Saffron: An Old Medicinal Plant and a Potential Novel Functional Food
Abstract
:1. Chemical Composition of Saffron
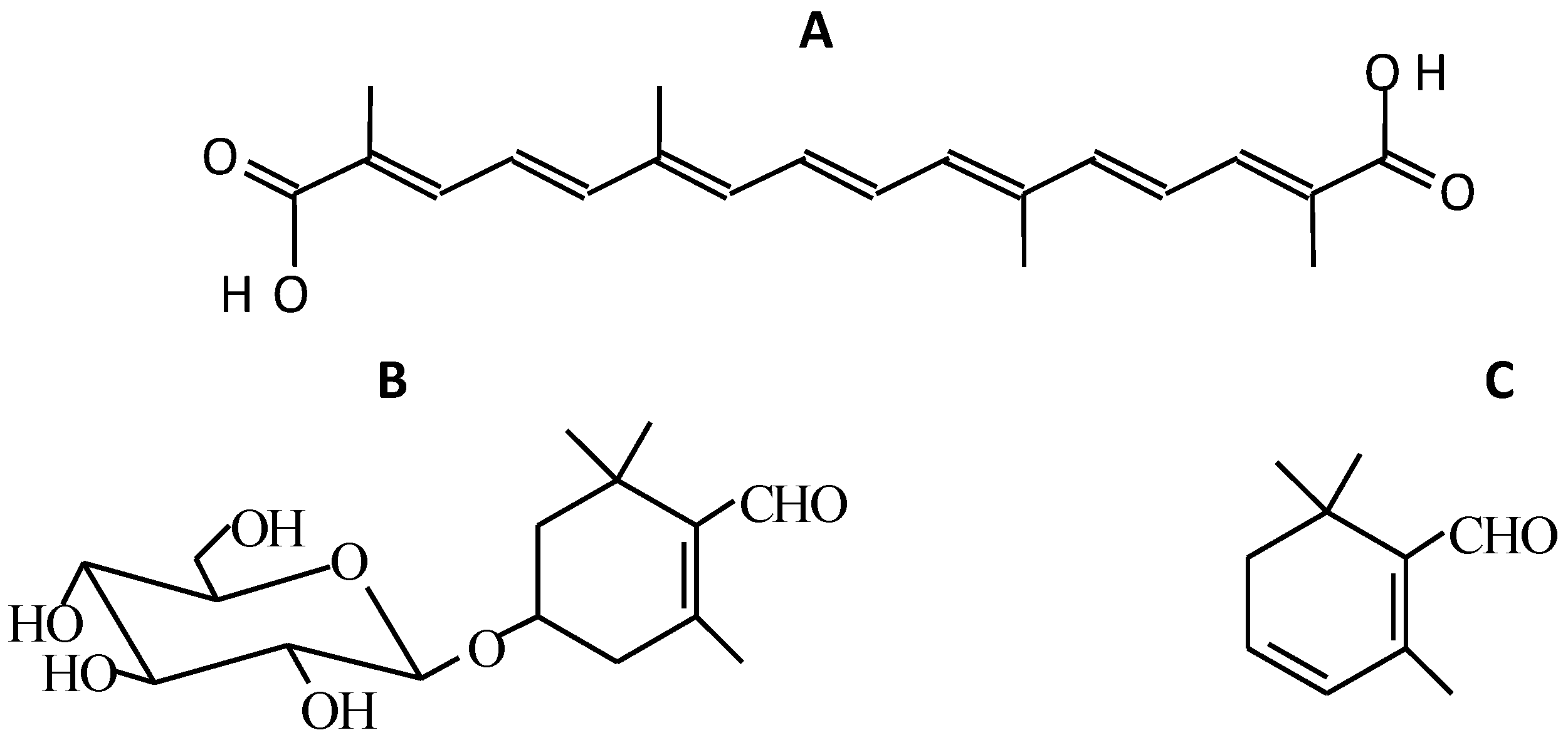
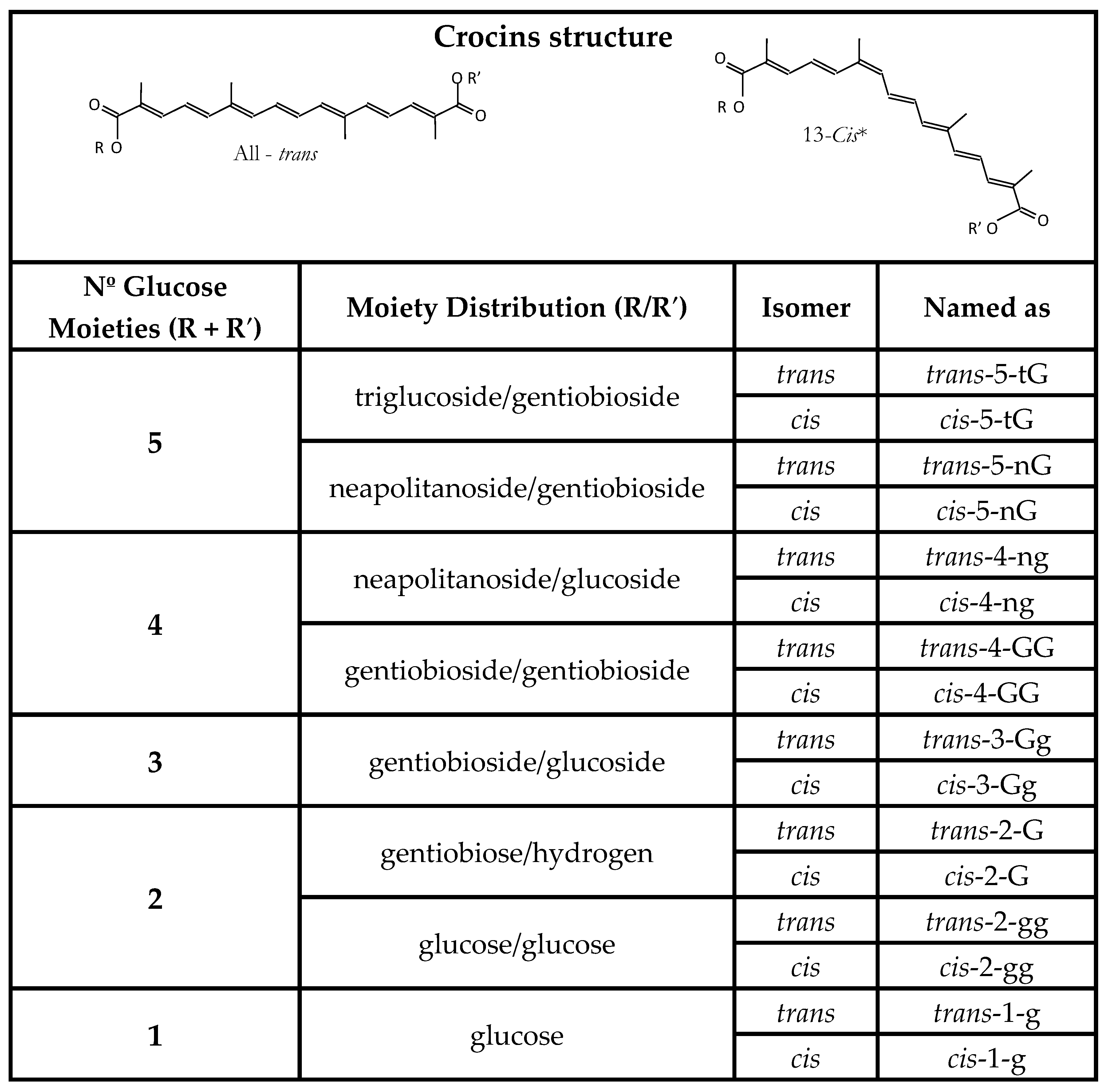
1.1. Compounds Responsible for the Colour
1.2. Compounds Responsible for the Bitter Taste
1.3. Compounds Responsible for the Aroma
1.4. The Need to Analyze the Main Components of Saffron
2. Bioaccessibility, Bioavailability and Bioactivity of Saffron’s Compounds
3. Relationship between Bioactivity and Antioxidant Capacity of Saffron
4. Therapeutic Properties of Saffron
4.1. Activity of Saffron in the Central Nervous System (CNS) and the Peripheral Nervous System
4.1.1. Effect on Memory and Learning
4.1.2. Effect on Alzheimer’s Disease
4.1.3. Effect on Parkinson’s Disease
4.1.4. Effect on Cerebral Ischemia
4.1.5. Effect on CNS Tumours
4.1.6. Age Related Macular Degeneration
4.1.7. Effect on Multiple Sclerosis (MS)
4.1.8. Effect on Nerve Damage Secondary to Diabetes Mellitus (DM)
4.1.9. Antidepressant and Anxiolytic Effects
4.2. Effect on the Cardiovascular System
4.3. Effect on Liver Function
4.4. Antineoplastic Effect
- In dermatological tumors in mice, the oral administration of saffron inhibits the formation of papillomas and simultaneously decreases their size [138].
- There are studies, both in vitro and in vivo, with pancreatic tumor cells which indicate that crocetin and its esters could have a potent antitumor action via the inhibition of apoptosis [139]. When saffron extract (300 mg/kg), crocin (200 mg/kg) and crocetin (100 mg/kg) were tested on prostate cancer, the tumor size decreased. In particular, crocetin, reduced tumor mass by 75–85% [140]. In colorectal cancer cell lines (HCT-116, SW-480 and HT-29), crocetin esters inhibited the growth of malignant cells, while not affecting the growth of normal cells [141].
4.5. Anti-Adiposity Effects
4.6. Other Effects
5. Saffron as a Functional Food. Dosage for Nutritional Intervention
6. Conclusions and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Carmona, M.; Zalacain, A.; Alonso, G.L. The Chemical Composition of Saffron: Color, Taste and Aroma; Bomarzo SL: Albacete, Spain, 2006. [Google Scholar]
- International Organization for Standardization (ISO). ISO 3632-1 Saffron (Crocus sativus L.). Part 1 (Specification) and Part 2 (Test Methods); ISO: Geneva, Switzerland, 2011. [Google Scholar]
- Código Alimentario Español; Boletín Oficial del Estado Español No. 248; Agencia Estatal Boletín Oficial del Estado: Madrid, Spain, 1967; p. 14384.
- Alonso, G.L.; Zalacain, A.; Carmona, C. Saffron. In Handbook of Herbs and Spices, 2nd ed.; Woodhead Publishing Limited: Philadelphia, PA, USA, 2012; Volume 1, pp. 469–498. [Google Scholar] [CrossRef]
- Samarghandian, S.; Borji, A. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacogn. Res. 2014, 6, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Díaz, J.; Sánchez, A.M.; Martínez-Tomé, M.; Winterhalter, P.; Alonso, G.L. A contribution to nutritional studies on Crocus sativus flowers and their value as food. J. Food Compos. Anal. 2013, 31, 101–108. [Google Scholar] [CrossRef]
- Alavizadeh, S.H.; Hosseinzadeh, H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem. Toxicol. 2014, 64, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, C.; Conquer, J.; Costa, D.; Hollands, W.; Iannuzzi, C.; Isaac, R.; Jordan, J.K.; Ledesma, N.; Ostroff, C.; Serrano, J.M.; et al. An evidence-based systematic review of saffron (Crocus sativus) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2011, 8, 58–114. [Google Scholar] [CrossRef] [PubMed]
- Ferrence, S.C.; Bendersky, G. Therapy with saffron and the goddess at Thera. Perspect. Biol. Med. 2004, 47, 199–226. [Google Scholar] [CrossRef] [PubMed]
- Colegio Oficial de Farmacéuticos de España. Catálogo de Plantas Medicinales; Colegio Oficial de Farmacéuticos de España: Madrid, Spain, 2011. [Google Scholar]
- Licón, C.; Carmona, M.; Llorens, S.; Berruga, M.I.; Alonso, G.L. Potential healthy effects of saffron spice (Crocus sativus L. stigmas) consumption. In Functional Plant Science and Biotechnology; Special Issue 2; Global Science Books, Ltd.: Ikenobe, Kagawa ken, Japan, 2010; pp. 64–73. [Google Scholar]
- Razak, S.I.A.; Anwar Hamzah, M.S.; Yee, F.C.; Kadir, M.R.A.; Nayan, N.H.M. A Review on Medicinal Properties of Saffron toward Major Diseases. J. Herbs Spices Med. Plants 2017, 23, 98–116. [Google Scholar] [CrossRef]
- Decker, F. Beiträge zur Kenntnis des Crocetins. Arch. Pharm. 1914, 252, 139–160. [Google Scholar] [CrossRef]
- Karrer, P.; Salomon, H. Über die Safranfarbstoffe II. (VI. Mitteilung über Pflanzenfarbstoffe). Helv. Chim. Acta 1928, 11, 513–525. [Google Scholar] [CrossRef]
- Karrer, P.; Salomon, H. Zur Kenntnis der Safranfarbstoffe III. (VI. Mitteilung über Pflanzenfarbstoffe). Helv. Chim. Acta 1928, 11, 711–713. [Google Scholar] [CrossRef]
- Karrer, P.; Helfenstein, A. Pflanzenfarbstoffe XX. Über die Safranfarbstoffe VI. Helv. Chim. Acta 1930, 13, 392–397. [Google Scholar] [CrossRef]
- IUPAC. Nomenclature of Carotenoids (Rules Approved 1974); Issued by the IUPAC Commision on the Nomenclature of Organic Chemistry and IUB Commision on Biochemical Nomenclature; Buters Worths: London, UK, 1974. [Google Scholar]
