.89 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (CDCl3): δ 206.0, 205.9, 173.0, 172.0, 171.5, 170.4, 170.2, 170.0, 169.8, 99.4, 98.6, 97.2, 75.2, 74.6, 72.3, 71.8, 71.4, 70.8, 70.0, 68.7, 68.1, 67.3, 67.0, 66.5, 37.8 (two), 34.0, 31.8, 31.3, 29.8, 29.7, 29.3 (two), 29.2, 28.2, 28.0, 26.1, 24.5, 22.6, 22.3, 20.9, 20.8, 20.6, 17.5, 17.2 (two), 14.1, 13.9; HRESIMS calcd for C50H78O22Na [M + Na]+ 1053.4882; found, 1053.4895.
1-Octyloxy-2,3-O-isopropylidene-4-O-acetyl-α-l-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-4-O-hexanoyl-2-O-levulinoyl-α-l-rhamno-pyranoside (27). 83% for two steps; 1H-NMR (CDCl3): δ 5.19 (s, 1H, H-1′′′), 5.13 (dd, 1H, J = 3.2, 1.7 Hz, H-2′′), 5.05 (t, 1H, J = 10.1 Hz, H-4′), 5.03 (t, 1H, J = 10.0 Hz, H-4′′), 4.93 (dd, 1H, J = 3.2, 1.5 Hz, H-2′), 4.82 (d, 1H, J = 1.2 Hz, H-1′′), 4.80 (dd, 1H, J = 10.1, 8.1 Hz, H-4′′′), 4.66 (d, 1H, J = 1.3 Hz, H-1′), 4.18 (dd, 1H, J = 10.0, 3.4 Hz, H-3′′′), 4.05–4.09 (m, 2H, H-3′, H-3′′′), 4.01 (d, 1H, J = 5.5 Hz, H-2′′′), 3.82–3.87 (m, 1H, H-5′′′), 3.75–3.77 (m, 1H, H-5′′), 3.61–3.65 (m, 2H, H-5′, H-1-1), 3.37–3.43 (m, 1H, H-1-2), 2.67–2.87 (m, 4H, 2 × COCH2), 2.30–2.44 (m, 2H, COCH2), 2.24, 2.14, 2.12, 2.09 (each s, each 3H, each CH3CO), 1.56–1.63 (m, 4H, 2 × CH2), 1.53 (s, 3H, CH3), 1.32 (s, 3H, CH3), 1.26–1.30 (m, 14H, 7 × CH2), 1.18 (d, 3H, J = 6.2 Hz, H-6′), 1.17 (d, 3H, J = 6.2 Hz, H-6′′), 1.10 (d, 3H, J = 6.2 Hz, H-6′′′), 0.90 (t, 3H, J = 7.0 Hz, CH3), 0.89 (t, 3H, J = 7.0 Hz, CH3); 13C-NMR (CDCl3): δ 206.1, 172.9, 171.9, 170.3, 170.2 (two), 170.0, 109.6, 99.3, 97.2, 76.1, 75.6, 75.0, 74.2, 74.1, 72.6, 72.2, 72.0, 71.8, 70.8, 68.2, 67.3, 66.5, 64.6, 37.8, 34.0, 31.8, 31.3, 29.7, 29.3 (two), 29.2, 28.3, 27.6, 26.3, 26.1, 24.5, 22.6, 22.3, 21.0 (two), 20.9, 17.4, 17.2, 16.5, 14.1, 13.9; HRESIMS calcd for C49H78O20Na [M + Na]+ 1009.4984; found, 1009.4988.
1-Octyloxy-4-O-hexanoyl-2-O-levulinoyl-α-l-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-2,3-di-O-acetyl-4-O-levulinoyl-α-l-rhamnopyranoside (28). 88% for two steps; 1H-NMR (CDCl3): δ 5.16 (dd, 1H, J = 10.2, 3.4 Hz, H-3′′′), 5.12 (dd, 1H, J = 3.3, 1.7 Hz, H-2′′′), 5.08 (dd, 1H, J = 3.4, 1.8 Hz, H-2′′), 5.04 (t, 1H, J = 9.9 Hz, H-4′′′), 5.03 (t, 1H, J = 9.9 Hz, H-4′′), 5.02 (t, 1H, J = 9.9 Hz, H-4′), 4.99 (dd, 1H, J = 3.2, 1.6 Hz, H-2′), 4.97 (d, 1H, J = 1.7 Hz, H-1′′′), 4.84 (d, 1H, J = 1.3 Hz, H-1′′), 4.64 (d, 1H, J = 1.5 Hz, H-1′), 4.10 (dd, 1H, J = 10.2 3.4 Hz, H-3′′), 4.05 (dd, 1H, J = 10.0, 3.4 Hz, H-3′), 3.74–3.84 (m, 3H, H-5′, H-5′′, H-5′′′), 3.61–3.65 (m, 1H, H-1-1), 3.36–3.40 (m, 1H, H-1-2), 2.68–2.78 (m, 6H, 3 × COCH2), 2.53 (td, 2H, J = 6.4, 2.2 Hz, COCH2), 2.42–2.47 (m, 1H, COCH2-1), 2.31–2.36 (m, 1H, COCH2-2), 2.21, 2.18, 2.16, 2.15, 2.11, 2.01 (each s, each 3H, each CH3CO), 1.55–1.64 (m, 4H, 2 × CH2), 1.26–1.33 (m, 14H, 7 × CH2), 1.18 (d, 3H, J = 6.2 Hz, H-6′′′), 1.17 (d, 3H, J = 6.2 Hz, H-6′′), 1.14 (d, 3H, J = 6.2 Hz, H-6′), 0.89 (t, 3H, J = 7.0 Hz, CH3), 0.89 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (CDCl3): δ 206.1, 206.0, 173.0, 171.9, 171.8, 170.4, 170.2, 170.0, 169.9, 99.4, 98.6, 97.2, 75.2, 74.7, 72.2, 71.9, 71.8, 71.4, 71.0, 70.1, 68.4, 68.2 (two), 67.3, 67.1, 66.6, 37.6, 34.0, 31.8, 31.3, 29.7 (two), 29.3 (two), 28.2, 27.9, 26.1, 24.5, 22.6, 22.3, 20.9 (two), 20.8, 20.7, 17.5, 17.2, 17.1, 14.1, 13.9; HRESIMS calcd for C50H78O22Na [M + Na]+ 1053.4882; found, 1053.4893.
1-Octyloxy-2,3,4-tri-O-acetyl-α-l-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-4-O-hexanoyl-2-O-levulinoyl-α-l-rhamnopyranoside (29). 89% for two steps; 1H-NMR (CDCl3): δ 5.15 (dd, 1H, J = 10.2, 3.4 Hz, H-3′′′), 5.13 (dd, 1H, J = 3.2, 1.6 Hz, H-2′′′), 5.10 (dd, 1H, J = 3.2, 1.8 Hz, H-2′′), 5.07 (t, 1H, J = 9.8 Hz, H-4′′′), 5.01–5.06 (m, 2H, H-4′, H-4′′), 4.98–4.99 (m 2H, H-2′, H-1′′′), 4.84 (d, 1H, J = 1.7 Hz, H-1′′), 4.65 (d, 1H, J = 1.4 Hz, H-1′), 4.11 (dd, 1H, J = 10.0, 3.4 Hz, H-3′′), 4.06 (dd, 1H, J = 10.0, 3.1 Hz, H-3′), 3.75–3.78 (m, 3H, H-5′, H-5′′, H-5′′′), 3.61–3.65 (m, 1H, H-1-1), 3.37–3.41 (m, 1H, H-1-2), 2.77 (t, 2H, J = 6.1 Hz, COCH2), 2.66–2.72 (m, 2H, CH2CO), 2.30–2.78 (m, 2H, CH2CO), 2.22, 2.17, 2.15, 2.13, 2.05, 1.97 (each s, each 3H, each CH3CO), 1.55–1.66 (m, 4H, 2 × CH2), 1.28–1.33 (m, 14H, 7 × CH2), 1.18 (d, 3H, J = 6.2 Hz, H-6′), 1.17 (d, 6H, J = 6.2 Hz, H-6′′, H-6′′′), 0.90 (t, 6H, J = 7.0 Hz, 2 × CH3); 13C-NMR (CDCl3): δ 206.0, 173.0, 171.9, 170.4, 170.2, 170.1, 170.0, 169.7, 99.4, 98.7, 97.2, 75.2, 74.8, 72.3, 71.9, 71.8, 71.4, 70.9, 70.0, 68.7, 68.2, 67.3, 67.1, 66.5, 37.7, 34.0, 31.8, 31.3, 29.7 (two), 29.3 (two), 29.2, 28.2, 26.1, 24.5, 22.6, 22.3, 20.9 (two), 20.8, 20.7, 17.5, 17.2 (two), 14.1, 13.9; HRESIMS calcd for C47H74O21Na [M + Na]+ 997.4620; found, 997.4630.