- Naess, S.N.; Elgsaeter, A.; Foss, B.J.; Li, B.; Sliwka, H.-R.; Patali, V.; Melø, T.B.; Naqvi, K.R. Hydrophilic carotenoids: Surface properties and aggregation of crocin as a biosurfactant. Helv. Chim. Acta 2006, 89, 45–53. [Google Scholar] [CrossRef]
- Pfander, H.; Wittwer, F. Carotenoid-Glycoside (3. Mitteilung) Untersuchungen zur carotinoid-Zusammensetzung im Safran. Helv. Chim. Acta 1975, 58, 1608–1620. [Google Scholar] [CrossRef] [PubMed]
- Speranza, G.; Dada, G.; Manitto, P.; Monti, D.; Grammatica, P. 13-Cis crocin: A new crocinoid of saffron. Gazz. Chim. Ital. 1984, 114, 189–192. [Google Scholar]
- Tarantilis, P.A.; Tsoupras, G.; Polissiou, M. Determination of saffron (Crocus sativus L.) components in crude plant extract using high-performance liquid chromatography-UV-visible photodiode-array detection-mass spectrometry. J. Chromatogr. A 1995, 699, 107–118. [Google Scholar] [CrossRef]
- Carmona, M.; Zalacain, A.; Sánchez, A.M.; Novella, J.L.; Alonso, G.L. Crocetin Esters, Picrocrocin and Its Related Compounds Present in Crocus sativus Stigmas and Gardenia jasminoides Fruits. Tentative Identification of Seven New Compounds by LC-ESI-MS. J. Agric. Food Chem. 2006, 54, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Corradi, C.; Micheli, G. Determinazione spettrofotometrica del podere colorante americante dello zafferano. Boll. Chim. Farm. 1979, 118, 553–562. [Google Scholar]
- Sujata, V.; Ravishankar, G.A.; Venkataraman, L.V. Methods for the analysis of the saffron metabolites crocin, crocetins, picrocrocin and safranal for the determination of the quality of the spice using thin-layer chromatography, high-performance liquid chromatography and gas chromatography. J. Chromatogr. A 1992, 624, 497–502. [Google Scholar] [CrossRef]
- Sánchez, A.M.; Carmona, M.; Zalacain, A.; Carot, J.M.; Jabaloyes, J.M.; Alonso, G.L. Rapid determination of crocetin esters and picrocrocin from saffron spice (Crocus sativus L.) using UV-visible spectrophotometry for quality control. J. Agric. Food Chem. 2008, 56, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, M.V.; Serrano-Díaz, J.; Tarantilis, P.A.; López-Córcoles, H.; Carmona, M.; Alonso, G.L. Determination of saffron quality by high-performance liquid chromatography. J. Agric. Food Chem. 2014, 62, 8068–8074. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.M.; Carmona, M.; del Campo, C.P.; Alonso, G.L. Solid-phase extraction for picrocrocin determination in the quality control of saffron spice (Crocus sativus L.). Food Chem. 2009, 116, 792–798. [Google Scholar] [CrossRef]
- Khun, R.; Winterstein, A. Über die Konstitution des Pikro-crocins und seine Beziehung zu den Carotin-Farbstoffen des Safrans. Eur. J. Inorg. Chem. 1934, 67, 344–347. [Google Scholar] [CrossRef]
- Sánchez, A.M.; Carmona, M.; Jarén-Galán, M.; Mosquera, M.I.; Alonso, G.L. Picrocrocin kinetics in aqueous saffron spice extracts (Crocus sativus L.) upon thermal treatment. J. Agric. Food Chem. 2011, 59, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Carmona, M.; Zalacain, A.; Salinas, M.R.; Alonso, G.L. A new approach to saffron aroma. Crit. Rev. Food Sci. Nutr. 2007, 47, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Official Journal of European Communities. 2000/C 173/05. 22 June 2000, pp. 4–8. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:C:2000:173:TOC (accessed on 23 December 2017).
- García-Rodríguez, M.V.; López-Córcoles, H.; Alonso, G.L.; Pappas, C.S.; Polissiou, M.G.; Tarantilis, P.A. Document Comparative evaluation of an ISO 3632 method and an HPLC-DAD method for safranal quantity determination in saffron. Food Chem. 2017, 221, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Anastasaki, E.; Kanakis, C.; Pappas, C.; Maggi, L.; del Campo, C.P.; Alonso, G.L.; Polissiou, M.G. Document Geographical differentiation of saffron by GC-MS/FID and chemometrics. Eur. Food Res. Technol. 2009, 229, 899–905. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Serrano, J.; Goñi, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef]
- Heaney, R.P. Factors influencing the measurement of bioavailability, taking calcium as a model. J. Nutr. 2001, 131, 1344–1348. [Google Scholar]
- Granado, F.; Olmedilla, B.; Herrero, C.; Pérez-Sacristán, B.; Blanco, I.; Blázquez, S. Bioavailability of carotenoids and tocopherols from broccoli: In vivo and in vitro assessment. Exp. Biol. Med. 2006, 231, 1733–1738. [Google Scholar] [CrossRef]
- Erdman, J.W., Jr.; Bierer, T.L.; Gugger, E.T. Absorption and transport of carotenoids. Ann. N. Y. Acad. Sci. 1993, 691, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.S. Absorption, metabolism, and transport of carotenoids. FASEB J. 1996, 10, 542–551. [Google Scholar] [PubMed]
- Chryssanthi, D.G.; Lamari, F.N.; Georgakopoulos, C.D.; Cordopatis, P. A new validated SPE-HPLC method for monitoring crocetin in human plasma—Application after saffron tea consumption. J. Pharm. Biomed. Anal. 2011, 55, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Qian, Z.; Du, P.; Fu, J. Pharmacokinetic properties of crocin (crocetin digentiobiose ester) following oral administration in rats. Phytomedicine 2007, 14, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Kyriakoudi, A.; Tsimidou, M.Z.; O’Callaghan, Y.C.; Galvin, K.; O’Brien, N.M. Changes in total and individual crocetin esters upon in vitro gastrointestinal digestion of saffron aqueous extracts. J. Agric. Food Chem. 2013, 61, 5318–5327. [Google Scholar] [CrossRef] [PubMed]
- Kyriakoudi, A.; O’Callaghan, Y.C.; Galvin, K.; Tsimidou, M.Z.; O’Brien, N.M. Cellular Transport and Bioactivity of a Major Saffron Apocarotenoid, Picrocrocin (4-(β-d-Glucopyranosyloxy)-2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde). J. Agric. Food Chem. 2015, 63, 8662–8668. [Google Scholar] [CrossRef] [PubMed]
- Lautenschläger, M.; Sendker, J.; Hüwel, S.; Galla, H.J.; Brandt, S.; Düfer, M.; Riehemann, K.; Hensel, K. Intestinal formation of trans-crocetin from saffron extract (Crocus sativus L.) and in vitro permeation through intestinal and blood brain barrier. Phytomedicine 2015, 22, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fei, F.; Zhen, L.; Zhu, X.; Wang, J.; Li, S.; Geng, J.; Sun, R.; Yu, X.; Chen, T.; et al. Sensitive analysis and simultaneous assessment of pharmacokinetic properties of crocin and crocetin after oral administration in rats. J. Chromatogr. B 2017, 1044–1045, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, F.; Yoshida, A.; Umigai, N.; Kubo, K.; Lee, M.C.I. Crocetin reduces the oxidative stress induced reactive oxygen species in the stroke-prone spontaneously hypertensive rats (SHRSPs) brain. J. Clin. Biochem. Nutr. 2011, 49, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Linardaki, Z.I.; Orkoula, M.G.; Kokkosis, A.G.; Lamari, F.N.; Margarity, M. Investigation of the neuroprotective action of saffron (Crocus sativus L.) in aluminum-exposed adult mice through behavioral and neurobiochemical assessment. Food Chem. Toxicol. 2013, 52, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Nakano, T.; Takahashi, M.; Nagao, A. Orally administered crocetin and crocins are absorbed into blood plasma as crocetin and its glucuronide conjugates in mice. J. Agric. Food Chem. 2005, 53, 7302–7306. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Qian, Z.; Tang, F.; Sheng, L. Suppression of vascular cell adhesion molecule-1 expression by crotetin contributes to attenuation of atherosclerosis in hypercholesterolemic rabbits. Biochem. Pharmacol. 2005, 70, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Si, P.; Wang, H.; Tahir, U.; Chen, K.; Xiao, J.; Duan, X.; Huang, R.; Xiang, G. Crocetin induces apoptosis of BGC-823 human gastric cancer cells. Mol. Med. Rep. 2014, 9, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Umigai, N.; Murakami, K.; Ulit, M.V.; Antonio, L.S.; Shirotori, M.; Morikawa, H.; Nakano, T. The pharmacokinetic profile of crocetin in healthy adult human volunteers after a single oral administration. Phytomedicine 2011, 18, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Mohamadpour, A.H.; Ramezani, M.; Anaraki, N.T.; Malaekeh-Nikouei, B.; Farzad, S.A.; Hosseinzadeh, H. Development and validation of hplc method for determination of crocetin, a constituent of saffron, in human serum samples. Iran J. Basic Med. Sci. 2013, 16, 47–55. [Google Scholar]
- Alessio, H.M. Exercise-induced oxidative stress. Med. Sci. Sports Exerc. 1993, 25, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Mizuma, H.; Tanaka, M.; Nozaki, S.; Mizuno, K.; Tahara, T.; Ataka, S.; Sugino, T.; Shirai, T.; Kajimoto, Y.; Kuratsune, H.; et al. Daily oral administration of crocetin attenuates physical fatigue in human subjects. Nutr. Res. 2009, 29, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, C.D.; Tarantilis, P.A.; Tajimir-Riahi, H.A.; Polissiou, M.G. Crocetin, dimethylcrocetin, and safranal bindhumanserum albumin: Stability and antioxidative properties. J. Agric. Food Chem. 2007, 55, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Martínez Coyuela, M. Oxygen free radicals and human disease. Biochimie 1995, 77, 147–161. [Google Scholar] [CrossRef]
- Scannevin, R.H.; Chollate, S.; Jung, M.-Y.; Shackett, M.; Patel, H.; Bista, P.; Zeng, W.; Ryan, S.; Yamamoto, M.; Lukashev, M.; et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J. Pharmacol. Exp. Ther. 2012, 341, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Lipton, S.A. Regulation of neuronal oxidative and nitrosative stress by endogenous protective pathways and disease processes. Antioxid. Redox Signal. 2011, 14, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Nassiri-Asl, M. Avicenna’s (Ibn Sina) the canon of medicine and saffron (Crocus sativus): A review. Phytother. Res. 2013, 27, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Bolhassani, A.; Khavari, A.; Bathaie, S.Z. Saffron and natural carotenoids: Biochemical activities and anti-tumor effects. Biochim. Biophys. Acta 2014, 1845, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Assimopoulou, A.N.; Sinakos, Z.; Papageorgiou, V.P. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother. Res. 2005, 19, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Mashmoul, M.; Azlan, A.; Khaza’ai, H.; Yusof, B.N.; Noor, S.M. Saffron: A Natural Potent Antioxidant as a Promising Anti-Obesity Drug. Antioxidants 2013, 2, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Farahmand, S.K.; Samini, F.; Samini, M.; Samarghandian, S. Safranal ameliorates antioxidant enzymes and suppresses lipid peroxidation and nitric oxide formation in aged male rat liver. Biogerontology 2013, 14, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, C.D.; Tarantilis, P.A.; Pappas, C.; Bariyanga, J.; Tajmir-Riahi, H.A.; Polissiou, M.G. An overview of structural features of DNA and RNA complexes with saffron compounds: Models and antioxidant activity. J. Photochem. Photobiol. B 2009, 95, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Oskoueian, E.; Hendra, R.; Jaafar, H.Z.E. Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules 2010, 15, 6244–6256. [Google Scholar] [CrossRef] [PubMed]