1-Octyloxy-2-O-acetyl-3,4-di-O-levulinoyl-α-l-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-4-O-hexanoyl-2-O-levulinoyl-α-l-rhamno-pyranoside (30). 84% for two steps; 1H-NMR (CDCl3): δ 5.16 (dd, 1H, J = 10.3, 3.4 Hz, H-3′′′), 5.12 (dd, 1H, J = 3.3, 1.7 Hz, H-2′′′), 5.06 (t, 1H, J = 9.8 Hz, H-4′′′), 5.05 (dd, 1H, J = 3.4, 1.7 Hz, H-2′′), 5.04 (t, 1H, J = 9.9 Hz, H-4′′), 5.03 (t, 1H, J = 9.9 Hz, H-4′), 4.98 (dd, 1H, J = 3.4, 1.6 Hz, H-2′), 4.47 (d, 1H, J = 1.7 Hz, H-1′′′), 4.83 (d, 1H, J = 1.4 Hz, H-1′′), 4.65 (d, 1H, J = 1.6 Hz, H-1′), 4.10 (dd, 1H, J = 10.1, 3.4 Hz, H-3′′), 4.05 (dd, 1H, J = 10.1, 3.4 Hz, H-3′), 3.73–3.83 (m, 3H, H-5′, H-5′′, H-5′′′), 3.61–3.65 (m, 1H, H-1-1), 3.37–3.40 (m, 1H, H-1-2), 2.31–2.78 (m, 14H, 7 × COCH2), 2.21, 2.18, 2.16, 2.16, 2.14, 2.12 (each s, each 3H, each COCH3), 1.55–1.65 (m, 4H, 2 × CH2), 1.25–1.33 (m, 14H, 7 × CH2), 1.18 (d, 3H, J = 6.2 Hz, H-6′), 1.17 (d, 3H, J = 6.2 Hz, H-6′′), 1.16 (d, 3H, J = 6.2 Hz, H-6′′′), 0.90 (t, 3H, J = 7.0 Hz, CH3), 0.89 (t, 3H, J = 7.0 Hz, CH3); 13C-NMR (CDCl3): δ 206.4 (two), 173.0, 172.0, 171.9, 171.4, 170.4, 170.2, 170.0, 99.4, 98.7, 97.2, 75.2, 74.8, 72.2, 71.9, 71.8, 71.4, 70.8, 70.0, 68.7, 68.1, 67.3, 67.1, 66.5, 37.8, 37.7 (two), 34.0, 31.8, 31.3, 29.7, 29.3 (two), 29.2, 28.2, 27.9, 27.8, 26.1, 24.5, 22.6, 22.3, 20.9 (two), 20.8, 17.5, 17.2, 17.1, 14.1, 13.9; HRESIMS calcd for C53H82O23Na [M + Na]+ 1109.5145; found, 1109.5156.
3.2.4. General Method A for Removal of the Lev Protecting Groups
To a stirred solution of Lev protected compound (1.0 mmol) in 20 mL dry CH2Cl2-MeOH (v:v = 1:1) hydrazine acetate (10 mmol)was added. After stirring at room temperature for 5 h, the reaction mixture was concentrated. The residue was purified by silica gel column chromatography (1:1, petroleum ether–EtOAc) to afford a white solid 2, 3, and 5–7, respectively.
1-Octyloxy-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-4-O-hexanoyl-α-l-rhamnopyranoside (2). 2 was prepared from 25 (515 mg, 0.50 mmol) and hydrazine acetate (921 mg, 10 mmol) following the general method A in 88% yield. 1H-NMR (CDCl3): δ 5.41 (d, 1H, J = 2.3 Hz, H-1′′′), 4.99 (t, 1H, J = 9.8 Hz, H-4′), 4.97(t, 1H, J = 9.8 Hz, H-4′′), 4.95 (s, 1H, H-1′′), 4.91 (dd, 1H, J = 3.5, 1.8 Hz, H-2′′), 4.86 (t, 1H, J = 9.9 Hz, H-4′′′), 4.74 (d, 1H, J = 1.7 Hz, H-1′), 4.44 (dd, 1H, J = 4.0, 2.5 Hz, H-2′′′), 4.05–4.08 (m, 2H, H-3′, H-3′′), 3.96–3.98 (m, 1H, H-5′′), 3.93 (dd, 1H, J = 3.1, 1.7 Hz, H-2′), 3.83 (dd, 1H, J = 9.8, 4.1 Hz, H-3′′′), 3.76–3.79 (m, 1H, H-5′′′), 3.63–3.67 (m, 1H, H-1-1), 3.40–3.44 (m, 1H, H-5′), 3.37–3.39 (m, 1H, H-1-2), 2.32 (t, 2H, J = 6.4 Hz, COCH2), 2.17, 2.16, 2.15, 1.73 (each s, each 3H, each CH3CO), 1.61–1.66 (m, 2H, CH2), 1.55–1.59 (m, 2H, CH2), 1.26–1.33 (m, 16H, CH2), 1.21 (d, 3H, J = 6.1 Hz, H-6′′′), 1.20 (d, 3H, J = 6.2 Hz, H-6′), 1.18 (d, 3H, J = 6.3 Hz, H-6′′), 0.90 (t, 6H, J = 7.0 Hz, 2 × CH3); 13C-NMR (CDCl3): δ 172.6, 171.6, 170.5, 170.2, 124.3, 99.4, 98.8, 97.4, 78.3, 77.5, 74.3, 72.8, 71.3 (two), 71.2, 70.8, 69.2, 67.8, 67.7, 66.9, 66.2, 34.3, 31.8, 31.3, 29.4, 29.3, 29.2, 26.1, 25.2, 24.6, 22.6, 22.3, 21.0 (two), 20.7, 17.5, 17.4, 17.3, 14.1, 13.9; HRESIMS calcd for C40H66O18Na [M + Na]+ 857.4147; found, 857.4160.