- Olmedilla, B.; Granado, F. Carotenoids and retinol-equivalents in food composition tables from European countries (EPIC study). Eur. J. Clin. Nutr. 2000, 54, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Escribano, J.; Alonso, G.L.; Coca-Prados, M.; Fernández, J.A. Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Lett. 1996, 100, 23–30. [Google Scholar] [CrossRef]
- Hausenblas, H.A.; Saha, D.; Dubyak, P.J.; Anton, S.D. Saffron (Crocus sativus L.) and major depressive disorder: A meta-analysis of randomized clinical trials. J. Integr. Med. 2013, 11, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.N.; Park, Y.-M.; Jung, H.-J.; Lee, J.Y.; Min, B.D.; Park, S.-U.; Jung, W.-S.; Cho, K.-H.; Park, J.-H.; Kang, I.; et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur. J. Pharmacol. 2010, 648, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Van Horssen, J.; Witte, M.E.; Schreibelt, G.; de Vries, H.E. Radical changes in multiple sclerosis pathogenesis. Biochim. Biophys. Acta 2011, 1812, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Gout, B.; Bourges, C.; Paineau-Dubreuil, S. Satiereal, a Crocus sativus L extract, reduces snacking and increases satiety in a randomized placebo-controlled study of mildly overweight, healthy women. Nutr. Res. 2010, 30, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Talebzadeh, F. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia 2005, 76, 722–724. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Sadeghnia, H.R. Protective effect of safranal on pentylenetetrazol-induced seizures in the rat: Involvement of GABAergic and opioids systems. Phytomedicine 2007, 14, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Ziaee, T.; Sadeghi, A. The effect of saffron, Crocus sativus stigma, extract and its constituents, safranal and crocin on sexual behaviors in normal male rats. Phytomedicine 2008, 15, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Shoyama, Y.; Saito, H.; Abe, K. Crocin (crocetin di-gentiobiose ester) prevents the inhibitory effect of ethanol on long-term potentiation in the dentate gyrus in vivo. J. Pharmacol. Exp. Ther. 1994, 271, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Saito, H.; Abe, K.; Shoyama, Y. The Effects of Ethanol and Crocin on the Induction of Long-Term Potentiation in the CA1 Region of Rat Hippocampal Slices. Jpn. J. Pharmacol. 1995, 67, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Saito, H. Effects of saffron extract and its constituent crocin on learning behaviour and long-term potentiation. Phytother. Res. 2000, 14, 149–152. [Google Scholar] [CrossRef]
- Zheng, Y.-Q.; Liu, J.-X.; Wang, J.-N.; Xu, L. Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res. 2007, 1138, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Abootorabi, A.; Sadeghnia, H.R. Protective effect of Crocus sativus stigma extract and crocin (trans-crocin 4) on methyl methanesulfonate-induced DNA damage in mice organs. DNA Cell Biol. 2008, 27, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Ghazavi, A.; Mosaycbi, G.; Salehi, H.; Abtahi, H. Effect of ethanol extract of Saffron (Crocus sativus L.) on the inhibition of Experimental Autoimmune Encephalomyelitis in C57BL/6 mice. Pak. J. Biol. Sci. 2009, 12, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Rajaei, Z.; Hadjzadeh, M.A.; Nemati, H.; Hosseini, M. Crocin improves spatial learning and memory deficits in the Morris water maze via attenuating cortical oxidative damage in diabetic rats. Neurosci. Lett. 2017, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, V.M.; Buyukturkoglu, K.; Inglese, M.; Sumowski, J.F. Protective personality traits: High openness and low neuroticism linked to better memory in multiple sclerosis. Mult. Scler. 2017, 23, 1786–1790. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Sugiura, M.; Shoyama, Y.; Saito, H. Crocin antagonizes ethanol inhibition of NMDA receptor-mediated responses in rat hippocampal neurons. Brain Res. 1998, 787, 132–138. [Google Scholar] [CrossRef]
- Pitsikas, N.; Zisopoulou, S.; Tarantilis, P.A.; Kanakis, C.D.; Polissiou, M.G.; Sakellaridis, N. Effects of the active constituents of Crocus sativus L., crocins on recognition and spatial rats’ memory. Behav. Brain Res. 2007, 183, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S.; Hatami, H.; Dehghan, G. Saffron ethanolic extract attenuates oxidative stress, spatial learning, and memory impairments induced by local injection of ethidium bromide. Res. Pharm. Sci. 2015, 10, 222–232. [Google Scholar] [PubMed]
- Papandreou, M.A.; Kanakis, C.D.; Polissiou, M.G.; Efthimiopoulos, S.; Cordopatis, P.; Margarity, M.; Lamari, F.N. Inhibitory activity on amyloid-beta aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J. Agric. Food Chem. 2006, 54, 8762–8768. [Google Scholar] [CrossRef] [PubMed]
- Finley, J.W.; Gao, S. A Perspective on Crocus sativus L. (Saffron) Constituent Crocin: A Potent Water-Soluble Antioxidant and Potential Therapy for Alzheimer’s Disease. J. Agric. Food Chem. 2017, 65, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Geromichalos, G.D.; Lamari, F.N.; Papandreou, M.A.; Trafalis, D.T.; Margarity, M.; Papageorgiou, A.; Sinakos, Z. Saffron as a source of novel acetylcholinesterase inhibitors: Molecular docking and in vitro enzymatic studies. J. Agric. Food Chem. 2012, 60, 6131–6138. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Shafiee-Sabet, M.; Harirchian, M.H.; Togha, M.; Cheraghmakani, H.; Razeghi, S.; Hejazi, S.; Yousefi, M.H.; Alimardani, R.; Jamshidi, A.; et al. Saffron in the treatment of patients with mild to moderate Alzheimer’s disease: A 16-week, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2010, 35, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Shafiee Sabet, M.; Harirchian, M.H.; Togha, M.; Cheraghmakani, H.; Razeghi, S.; Hejazi, S.S.; Yousefi, M.H.; Alimardani, R.; Jamshidi, A.; et al. A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer’s disease. Psychopharmacology 2010, 207, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Farokhnia, M.; Shafiee Sabet, M.; Iranpour, N.; Gougol, A.; Yekehtaz, H.; Alimardani, R.; Farsad, F.; Kamalipour, M.; Akhondzadeh, S. Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer’s disease: A double-blind randomized clinical trial. Hum. Psychopharmacol. 2014, 29, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.S.; Ansari, M.A.; Ahmad, M.; Saleem, S.; Yousuf, S.; Hoda, M.N.; Islam, F. Neuroprotection by crocetin in a hemi-parkinsonian rat model. Pharmacol. Biochem. Behav. 2005, 81, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Purushothuman, S.; Nandasena, C.; Peoples, C.L.; El Massri, N.; Johnstone, D.M.; Mitrofanis, J.; Stone, J. Saffron pre-treatment offers neuroprotection to Nigral and retinal dopaminergic cells of MPTP-Treated mice. J. Park. Dis. 2013, 3, 77–83. [Google Scholar] [CrossRef]
- Vakili, A.; Einali, M.R.; Bandegi, A.R. Protective Effect of Crocin against Cerebral Ischemia in a Dose-dependent Manner in a Rat Model of Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2014, 23, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Ahmad, M.; Ahmad, A.S.; Yousuf, S.; Ansari, M.A.; Khan, M.B.; Ishrat, T.; Islam, F. Effect of saffron (Crocus sativus) on neurobehavioral and neurochemical changes in cerebral ischemia in rats. J. Med. Food 2006, 9, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Shimeno, H.; Mishima, K.; Iwasaki, K.; Fujiwara, M.; Tanaka, H.; Shoyama, Y.; Toda, A.; Eyanagi, R.; Soeda, S. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim. Biophys. Acta 2007, 1770, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Gainer, J.L.; Sheehan, J.P.; Larner, J.M.; Jones, D.R. Trans sodium crocetinate with temozolomide and radiation therapy for glioblastoma multiforme. J. Neurosurg. 2017, 126, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.; Sherman, J.; Cifarelli, C.; Jagannathan, J.; Dassoulas, K.; Olson, C.; Rainey, J.; Han, S. Effect of trans sodium crocetinate on brain tumor oxgenation. J. Neurosurg. 2009, 111, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Bisti, S.; Maccarone, R.; Falsini, B. Saffron and retina: Neuroprotection and pharmacokinetics. Vis. Neurosci. 2014, 31, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Piccardi, M.; Marangoni, D.; Minnella, A.M.; Savastano, M.C.; Valentini, P.; Ambrosio, L.; Capoluongo, E.; Maccarone, R.; Bisti, S.; Falsini, B. A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: Sustained benefits to central retinal function. Evid. Based Complement. Altern. Med. 2012, 2012, 429124. [Google Scholar] [CrossRef] [PubMed]
- Falsini, B.; Piccardi, M.; Minnella, A.; Savastano, C.; Capoluongo, E.; Fadda, A.; Balestrazzi, E.; Maccarone, R.; Bisti, S. Influence of saffron supplementation on retinal flicker sensitivity in early age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6118–6124. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Tsuruma, K.; Imai, S.; Nakanishi, T.; Umigai, N.; Shimazawa, M.; Hara, H. Crocetin prevents retinal degeneration induced by oxidative and endoplasmic reticulum stresses via inhibition of caspase activity. Eur. J. Pharmacol. 2011, 650, 110–119. [Google Scholar] [CrossRef] [PubMed]
- González-González, L.; Pérez-Cortéz, J.G.; Flores-Aldana, M.; Macías-Morales, N.; Hernández-Girón, C. Antioxidant use as dietary therapy in patients with multiple sclerosis. Medwave 2015, 15, e6065. [Google Scholar] [CrossRef] [PubMed]
- Flores-Alvarado, L.J.; Gabriel-Ortiz, G.; Pacheco-Moisés, F.P.; Bitzer-Quintero, O.K. Pathogenic mechanisms of neuronal damage in multiple sclerosis. Investig. Clin. 2015, 56, 201–214. [Google Scholar] [PubMed]