1-Octyloxy-3,4-di-O-acetyl-α-L-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-4-O-hexanoyl-α-l-rhamnopyranoside (3). Analogously, 3 was prepared from 26 (773 mg, 0.75 mmol) and hydrazine acetate (1.38 g, 15 mmol) following the general method A in 85% yield. 1H-NMR (CDCl3): δ 5.08 (t, 1H, J = 10.0 Hz, H-4′), 5.06 (t, 1H, J = 10.0 Hz, H-4′′′), 5.05 (t, 1H, J = 10.0 Hz, H-4′′), 5.03 (dd, 1H, J = 9.9, 3.3 Hz, H-3′′′), 5.02 (dd, 1H, J = 3.3, 1.7 Hz, H-2′′), 4.97 (d, 1H, J = 1.6 Hz, H-1′′′), 4.88(d, 1H, J = 1.4 Hz, H-1′′), 4.77 (d, 1H, J = 1.4 Hz, H-1′), 4.15 (dd, 1H, J = 9.9, 3.4 Hz, H-3′′), 3.98 (dd, 1H, J = 3.3, 1.7 Hz, H-2′′′), 3.95–3.97 (m, 1H, H-5′′), 3.95 (dd, 1H, J = 3.2, 1.8 Hz, H-2′), 3.92 (dd, 1H, J = 9.8, 3.3 Hz, H-3′), 3.77–3.83 (m, 2H, H-5′, H-5′′′), 3.67 (dt, 1H, J = 9.5, 6.5 Hz, H-1-1), 3.41 (dt, 1H, J = 9.6, 6.7 Hz, H-1-2), 2.45 (dt, 1H, J = 15.7, 7.4 Hz, COCH2-1), 2.34 (dt, 1H, J = 15.7, 7.9 Hz, COCH2-2), 2.18, 2.10, 2.07, 2.04 (each s, each 3H, each CH3CO), 1.63–1.66 (m, 2H, CH2), 1.56–1.59 (m, 2H, CH2), 1.29–1.33 (m, 14H, 7 × CH2), 1.21 (d, 3H, J = 6.2 Hz, H-6′′′), 1.19 (d, 3H, J = 6.2 Hz, H-6′′), 1.18 (d, 3H, J = 6.3 Hz, H-6′), 0.90 (t, 3H, J = 7.2 Hz, CH3), 0.89 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (CDCl3): δ 173.4, 170.3 (two), 170.0, 169.9, 101.0, 99.3 (two), 78.9, 74.7, 72.3, 71.8, 71.5, 71.4, 71.1 (two), 69.5, 68.0, 67.2, 67.1, 66.2, 34.1, 31.8, 31.3, 29.4, 29.3, 29.2, 26.1, 24.5, 22.6, 22.3, 21.0, 20.9, 20.8 (two), 17.4, 17.2, 14.1, 13.9; HRESIMS calcd for C40H66O18Na [M + Na]+ 857.4147; found, 857.4161.
1-Octyloxy-2,3-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-4-O-hexanoyl-α-l-rhamnopyranoside (5). Analogously, 5 was prepared from 28 (515 mg, 0.50 mmol) and hydrazine acetate (921 mg, 10 mmol) following the general method A in 87% yield. 1H-NMR (CDCl3): δ 5.07 (t, 1H, J = 9.7 Hz, H-4′), 5.06 (dd, 1H, J = 9.7, 3.3 Hz, H-3′′), 5.05 (t, 1H, J = 9.7 Hz, H-4′′), 4.98–5.01 (m, 2H, H-2′′, H-2′′′), 4.89 (d, 1H, J = 1.7 Hz, H-1′′′), 4.87 (d, 1H, J = 1.4 Hz, H-1′′), 4.77 (d, 1H, J = 1.4 Hz, H-1′), 4.10 (dd, 1H, J = 10.0, 3.5 Hz, H-3′′), 3.95 (dd, 1H, J = 3.3, 1.2 Hz, H-2′), 3.91–3.94 (m, 2H, H-3′, H-5′′′), 3.73–3.80 (m, 2H, H-5′, H-5′′), 3.66 (dt, 1H, J = 9.6, 6.7 Hz, H-1-1), 3.56 (t, 1H, J = 9.7 Hz, H-4′′′), 3.40 (dt, 1H, J = 9.6, 6.5 Hz, H-1-2), 2.42 (dt, 1H, J = 15.8, 7.5 Hz, COCH2-1), 2.34 (dt, 1H, J = 15.7, 7.8 Hz, COCH2-2), 2.18, 2.13, 2.12, 2.08 (each s, each 3H, each CH3CO), 1.62–1.66 (m, 2H, CH2), 1.57–1.59 (m, 2H, CH2), 1.33 (d, 3H, J = 6.2 Hz, H-6′′′), 1.28–1.31 (m, 14H, 7 × CH2), 1.21 (d, 3H, J = 6.2 Hz, H-6′′), 1.19 (d, 3H, J = 6.2 Hz, H-6′), 0.90 (t, 3H, J = 7.1 Hz, CH3), 0.89 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (CDCl3): δ 173.0, 171.1, 170.5, 170.2, 170.0, 99.3, 99.2, 98.6, 78.8, 74.0, 72.2, 71.7, 71.3 (two), 71.0, 70.4, 69.6, 68.0, 67.3, 66.2, 34.1, 31.8, 31.3, 29.4, 29.3, 29.2, 26.1, 24.5, 22.6, 22.3, 20.9 (two), 20.8, 20.7, 17.4 (three), 14.1, 13.9; HRESIMS calcd for C40H66O18Na [M + Na]+ 857.4147; found, 857.4160.
1-Octyloxy-2,3,4-tri-O-acetyl-α-l-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-4-O-hexanoyl-α-l-rhamnopyranoside (6). Analogously, 6 was prepared from 29 (488 mg, 0.50 mmol) and hydrazine acetate (461 mg, 5 mmol) following the general method A in 88% yield. 1H-NMR (CDCl3): δ 5.16 (dd, 1H, J = 10.2, 3.3 Hz, H-3′′′), 5.09 (t, 1H, J = 9.9 Hz, H-4′), 5.02–5.06 (m, 4H, H-4′′, H-4′′′, H-2′′′, H-2′′), 4.89, 4.88, 4.77 (each brs, each 1H, H-1′, H-1′′, H-1′′′), 4.08 (dd, 1H, J = 9.9, 3.4 Hz, H-3′′), 3.95 (dd, 1H, J = 3.3, 1.2 Hz, H-2′), 3.92 (dd, 1H, J = 9.9, 3.3 Hz, H-3′), 3.91–3.93 (m, 1H, H-5′′′), 3.82–3.84 (m, 1H, H-5′′), 3.76–3.78 (m, 1H, H-5′), 3.66 (dt, 1H, J = 9.5, 2.8 Hz, H-1-1), 3.40 (dt, 1H, J = 9.6, 6.6 Hz, H-1-2), 2.44 (dt, 1H, J = 16.0, 7.5 Hz, COCH2-1), 2.34 (dt, 1H, J = 16.0, 7.7 Hz, COCH2-2), 2.18, 2.14, 2.13, 2.05, 1.98 (each s, each 3H, each CH3CO), 1.62–1.66 (m, 2H, CH2), 1.55–1.58 (m, 2H, CH2), 1.26–1.33 (m, 14H, 7 × CH2), 1.21 (d, 3H, J = 6.4 Hz, H-6′′′), 1.19 (d, 3H, J = 6.4 Hz, H-6′′), 1.18 (d, 3H, J = 6.3 Hz, H-6′), 0.90 (t, 3H, J = 7.1 Hz, CH3), 0.89 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (CDCl3): δ 173.0, 170.3, 170.2, 170.1, 170.0, 169.7, 99.2 (two), 98.8, 78.7, 74.8, 72.0, 71.7, 71.3, 71.0, 70.8, 68.6, 68.0, 67.4, 67.2, 66.2, 34.1, 31.8, 31.3, 29.4, 29.3, 29.2, 26.1, 24.5, 22.6, 22.3, 20.9 (two), 20.8, 20.7 (two), 17.4, 17.2, 14.1, 13.9; HRESIMS calcd for C42H68O19Na [M + Na]+ 899.4252; found, 899.4263.