- Deslauriers, A.M.; Afkhami-Goli, A.; Paul, A.M.; Bhat, R.K.; Acharjee, S.; Ellestad, K.K.; Noorbakhsh, F.; Michalak, M.; Power, C. Neuroinflammation and endoplasmic reticulum stress are coregulated by crocin to prevent demyelination and neurodegeneration. J. Immunol. 2011, 187, 4788–4799. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.C.; Hur, J.; Feldman, E.L. Diabetic neuropathy: One disease or two? Curr. Opin. Neurol. 2012, 25, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, B.; Bathaie, S.Z.; Fadai, F.; Ashtari, Z.; Ali Beigi, N.; Farhang, S.; Hashempour, S.; Shahhamzei, N.; Heidarzadeh, H. Safety evaluation of saffron stigma (Crocus sativus L.) aqueous extract and crocin in patients with schizophrenia. Avicenna J. Phytomed. 2015, 5, 413–419. [Google Scholar] [PubMed]
- Akhondzadeh, S.; Fallah-Pour, H.; Afkham, K.; Jamshidi, A.H.; Khalighi-Cigaroudi, F. Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: A pilot double-blind randomized trial [ISRCTN45683816]. BMC Complement. Altern. Med. 2004, 4. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Tahmacebi-Pour, N.; Noorbala, A.A.; Amini, H.; Fallah-Pour, H.; Jamshidi, A.H.; Khani, M. Crocus sativus L. in the treatment of mild to moderate depression: A double-blind, randomized and placebo-controlled trial. Phytother. Res. 2005, 19, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Noorbala, A.A.; Akhondzadeh, S.; Tahmacebi-Pour, N.; Jamshidi, A.H. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: A double-blind, randomized pilot trial. J. Ethnopharmacol. 2005, 97, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Shahmansouri, N.; Farokhnia, M.; Abbasi, S.-H.; Kassaian, S.E.; Noorbala Tafti, A.A.; Gougol, A.; Yekehtaz, H.; Forghani, S.; Mahmoodian, M.; Saroukhani, S.; et al. A randomized, double-blind, clinical trial comparing the efficacy and safety of Crocus sativus L. with fluoxetine for improving mild to moderate depression in post percutaneous coronary intervention patients. J. Affect. Disord. 2014, 155, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Talaei, A.; Hassanpour Moghadam, M.; Sajadi Tabassi, S.A.; Mohajeri, S.A. Crocin, the main active saffron constituent, as an adjunctive treatment in major depressive disorder: A randomized, double-blind, placebo-controlled, pilot clinical trial. J. Affect. Disord. 2015, 174, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Ghajar, A.; Neishabouri, S.M.; Velayati, N.; Jahangard, L.; Matinnia, N.; Haghighi, M.; Ghaleiha, A.; Afarideh, M.; Salimi, S.; Meysamie, A.; et al. Crocus sativus L. versus Citalopram in the Treatment of Major Depressive Disorder with Anxious Distress: A Double-Blind, Controlled Clinical Trial. Pharmacopsychiatry 2016, 50, 152–160. [Google Scholar] [CrossRef] [PubMed]
- De Monte, C.; Carradori, S.; Chimenti, P.; Secci, D.; Mannina, L.; Alcaro, F.; Petzer, A.; N’Da, C.I.; Gidaro, M.C.; Costa, G.; et al. New insights into the biological properties of Crocus sativus L.: Chemical modifications, human monoamine oxidases inhibition and molecular modeling studies. Eur. J. Med. Chem. 2014, 82, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Agha-Hosseini, M.; Kashani, L.; Aleyaseen, A.; Ghoreishi, A.; Rahmanpour, H.; Zarrinara, A.R.; Akhondzadeh, S. Crocus sativus L. (saffron) in the treatment of premenstrual syndrome: A double-blind, randomised and placebo-controlled trial. BJOG 2008, 115, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Jahanian, Z. Effect of Crocus sativus L. (saffron) stigma and its constituents, crocin and safranal, on morphine withdrawal syndrome in mice. Phytother. Res. 2010, 24, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Amin, B.; Hosseinsadeh, H. Evaluation of aqueous and ethanolic extracts of saffron, Crocus sativus L., and its constituents, safranal and crocin in allodynia and hyperalgesia induced by chronic constriction injury model of neuropathic pain in rats. Fitoterapia 2012, 83, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Naghibi, S.M.; Hosseini, M.; Khani, F.; Rahimi, M.; Vafaee, F.; Rakhshandeh, H.; Aghaie, A. Effect of Aqueous Extract of Crocus sativus L. on Morphine-Induced Memory Impairment. Adv. Pharmacol. Sci. 2012, 2012, 494367. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Serrano-Díaz, J.; Nava, E.; D’Alessandro, A.M.; Alonso, G.L.; Carmona, M.; Llorens, S. Crocetin, a carotenoid derived from saffron (Crocus sativus L.), improves acetylcholine-induced vascular relaxation in hypertension. J. Vasc. Res. 2014, 51, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Llorens, S.; Mancini, A.; Serrano-Díaz, J.; D’Alessandro, A.M.; Nava, E.; Alonso, G.L.; Carmona, M. Effects of crocetin esters and crocetin from Crocus sativus L. on aortic contractility in rat genetic hypertension. Molecules 2015, 20, 17570–17584. [Google Scholar] [CrossRef] [PubMed]
- Gainer, J.L.; Jones, J.R. The use of crocetin in experimental atherosclerosis. Experientia 1975, 31, 548–549. [Google Scholar] [CrossRef] [PubMed]
- Bagur, M.J.; Alonso Salinas, G.L.; Jiménez-Monreal, A.M.; Serrano-Heras, G.; Martínez-Tomé, M.; Murcia, M.A.; Alonso, G.L. Effect of Saffron Intake on Lipid Profile in Multiple Sclerosis Patients. 2018; unpublished data sent for publication. [Google Scholar]
- Wang, Y.; Sun, J.; Liu, C.; Fang, C. Protective effects of crocetin pretreatment on myocardial injury in an ischemia/reperfusion rat model. Eur. J. Pharmacol. 2014, 741, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Gainer, J.L. Trans-sodium crocetinate for treating hypoxia/ischemia. Expert Opin. Investig. Drugs 2008, 17, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Giassi, L.J.; Gainer, J.L. TSC and hemorrhagic shock: A Review. Adv. Exp. Med. Biol. 2003, 540, 55–60. [Google Scholar] [PubMed]
- Yang, R.; Vernon, K.; Thomas, A.; Morrison, D.; Qureshi, N.; Van Way, C.W., III. Crocetin reduces activation of hepatic apoptotic pathways and improves survival in experimental hemorrhagic shock. J. Parenter. Enteral. Nutr. 2011, 35, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Giassi, L.J.; Poynter, A.K.; Gainer, J.L. Trans sodium crocetinate for hemorrhagic shock: Effect of time delay in initiating therapy. Shock 2002, 18, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Lari, P.; Abnous, K.; Imenshahidi, M.; Rashedinia, M.; Razavi, M.; Hosseinzadeh, H. Evaluation of diazinon-induced hepatotoxicity and protective effects of crocin. Toxicol. Ind. Health 2015, 31, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Bathaie, S.Z.; Hoshyar, R.; Miri, H.; Sadeghizadeh, M. Anticancer effects of crocetin in both human adenocarcinoma gastric cancer cells and rat model of gastric cancer. Biochem. Cell Biol. 2013, 91, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Shoshtari, M.E.; Sargolzaei, J.; Hossinimoghadam, H.; Farahzad, J.A.; Samarghandian, S. Anti-tumor activity of safranal against neuroblastoma cells. Pharmacogn. Mag. 2014, 10, S419–S424. [Google Scholar]
- Bakshi, H.; Sam, S.; Rozati, R.; Sultan, P.; Islam, T.; Rathore, B.; Lone, Z.; Sharma, M.; Triphati, J.; Saxena, R.C. DNA fragmentation and cell cycle arrest: A hallmark of apoptosis induced by crocin from Kashmiri saffron in a human pancreatic cancer cell line. Asian Pac. J. Cancer Prev. 2010, 11, 675–679. [Google Scholar] [PubMed]
- Sun, Y.; Xu, H.J.; Zhao, Y.X.; Wang, L.Z.; Sun, L.R.; Wang, Z.; Sun, X.F. Crocin Exhibits Antitumor Effects on Human Leukemia HL-60 Cells In Vitro and In Vivo. Evid. Based Complement. Altern. Med. 2013, 2013, 690164. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Huang, W.F.; Wang, Q.L.; Cai, F.W.; Hu, B.; Du, J.C.; Wang, J.; Chen, R.; Cai, X.J.; Feng, J.; et al. Crocetin induces cytotoxicity in colon cancer cells via p53-independent mechanisms. Asian Pac. J. Cancer Prev. 2012, 13, 3757–3761. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Tavakkol Afshari, J.; Davoodi, S. Suppression of pulmonary tumor promotion and induction of apoptosis by Crocus sativus L. extraction. Appl. Biochem. Biotechnol. 2011, 164, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Borji, A.; Farahmand, S.K.; Afshari, R.; Davoodi, S. Crocus sativus L. (saffron) stigma aqueous extract induces apoptosis in alveolar human lung cancer cells through caspase-dependent pathways activation. Biomed. Res. Int. 2013, 2013, 417928. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.M.; Mancini, A.; Lizzi, A.R.; De Simone, A.; Marroccella, C.E.; Gravina, G.L.; Tatone, C.; Festuccia, C. Crocus sativus stigma extract and its major constituent crocin possess significant antiproliferative properties against human prostate cancer. Nutr. Cancer. 2013, 65, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Gutheil, W.G.; Reed, G.; Ray, A.; Anant, S.; Dhar, A. Crocetin: An agent derived fromsaffron for prevention and therapy for cancer. Curr. Pharm. Biotechnol. 2012, 13, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Abdullaev, F.I. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.). Exp. Biol. Med. 2002, 227, 20–25. [Google Scholar] [CrossRef]
- Das, I.; Das, S.; Saha, T. Saffron suppresses oxidative stress in DMBA-induced skin carcinoma: A histopathological study. Acta Histochem. 2010, 112, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Mehta, S.; Dhar, G.; Dhar, K.; Banerjee, S.; Van Veldhuizen, P.; Campbell, D.R.; Banerjee, S.K. Crocetin inhibits pancreatic cancer cell proliferation and tumor progression in a xenograft mouse model. Mol. Cancer Ther. 2009, 8, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Festuccia, C.; Mancini, A.; Gravina, G.L.; Scarsella, L.; Llorens, S.; Alonso, G.L.; Tatone, C.; Di Cesare, E.; Jannini, E.A.; Lenzi, A.; et al. Antitumor effects of saffron-derived carotenoids in prostate cancer cell models. Biomed. Res. Int. 2014, 2014, 135048. [Google Scholar] [CrossRef] [PubMed]