1-Octyloxy-2-O-acetyl-α-l-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-L-rhamnopyranosyl-(1→3)-4-O-hexanoyl-α-l-rhamnopyranoside (7). Analogously, 7 was prepared from 30 (544 mg, 0.50 mmol) and hydrazine acetate (1.38 g, 15 mmol) following the general method A in 78% yield. 1H-NMR (CDCl3): δ 5.05 (t, 1H, J = 9.7 Hz, 1H, H-4′), 5.04 (t, 1H, J = 9.7 Hz, H-4′′), 4.98 (dd, 1H, J = 3.4, 1.8 Hz, H-2′′), 4.89 (dd, 1H, J = 3.6, 1.5 Hz, H-2′′′), 4.87 (d, 1H, J = 1.8 Hz, H-1′′′), 4.87 (d, 1H, J = 1.6 Hz, H-1′′), 4.75 (d, 1H, J = 1.8 Hz, H-1′), 4.04 (dd, 1H, J = 9.7, 3.4 Hz, H-3′′), 3.94 (dd, 1H, J = 9.9, 3.3 Hz, H-3′), 3.93 (dd, 1H, J = 3.3, 1.5 Hz, H-2′), 3.92 (dq, 1H, J = 9.7, 6.3 Hz, H-5′), 3.78 (dd, 1H, J = 9.4, 3.5 Hz, H-3′′′), 3.76 (dq, 1H, J = 9.7, 6.3 Hz, H-5′′), 3.64–3.67 (m, 1H, H-1-1), 3.58–3.62 (m, 1H, H-5′′′), 3.43 (t, 1H, J = 9.6 Hz, H-4′′′), 3.40 (ddd, 1H, J = 10.4, 6.8, 6.8 Hz, H-1-2), 2.40–2.44 (m, 1H, COCH2-1), 2.34–2.37 (m, 1H, COCH2-2), 2.14, 2.13, 2.12 (each s, each 3H, each COCH3), 1.61–1.63 (m, 4H, 2 × CH2), 1.27–1.33 (m, 14H, 7 × CH2), 1.21 (d, 3H, J = 6.4 Hz, H-6′′′), 1.19 (d, 6H, J = 6.4 Hz, H-6′′, H-6′), 0.89 (t, 6H, J = 7.1 Hz, 2 × CH3); 13C-NMR (CDCl3): δ 173.0, 170.5, 170.4, 170.2, 99.3, 99.2, 99.2, 78.7, 74.7, 73.3, 72.5, 72.3, 71.9, 71.8, 71.0, 69.8, 69.1, 68.2, 67.3, 66.2, 34.1, 31.8, 31.3, 29.4, 29.3, 29.2, 28.1, 24.5, 22.6, 22.3, 21.0, 20.9, 20.8, 17.4, 17.2, 17.4, 14.1, 14.9; HRESIMS calcd for C38H64O17Na [M + Na]+ 815.4041; found, 815.4058.
3.2.5. 1-Octyloxy-4-O-acetyl-α-l-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-4-O-hexanoyl-α-l-rhamnopyranoside (4)
To a solution of compound 30 (400 mg, 0.43 mmol) in 10 mL dry CH2Cl2-MeOH (V:V = 1:1) hydrazine acetate was added (396 mg, 4.3 mmol) and stirred at room temperature for 5 h under argon. Then the reaction mixture was concentrated under reduced pressure, and then the mixture was diluted with EtOAc (100 mL), washed with saturated aqueous NaHCO3 (2 × 50 mL), 1 mol·L−1 HCl (2 × 50 mL), and brine (2 × 50 mL), dried over Na2SO4, and concentrated in vacuo. The above residue was dissolved in 80% HOAc (20 mL) and stirred for 2 h at 100 °C, then the reaction mixture was concentrated in vacuo. The residue was purified by silica gel column chromatography (3:1, CH2Cl2—EtOAc) to afford a white solid 4 (265 mg, 78% for two steps); 1H-NMR (CDCl3): δ 5.07 (t, 1H, J = 10.0 Hz, H-4′), 5.04 (brs, 1H, H-1′′′) 5.03 (t, 1H, J = 10.0 Hz, H-4′′), 4.98 (dd, 1H, J = 3.3, 1.9 Hz, H-2′′), 4.86 (d, 1H, J = 1.4 Hz, H-1′′), 4.80 (d, 1H, J = 1.7 Hz, H-1′), 4.79 (t, 1H, J = 10.0 Hz, H-4′′′), 4.18 (dd, 1H, J = 10.0, 3.4 Hz, H-3′′), 3.98 (dd, 1H, J = 9.9, 3.2 Hz, H-3′), 3.97 (dd, 1H, J = 3.2, 1.3 Hz, H-2′), 3.90 (dd, 1H, J = 9.9, 3.3 Hz, H-3′′′), 3.86 (dd, 1H, J = 3.2, 1.9 Hz, H-2′′′), 3.70–3.81 (m, 3H, H-5′, H-5′′, H-5′′′), 3.65 (dt, 1H, J = 9.5, 2.8 Hz, H-1-1), 3.41 (dt, 1H, J = 9.6, 6.7 Hz, H-1-2), 3.61, 3.19, 3.05 (each brs, each 1H, each OH), 2.46–2.50 (m,1 H, COCH2-1), 2.34–2.40 (m, 1H, COCH2-2), 2.15, 2.13, 2.08 (each s, each 3H, each CH3CO), 1.61–1.66 (m, 2H, CH2), 1.55–1.58 (m, 2H, CH2), 1.26–1.33 (m, 14H, 7 × CH2), 1.20, 1.18, 1.15 (each d, each 3H, J = 6.4 Hz, H-6′, H-6′′, H-6′′′), 0.90 (t, 3H, J = 7.2 Hz, CH3), 0.89 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (CDCl3): δ 173.6, 172.1, 170.2, 170.0, 101.5, 99.4, 99.3, 79.1, 75.1, 75.0, 72.3, 71.9, 71.8, 71.0, 70.9, 69.8, 68.0, 67.2, 66.5, 66.2, 34.1, 31.8, 31.3, 29.4, 29.3, 29.2, 26.1, 24.5, 22.6, 22.3, 21.1, 21.0, 20.8, 17.4 (two), 17.1, 14.1, 13.9; HRESIMS calcd for C38H64O17Na [M + Na]+ 815.4041; found, 815.4055.
3.2.6. 1-Octyloxy-α-L-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-4-O-hexanoyl-α-l-rhamnopyranoside (8)
To a solution of compounds 30 (543 mg, 0.50 mmol) in 20 mL dry CH2Cl2–MeOH (V:V = 1:1), hydrazine acetate (1.38 g, 15 mmol) was added and stirred at 40 °C for 18 h under argon. The reaction mixture was concentrated in vacuo, and then the residue was purified by silica gel column chromatography (2:1, CH2Cl2–EtOAc) to afford a white solid 8 (0.49 g, 71%). 1H-NMR (CDCl3): δ 5.06 (t, 1H, J = 9.9 Hz, H-4′), 5.03 (t, 1H, J = 10.0 Hz, H-4′′), 4.97 (dd, 1H, J = 3.2, 1.1 Hz, H-2′′), 4.96 (brs, 1H, H-1′′), 4.87 (brs, 1H, H-1′′′), 4.77 (brs, 1H, H-1′), 4.18 (dd, 1H, J = 9.9, 3.3 Hz, H-3′′), 3.95–3.99 (m, 1H, H-5′′), 3.95 (dd, 1H, J = 3.2, 1.5 Hz, H-2′), 3.91 (dd, 1H, J = 9.7, 3.1 Hz, H-3′), 3.82 (dd, 1H, J = 3.3, 1.2 Hz, H-2′′′), 3.76–3.79 (m, 1H, H-5′), 3.63–3.67 (m, 2H, H-3′′′, H-1-1), 3.55–3.58 (m, 1H, H-5′′′), 3.39–3.46 (m, 2H, H-4′′′, H-1-2), 2.49 (dt, 1H, J = 15.7, 7.5 Hz, COCH2-1), 2.36 (dt, 1H, J = 15.7, 7.9 Hz, COCH2-2), 2.14, 2.11 (each s, each 3H, each CH3CO), 1.62–1.66 (m, 2H, CH2), 1.55–1.58 (m, 2H, CH2), 1.28–1.31 (m, 14H, 7 × CH2), 1.26 (d, 3H, J = 6.2 Hz, H-6′′′), 1.19 (d, 3H, J = 6.2 Hz, H-6′′), 1.18 (d, 3H, J = 6.2 Hz, H-6′), 0.90 (t, 3H, J = 7.2 Hz, CH3), 0.89 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (CDCl3): δ 174.0, 170.4 (two), 101.9, 99.3 (two), 78.6, 75.0, 72.7, 72.2, 71.9, 71.3, 71.1, 70.9, 69.1, 68.0, 67.1, 66.2, 34.1, 31.8, 31.3, 29.4, 29.3, 29.2, 26.1, 24.5, 22.6, 22.2, 20.9 (two), 17.4 (two), 17.2, 14.1, 13.9; HRESIMS calcd for C36H62O16Na [M + Na]+ 773.3936; found, 773.3942.
3.2.7. 1-Octyloxy-4-O-hexanoyl-2-O-acetyl-α-l-rhamnopyranoside (32)
To a solution of the known compound 31 (2.0 g, 5.4 mmol) in anhydrous N,N-dimethylformamide (20 mL), and triethylorthoacetate (1.5 mL, 7.9 mmol) were added, followed by a catalytic amount of camphorsulfonicacid (250 mg, 1.1 mmol). The mixture was stirred for 5 h at r.t. When TLC (1:1, petroleum ether-EtOAc) showed complete conversion, the mixture was diluted with EtOAc (200 mL). The organic layer was shaken with 1 mol·L−1 HCl (3 × 100 mL), followed by washing with water (3 × 100 mL), and brine (2 × 100 mL), dried over Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (8:1, petroleum ether—EtOAc) to generate 32 (1.9 g, 86%). 1H-NMR (CDCl3): δ 5.05 (dd, 1H, J = 3.6, 1.6 Hz, H-2′), 4.86 (t, 1H, J = 9.8 Hz, H-4′), 4.77 (d, 1H, J = 1.3 Hz, H-1′), 4.03 (dd, 1H, J = 9.9, 3.6 Hz, H-3′), 3.79–3.83 (m, 1H, H-5′), 3.66 (dt, 1H, J = 9.9, 6.8 Hz, H-1-1), 3.40 (dt, 1H, J = 9.9, 6.5 Hz, H-1-2), 2.36–2.39 (m, 2H, COCH2), 2.16 (s, 3H, CH3CO), 1.63–1.68 (m, 2H, H-2), 1.56–1.60 (m, 2H, H-3), 1.26–1.35 (m, 14H, 7 × CH2), 1.21 (d, 3H, J = 6.3 Hz, H-6′), 0.90 (t, 3H, J = 7.1 Hz, CH3); 13C-NMR (CDCl3) δ 174.4, 170.6, 97.1 (C-1′), 74.5, 72.9, 68.6, 68.2, 65.8, 34.3, 31.8, 31.2, 29.4, 29.3, 29.2, 26.1, 24.6, 22.6, 22.3, 21.0, 17.4, 14.1, 13.9; HRESIMS calcd for C22H40O7Na [M + Na]+ 439.2672; found, 439.2688.
3.2.8. 1-Octyloxy-2,4-di-O-acetyl-3-O-levulinoyl-α-l-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-4-O-hexanoyl-2-O-acetyl-α-l-rhamnopyranoside (33)
Analogously, 33 was prepared from 32, 24 and 18 as described for 25 in 82% yield. 1H-NMR (CDCl3): δ 5.14 (dd, 1H, J = 10.2, 3.4 Hz, H-3′′′), 5.13 (dd, 1H, J = 3.6, 1.7 Hz, H-2′′′), 5.07 (t, 2H, J = 9.8 Hz, H-4′′, H-4′′′), 5.04 (t, 1H, J = 9.7 Hz, H-4′), 5.03 (dd, 1H, J = 3.4, 1.6 Hz, H-2′′), 4.97 (dd, 1H, J = 3.4, 1.7 Hz, H-2′), 4.85 (d, 1H, J = 1.5 Hz, H-1′′′), 4.84 (d, 1H, J = 1.6 Hz, H-1′′), 4.69 (d, 1H, J = 1.5 Hz, H-1′), 3.74–3.83 (m, 3H, H-5′, H-5′′, H-5′′′), 3.61–3.65 (m, 1H, H-1-1), 3.37–3.41 (m, 1H, H-1-2), 2.73–2.77 (m, 1H, COCH2-1), 2.60–2.66 (m, 1H, COCH2-2), 2.51–2.56 (m, 1H, COCH2-1′), 2.39–2.45 (m, 1H, COCH2), 2.30–2.36 (m, 1H, COCH2-2′), 2.18, 2.16, 2.14, 2.12, 2.09 (each s, each 3H, each CH3CO), 1.55–1.65 (m, 4H, 2 × CH2), 1.22–1.32 (m, 14H, 7 × CH2), 1.19 (d, 3H, J = 6.2 Hz, H-6′′′), 1.17 (d, 6H, J = 6.2 Hz, H-6′′, H-6′), 0.90 (t, 3H, J = 7.1 Hz, CH3), 0.89 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (CDCl3): δ 206.1, 172.9, 171.2, 170.5, 170.3 (two), 170.2, 170.1, 99.2, 98.8, 97.2, 75.5, 74.7, 72.2, 72.0, 71.8, 71.4, 70.5, 70.0, 68.7, 68.2, 67.4, 67.1, 66.5, 37.6, 34.0, 31.8, 31.3, 29.7, 29.3 (two), 29.2, 27.8, 26.1, 24.5, 22.6, 22.3, 22.1, 20.9 (two), 20.8 (two), 17.4 (two), 14.1, 13.9; HRESIMS calcd for C47H74O21Na [M + Na]+ 997.4620; found, 997.4633.
3.2.9. 1-Octyloxy-2,4-di-O-acetyl-α-L-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-4-O-hexanoyl-2-O-acetyl-α-l-rhamnopyranoside (9)
Analogously, 9 was prepared from 33 as described for 2 in 90% yield. 1H-NMR (CDCl3): δ 5.12 (dd, 1H, J = 3.4, 1.6 Hz, H-2′′′), 5.07 (t, 1H, J = 9.8 Hz, H-4′′′), 5.03 (t, 1H, J = 10.0 Hz, H-4′′), 4.93 (dd, 1H, J = 3.1, 1.8 Hz, H-2′′), 4.89 (s, 1H, H-1′′′), 4.87 (dd, 1H, J = 3.5, 1.4 Hz, H-2′), 4.83 (d, 1H, J = 1.1 Hz, H-1′′), 4.81 (t, 1H, J = 9.8 Hz, H-4′), 4.69 (s, 1H, H-1′), 4.06 (dd, 1H, J = 9.9, 3.3 Hz, H-3′′), 3.93 (dd, 1H, J = 10.0, 3.4 Hz, H-3′), 3.85 (dd, 1H, J = 10.0, 3.4 Hz, H-3′′′), 3.71–3.78 (m, 3H, H-5′, H-5′′, H-5′′′), 3.61–3.65 (m, 1H, H-1-1), 3.37–3.41 (m, 1H, H-1-2), 2.38–2.44 (m, 1H, COCH2-1), 2.30–2.35 (m, 1H, COCH2-2), 2.19, 2.15, 2.15, 2.14, 2.13 (each s, each 3H, each CH3CO), 1.54–1.65 (m, 4H, 2 × CH2), 1.28–1.34 (m, 14H, 7 × CH2), 1.18 (d, 3H, J = 6.2 Hz, H-6′′′), 1.17 (d, 3H, J = 6.2 Hz, H-6′′), 1.16 (d, 3H, J = 6.2 Hz, H-6′), 0.90 (t, 3H, J = 7.1 Hz, CH3), 0.89 (t, 3H, J = 7.0 Hz, CH3); 13C-NMR (CDCl3): δ 172.9, 171.6, 170.5 (two), 170.3, 170.2, 99.2, 99.0, 97.2, 75.5, 74.5, 74.3, 72.7, 72.2, 72.1, 71.8, 71.7, 68.2, 68.1, 67.4, 66.7, 66.5, 34.0, 31.8, 31.3, 29.3 (two), 29.2, 26.1, 24.5, 22.6, 22.3, 21.1, 21.0, 20.9, 20.8, 17.4, 17.2, 17.1, 14.1, 13.9; HRESIMS calcd for C42H68O19Na [M + Na]+ 899.4252; found, 899.4266.
3.2.10. O-Oct-1-yl-α-L-rhamnopyranosyl-(1→3)-α-L-rhamnopyranosyl-(1→3)-α-L-rhamnopyranoside (10)
To a stirred solution of 9 (420 mg, 0.51 mmol) in anhydrous methanol (10 mL) sodium methoxide was added (54 mg, 1.0 mmol). The reaction mixture was stirred at at 50 °C for 24 h, after which the reaction mixture was neutralized with Dowex 50 × 8 (H+) resin until pH 7, filtered and concentrated in vacuo to furnish a crude product, that was purified via silica gel column chromatography (15:1, trichloromethane—methanol) to yield 10 (0.25 g, 86%); 1H-NMR (CD3OD): δ 5.03 (d, 1H, J = 1.6 Hz, H-1′′′), 5.01 (d, 1H, J = 1.6 Hz, H-1′′), 4.65 (d, 1H, J = 1.7 Hz, H-1′), 4.08 (dd, 1H, J = 3.2, 1.8 Hz, H-2′), 3.99 (dd, 1H, J = 3.4, 1.7 Hz, H-2′′), 3.88 (dd, 1H, J = 3.4, 1.6 Hz, H-2′′′), 3.87 (dd, 1H, J = 9.8, 3.3 Hz, H-3′′′), 3.80–3.85 (m, 2H, H-5′′, H-5′′′), 3.79 (dd, 1H, J = 9.5, 3.4 Hz, H-3′′), 3.76 (dd, 1H, J = 9.6, 3.2 Hz, H-3′), 3.67–3.71 (m, 1H, H-5′), 3.60–3.64 (m, 1H, H-1-1), 3.53 (t, 1H, J = 9.5 Hz, H-4′), 3.51 (t, 1H, J = 9.6 Hz, H-4′′), 3.41–3.44 (m, 1H, H-1-2), 3.40 (t, 1H, J = 9.5 Hz, H-4′′′), 1.58–1.60 (m, 2H, CH2), 1.30–1.43 (m, 10H, 5 × CH2), 1.28 (d, 3H, J = 6.2 Hz, H-6′′′), 1.27 (d, 6H, J = 6.2 Hz, H-6′′, H-6′), 0.92 (t, 3H, J = 7.1 Hz, CH3); 13C-NMR (CD3OD): δ 102.7, 102.5, 100.2, 78.6, 78.0, 72.7, 71.9, 71.8, 70.8, 70.5, 68.9, 68.7 (two), 67.2, 31.6, 29.2, 29.1, 29.0, 26.0, 22.3, 16.6, 13.0; HRMALDIMS calcd for C26H48O13Na 591.2993; found 591.2997.
3.2.11. 1-Octyloxy-2,4-di-O-acetyl-3-O-(p-methoxybenzyl)-α-l-rhamnopyranosyl-(1→3)-4-O-hexanoyl-2-levulinoyl-α-l-rhamnopyranoside (35)
To a solution of compound
15 (1.0 g, 2.3 mmol),
34 [
20] (1.5 g, 3.2 mmol) and 4 Å molecular sieves in dry CH
2Cl
2 (30 mL)
N-iodosuccinimide (0.90 g, 4.8 mmol) and silver trifluoromethanesulfonate (0.12 g, 0.45 mmol) were added at 0 °C under argon. The reaction mixture was allowed to stir for 2 h under this condition, while warmed to room temperature until TLC indicated that the reaction was complete. Then the residue was diluted with CH
2Cl
2 (100 mL), and washed with aqueous Na
2S
2O
3 (50 mL), saturated aqueous NaHCO
3 (2 × 50 mL), and brine (2 × 50 mL), dried over Na
2SO
4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (5:1, petroleum ether–EtOAc) to give a white solid
35 (1.6 g, 92%);
1H-NMR (CDCl
3):
δ 7.19 (d, 2H,
J = 8.6 Hz, Ar-H), 6.85 (d, 2H,
J = 8.6 Hz, Ar-H), 5.11–5.13 (m, 2H, H-2′, H-2′′), 5.05 (t, 1H,
J = 9.8 Hz, H-4′), 4.96 (t, 1H,
J = 9.8 Hz, H-4′′), 4.85 (d, 1H,
J = 1.1 Hz, H-1′′), 4.66 (brs, 1H, H-1′′), 4.53 (d, 1H,
J = 11.6 Hz, Ar-CH
2-1), 4.37 (d, 1H,
J = 11.6 Hz, Ar-CH
2-2), 4.07 (dd, 1H,
J = 9.8, 3.2 Hz, H-3′′), 3.79 (s, 3H, OCH
3), 3.73–3.77 (m, 3H, H-3′, H-5′, H-5′′), 3.61–3.65 (m, 1H, H-1-1), 3.37–3.40 (m, 1H, H-1-2), 2.71 (t, 2H,
J = 6.3 Hz, COCH
2), 2.60–2.65 (m, 2H, COCH
2), 2.36 (t, 2H,
J = 6.6 Hz, COCH
2), 2.18, 2.13, 2.02 (each s, each 3H, each CH
3CO), 1.63–1.66 (m, 2H, CH
2), 1.54–1.56 (m, 2H, CH
2), 1.28–1.33 (m, 14H, 7 × CH
2), 1.19 (d, 3H,
J = 6.4 Hz, H-6′), 1.14 (d, 3H,
J = 6.3 Hz, H-6′′), 0.90 (t, 3H,
J = 7.1 Hz, CH
3), 0.89 (t, 3H,
J = 7.2 Hz, CH
3);
13C-NMR (CDCl
3):
δ 205.9, 176.5, 173.0, 171.9, 170.2, 159.1, 133.7, 130.1, 129.3 (two), 113.3 (two), 99.8, 97.1, 75.2, 73.8, 72.3, 72.2, 72.0, 71.0, 68.8, 68.1, 67.3, 66.5, 55.2, 37.8, 34.1, 31.8, 31.3, 29.3 (two), 29.2, 28.5, 28.2, 26.1, 24.6, 22.6, 22.3, 20.9, 17.5, 17.3, 14.1, 13.9; HRESIMS calcd for C
43H
66O
15Na [M + Na]
+ 845.4299; found, 845.4283.
3.2.12. 1-Octyloxy-2,4-di-O-acetyl-α-L-rhamnopyranosyl-(1→3)- 4-O-hexanoyl-2-levulinoyl-α-l-rhamnopyranoside (36)
Reaction of compound 35 (2.0 g, 2.4 mmol), and DDQ (0.72 g, 3.2 mmol) in CH2Cl2 (60 mL) and H2O (4 mL) was essentially as described for 15 yielded 36 (1.6 g, 94%) as a white solid; 1H-NMR (CDCl3): δ 5.12 (dd, 1H, J = 3.3, 1.8 Hz, H-2′′), 5.05 (t, 1H, J = 9.9 Hz, H-4′), 4.90 (d, 1H, J = 1.2 Hz, H-1′′), 4.87 (dd, 1H, J = 3.5, 1.6 Hz, H-2′), 4.83 (t, 1H, J = 9.8 Hz, H-4′′), 4.66 (d, 1H, J = 1.6 Hz, H-1′), 4.07 (dd, 1H, J = 10.0, 3.4 Hz, H-3′′), 3.94–3.97 (m, 1H, H-3′), 3.74–3.82 (m, 2H, H-5′, H-5′′), 3.63 (dt, 1H, J = 9.5, 2.8 Hz, H-1-1), 3.39 (dt, 1H, J = 9.6, 6.6 Hz, H-1-2), 2.70–2.80 (m, 4H, 2 × COCH2), 2.46 (dt, 1H, J = 16.1, 7.5 Hz, COCH2-1), 2.34 (dt, 1H, J = 16.0, 7.6 Hz, COCH2-2), 2.22, 2.14, 2.13 (each s, each 3H, each CH3CO), 1.63–1.66 (m, 2H, CH2), 1.54–1.59 (m, 2H, CH2), 1.26–1.34 (m, 14H, CH2), 1.18 (d, 3H, J = 6.4 Hz, H-6′), 1.17 (d, 3H, J = 6.3 Hz, H-6′′), 0.90 (t, 3H, J = 7.2 Hz, CH3), 0.89 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (CDCl3): δ 206.1, 173.1, 171.9, 171.5, 170.2, 99.1, 97.1, 74.9, 74.3, 72.9, 72.2, 71.9, 68.1, 67.8, 66.8, 66.6, 37.8, 34.0, 31.8, 31.3, 29.8, 29.3 (two), 29.2, 28.2, 26.1, 24.5, 22.6, 22.3, 21.0, 20.9, 17.5, 17.2, 14.1, 13.9; HRESIMS calcd for C35H58O14Na [M + Na]+ 725.3724; found, 725.3730.
3.2.13. 1-Octyloxy-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-4-O-hexanoyl -α-l-rhamnopyranoside (11)
Analogously, 11 was prepared from 36 as described for 2 in 89% yield. 1H-NMR (CDCl3): δ 5.04 (t, 1H, J = 9.2 Hz, H-4′), 4.93 (s, 1H, H-1′′), 4.90 (dd, 1H, J = 3.2, 1.7 Hz, H-2′′), 4.86 (t, 1H, J = 9.7 Hz, H-4′′), 4.75 (s, 1H, H-1′), 4.02 (dd, 1H, J = 10.1, 3.1 Hz, H-3′), 3.92–3.97 (m, 3H, H-5′′, H-2′, H-3′′), 3.71–3.79 (m, 1H, H-5′), 3.62–3.67 (m, 1H, H-1-a), 3.37–3.42 (m, 1H, H-1-b), 2.34–2.48 (m, 2H, CH2CO), 2.13, 2.12 (each s, each 3H, each CH3CO), 1.61–1.66 (m, 2H, CH2), 1.53–1.58 (m, 2H, CH2), 1.25–1.35 (m, 14H, 7 × CH2), 1.20 (d, 3H, J = 6.2 Hz, H-6′′), 1.18 (d, 3H, J = 6.2 Hz, H-6′), 0.87–0.89 (m, 6H, 2 × CH3); 13C-NMR (CDCl3): δ 174.8, 172.8, 171.7, 100.6, 100.4, 79.7, 75.7, 74.2, 73.2, 72.4, 69.5, 69.4, 68.2, 67.6, 35.5, 33.2, 32.7, 30.8, 30.7, 30.6, 27.5, 26.0, 24.1, 23.7, 22.4 (two), 18.8 (two), 15.5, 15.3; HRESIMS calcd for C30H52O12Na [M + Na]+ 627.3356; found, 627.3370.
3.2.14. 1-Octyloxy-2,4-di-O-acetyl-3-O-levulinoyl-α-l-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-4-O-hexanoyl-2-O-levulinoyl-α-l-rhamnopyranoside (37)
Analogously, 37 was prepared from 36, 24, and 18 as described for 25 in 80% yield. 1H-NMR (CDCl3): δ 5.14 (dd, 1H, J = 10.2, 3.4 Hz, H-3′′′′), 5.08 (dd, 1H, J = 3.2, 1.8 Hz, H-2′′′′), 5.04 (t, 2H, J = 10.0 Hz, H-4′, H-4′′), 5.03 (t, 2H, J = 10.0 Hz, H-4′′′, H-4′′′′), 5.02 (dd, 1H, J = 3.2, 2.0 Hz), 4.99 (dd, 1H, J = 3.2, 1.8 Hz), 4.94 (d, 1H, J = 1.8 Hz), 4.93 (d, 1H, J = 3.2, 1.7 Hz), 4.84 (d, 1H, J = 1.4 Hz), 4.82 (d, 1H, J = 1.1 Hz), 4.66 (d, 1H, J = 1.3 Hz), 4.10 (dd, 1H, J = 10.0, 3.4 Hz), 4.05 (dd, 1H, J = 10.0, 3.3 Hz), 3.93 (dd, 1H, J = 10.0, 3.4 Hz), 3.74–3.82 (m, 3H), 3.66–3.70 (m, 1H), 3.61–3.65 (m, 1H, H-1-1), 3.37–3.41 (m, 1H, H-1-2), 2.31–2.79 (m, 10 H, 5 × COCH2), 2.22, 2.17, 2.17, 2.16, 2.15, 2.14, 2.12, 2.09 (each s, each 3H, each CH3CO), 1.60–1.65 (m, 2H, CH2), 1.54–1.58 (m, 2H, CH2), 1.26–1.34 (m, 14H, 7 × CH2), 1.19 (d, 6H, J = 6.2 Hz), 1.16 (d, 3H, J = 6.2 Hz), 1.14 (d, 3H, J = 6.2 Hz), 0.90 (t, 3H, J = 7.2 Hz, CH3), 0.89 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (CDCl3): δ 206.1, 205.9, 172.9, 171.9, 171.2, 170.4 (two), 170.3, 170.2, 170.1, 99.4, 99.2, 98.8, 75.4, 75.0, 74.8, 72.2, 72.0, 71.8 (two), 71.4, 70.6, 70.0, 68.7, 68.2, 67.3, 67.2, 67.1, 66.5, 37.8, 37.6, 34.0, 31.8, 31.3, 29.7, 29.3 (two), 29.2, 28.2, 27.8, 26.1, 24.5, 22.6, 22.3, 21.0 (two), 20.9 (two), 20.8, 20.7, 17.5, 17.2, 17.1 (two), 14.1, 13.9; HRESIMS calcd for C60H92O28Na [M + Na]+ 1283.5673; found, 1283.5690.
3.2.15. 1-Octyloxy-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-2,4-di-O-acetyl-α-l-rhamnopyranosyl-(1→3)-4-O-hexanoyl-α-l-rhamnopyranoside (12)
Analogously, 12 was prepared from 37 as described for 2 in 85% yield. 1H-NMR (CDCl3): δ 5.06 (t, 2H, J = 10.0 Hz, H-4′, H-4′′′′), 5.04 (dd, 1H, J = 3.3, 1.2 Hz, H-2′), 5.02 (t, 1H, J = 10.0 Hz, H-4′′′), 4.98 (dd, 1H, J = 3.3, 1.9 Hz, H-2′′′′), 4.94 (dd, 1H, J = 3.3, 1.2 Hz, H-2′′′), 4.89 (d, 1H, J = 1.6 Hz, H-1′′′), 4.87 (d, 1H, J = 1.2 Hz, H-1′′′′), 4.83 (brs, 1H, H-1′′), 4.81 (t, 1H, J = 9.9 Hz, H-4′′′), 4.76 (d, 1H, J = 1.1 Hz, H-1′), 4.05 (dd, 1H, J = 10.0, 3.3 Hz, H-3′′′′), 3.94 (dd, 1H, J = 3.3, 1.2 Hz, H-2′), 3.90–3.93 (m, 3H, H-3′, H-3′′, H-3′′′), 3.85–3.87 (m, 1H, H-5′′), 3.70–3.78 (m, 3H, H-5′, H-5′′′, H-5′′′′), 3.65 (dt, 1H, J = 9.5, 2.7 Hz, H-1-1), 3.41 (dt, 1H, J = 9.6, 6.5 Hz, H-1-2), 2.31–2.45 (m, 2H, COCH2), 2.17, 2.16, 2.15, 2.14, 2.13, 2.12 (each s, each 3H, each CH3CO), 1.61–1.65 (m, 2H, CH2), 1.55–1.58 (m, 2H, CH2), 1.28–1.33 (m, 14H, 7 × CH2), 1.21, 1.20, 1.18, 1.17 (each d, each 3H, J = 6.4 Hz, H-6′, H-6′′, H-6′′′, H-6′′′′), 0.90 (t, 3H, J = 7.2 Hz, CH3), 0.89 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (CDCl3): δ 173.0, 171.6, 170.4, 170.3 (two), 170.2, 170.1, 99.3 (two), 99.1 (two), 78.8, 75.3, 74.8, 74.3, 72.8, 72.1, 72.0, 71.7 (two), 71.6, 71.0, 68.1, 68.0, 67.5, 67.3, 66.7, 66.2, 34.1, 31.8, 31.3, 29.4, 29.3, 29.2, 26.1, 24.5, 22.6, 22.2, 21.0, 20.9 (three), 20.8, 20.7, 17.4 (two), 17.1, 17.0, 14.0, 13.9; HRESIMS calcd for C50H80O24Na [M + Na]+ 1087.4937; found, 1087.4946.
3.2.16. 1-Octyloxy-α-l-rhamnopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→3)-4-O-hexanoyl-α-l-rhamnopyranoside (13)
To a stirred solution of 37 (320 mg, 14.9 mmol) in dry CH3OH (10 mL) MeONa (65 mg, 1.2 mmol) was added at 0 °C. The reaction mixture was stirred at 0 °C for 2 h, after which the reaction mixture was neutralized with Dowex 50 × 8 (H+) resin until pH 7, filtered and concentrated in vacuo to furnish a crude product, which was purified via silica gel column chromatography (8:1, trichloromethane—methanol) to give 13 (185 mg, 82%) as a white solid. 1H-NMR (CD3OD): δ 5.11 (t, 1H, J = 10.0 Hz, H-4′), 5.03 (d, 1H, J = 1.6 Hz, H-1′′′′), 5.00 (d, 1H, J = 1.6 Hz, H-1′′′), 4.78 (d, 1H, J = 1.3 Hz, H-1′′), 4.69 (d, 1H, J = 1.6 Hz, H-1′), 4.08 (dd, 1H, J = 3.2, 1.8 Hz, H-2′′′), 3.99 (dd, 1H, J = 3.4, 1.7 Hz, H-2′′), 3.93 (dd, 1H, J = 3.2, 1.7 Hz, H-2′), 3.89 (dd, 1H, J = 9.8, 3.2 Hz, H-3′), 3.87 (dd, 1H, J = 9.6, 3.3 Hz, H-3′′′′), 3.76–3.85 (m, 7H, H-4′′′, H-4′′, H-2′′′′, H-3′′′, H-3′′, H-5′, H-5′′′′), 3.68–3.72 (m, 1H, H-1-1), 3.47–3.54 (m, 2H, H-5′′′, H-5′′), 3.43–3.47 (m, 1H, H-1-2), 3.40 (t, 1H, J = 9.5 Hz, H-4′′′′), 2.37 (td, 2H, J = 7.5, 1.7 Hz, COCH2), 1.59–1.67 (m, 4H, 2 × CH2), 1.32–1.44 (m, 14H, 7 × CH2), 1.28 (d, 6H, J = 6.2 Hz, H-6′, H-6′′), 1.27 (d, 3H, J = 6.2 Hz, H-6′′′), 1.15 (d, 3H, J = 6.2 Hz, H-6′′′′), 0.93 (t, 3H, J = 6.9 Hz, CH3), 0.92 (t, 3H, J = 7.0 Hz, CH3); 13C-NMR (CD3OD): δ 172.9, 102.7, 102.6, 102.5, 100.1, 78.6, 77.8, 77.2, 72.7, 72.5, 71.8, 71.7, 70.8 (three), 70.7, 70.5, 69.2, 68.9, 68.7, 67.4, 66.5. 33.8, 31.6, 31.1, 29.1, 29.0 (two), 25.9, 24.3, 22.3, 21.9, 16.5 (three), 16.4, 13.0, 12.9; HRESIMS calcd for C38H68O18Na [M + Na]+ 835.4303; found, 835.4306.
3.2.17. 1-Octyloxy-α-L-rhamnopyranosyl-(1→3)-α-L-rhamnopyranosyl-(1→3)-α-L-rhamnopyranosyl-(1→3)-α-L-rhamnopyranoside (14)
To a stirred solution of 37 (320 mg, 14.9 mmol) in dry CH3OH (10 mL) MeONa (81 mg, 1.5 mmol) was added at 50 °C. The reaction mixture was stirred at 50 °C for 24 h, after which the reaction mixture was neutralized with Dowex 50 × 8 (H+) resin until pH 7, filtered and concentrated in vacuo to furnish a crude product, which was purified via silica gel column chromatography (4:1, trichloromethane—methanol) to afford 14 (180 mg, 91%) as a white solid. 1H-NMR (CD3OD): δ 5.03 (d, 1H, J = 1.7 Hz, H-1′′′′), 5.02 (d, 1H, J = 1.6 Hz, H-1′′′), 5.01 (d, 1H, J = 1.3 Hz, H-1′′), 4.65 (d, 1H, J = 1.7 Hz, H-1′), 4.07–4.09 (m, 2H), 3.99 (dd, 1H, J = 3.4, 1.7 Hz, H-2′′), 3.86–3.90 (m, 4H), 3.80–3.85 (m, 2H), 3.79 (dd, 1H, J = 9.5, 3.4 Hz), 3.76 (dd, 1H, J = 9.6, 3.3 Hz), 3.67–3.71 (m, 1H, H-1-1), 3.60–3.65 (m, 1H), 3.50–3.56 (m, 3H), 3.39–3.44 (m, 2H), 1.58–1.63 (m, 2H, H-2), 1.35–1.42 (m, 2H, H-3), 1.32–1.35 (m, 8H, 4 × CH2), 1.28 (d, 6H, J = 6.2 Hz, H-6′, H-6′′), 1.27 (d, 6H, J = 6.2 Hz, H-6′′′, 6′′′′), 0.92 (t, 3H, J = 7.2 Hz, CH3); 13C-NMR (CD3OD): δ 106.6, 106.5, 106.4, 104.1, 82.5, 82.4, 82.0, 76.7, 75.8 (three), 74.8 (two), 74.7, 74.5 (two), 72.9 (two), 72.7 (two), 71.2, 35.5, 33.1, 33.0, 32.9, 29.9, 26.2, 20.5, 16.9; HRMALDIMS calcd for C32H58O17Na 737.3572; found 737.3576.