- García-Olmo, D.C.; Riese, H.H.; Escribano, J.; Ontañón, J.; Fernández, J.A.; Atiénzar, M.; García-Olmo, D. Effects of long-term treatment of colon adenocarcinoma with crocin, a carotenoid from saffron (Crocus sativus L.): An experimental study in the rat. Nutr. Cancer 1999, 35, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Qian, Z.; Zheng, S.; Xi, L. Mechanism of hypolipidemic effect of crocin in rats: Crocin inhibits pancreatic lipase. Eur. J. Pharmacol. 2006, 543, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Azimi-Nezhad, M.; Samini, F. Ameliorative effect of saffron aqueous extract on hyperglycemia, hyperlipidemia, and oxidative stress on diabetic encephalopathy in streptozotocin induced experimental diabetes mellitus. Biomed. Res. Int. 2014, 2014, 920857. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Qian, Z.; Xu, G.; Zhou, C.; Sun, S. Crocetin attenuates palmitate-induced insulin insensitivity and disordered tumor necrosis factor-alpha and adiponectin expression in rat adipocytes. Br. J. Pharmacol. 2007, 151, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Qian, Z.; Shi, Y.; Yang, L.; Xi, L. Crocetin improves the insulin resistance induced by high-fat diet in rats. Br. J. Pharmacol. 2008, 154, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Younesi, H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Alonso, G.L.; Salinas, M.R.; Garijo, J. Method to determine the authenticity of aroma of saffron (Crocus sativus L.). J. Food Prot. 1998, 61, 1525–1528. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G. Functional Foods: Biochemical and Processing Aspects; Technomic Publishing Co.: Lancaster, PA, USA, 1998. [Google Scholar]
- Goldberg, R. The business of agriceuticals. Nat. Biotechnol. 1999, 17, BV5–BV6. [Google Scholar] [CrossRef]
- Srivastava, R.; Ahmed, H.; Dixit, R.; Dharamveer; Saraf, S.A. Crocus sativus L.: A comprehensive Review. Pharmacogn. Rev. 2010, 4, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Modaghegh, M.-H.; Shahabian, M.; Esmaeili, H.-A.; Rajbai, O.; Hosseinzadeh, H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine 2008, 15, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E. Herbal medicinal products during pregnancy: Are they safe? BJOG 2002, 109, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Precious and magic saffron. Pharm. Care Res. 2014, 14, S1–S2. [Google Scholar]
- Bahmani, M.; Rafieian, M.; Baradaran, A.; Rafieian, S.; Rafieian-Kopaei, M. Nephrotoxicity and hepatotoxicity evaluation of Crocus sativus stigmas in neonates of nursing mice. J. Nephropathol. 2014, 3, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Khazdair, M.R.; Boskabady, M.H.; Hosseini, M.; Rezaee, R.; Tsatsakis, A.M. The effects of Crocus sativus (saffron) and its constituents on nervous system: A review. Avicenna J. Phytomed. 2015, 5, 376–391. [Google Scholar] [PubMed]
- Ziaee, T.; Razavi, B.M.; Hosseinzadeh, H. Saffron reduced toxic effects of its constituent, safranal, in acute and subacute toxicities in rats. Jundishapur J. Nat. Pharm. Prod. 2014, 9, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.L.; Willett, S.L.; Moss, M.E.; Miller, J.; Belinka, B.A., Jr. Binding of crocetin to plasma albumin. J. Pharm. Sci. 1982, 71, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Abdullaev, F.I.; Espinosa-Aguirre, J.J. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect. Prev. 2004, 28, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Betti, G.; Hensel, A. Saffron in phytotherapy: Pharmacology and clinical uses. Wien Med. Wochenschr. 2007, 157, 315–319. [Google Scholar] [CrossRef] [PubMed]
| Agent Supplied | Analysis Comp. | Time (Week) | Dose (mg/day) | Bioactivity | Ref. |
|---|---|---|---|---|---|
| Saffron ethanol (80%) extract | Bad analysis | 16 | 30 | Alzheimer‘s disease | [88] |
| Saffron ethanol (80%) extract | Bad analysis | 22 | 30 | Alzheimer‘s disease | [89] |
| Saffron ethanol (80%) extract | Bad analysis | 48 | 30 | Alzheimer‘s disease | [90] |
| trans Sodium crocetinate | Yes | undefined | 0.25 | Glioblastoma | [96] |
| Saffron | Yes | 12 | 20 | Macular degeneration | [98] |
| Saffron | No | 56 | 20 | Macular degeneration | [99] |
| Saffron | No | 12 | 20 | Macular degeneration | [100] |
| Saffron aqueous extract (SAE) and crocin (CR) | Yes | 12 | SAE: 30 CR: 30 | Schizophrenia | [106] |
| Saffron ethanol (80%) extract | No | 6 | 30 | Depression | [107] |
| Saffron ethanol (80%) extract | No | 6 | 30 | Depression | [108] |
| Saffron ethanol (80%) extract | No | 6 | 30 | Depression | [109] |
| Saffron ethanol (80%) extract | No | 6 | 30 | Depression | [110] |
| Crystallized crocin | No | 4 | 30 | Depression | [111] |
| Saffron | No | 6 | 30 | Depression | [112] |
| Saffron ethanol (80%) extract | No | 8 | 30 | Premenstrual syndrome | [114] |
| Saffron | Yes | 12 | 50 | Lipid Profile | [121] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
José Bagur, M.; Alonso Salinas, G.L.; Jiménez-Monreal, A.M.; Chaouqi, S.; Llorens, S.; Martínez-Tomé, M.; Alonso, G.L. Saffron: An Old Medicinal Plant and a Potential Novel Functional Food. Molecules 2018, 23, 30. https://doi.org/10.3390/molecules23010030
José Bagur M, Alonso Salinas GL, Jiménez-Monreal AM, Chaouqi S, Llorens S, Martínez-Tomé M, Alonso GL. Saffron: An Old Medicinal Plant and a Potential Novel Functional Food. Molecules. 2018; 23(1):30. https://doi.org/10.3390/molecules23010030
Chicago/Turabian StyleJosé Bagur, María, Gonzalo Luis Alonso Salinas, Antonia M. Jiménez-Monreal, Soukaina Chaouqi, Silvia Llorens, Magdalena Martínez-Tomé, and Gonzalo L. Alonso. 2018. "Saffron: An Old Medicinal Plant and a Potential Novel Functional Food" Molecules 23, no. 1: 30. https://doi.org/10.3390/molecules23010030







