Biochemical Analysis of the Role of Leucine-Rich Repeat Receptor-Like Kinases and the Carboxy-Terminus of Receptor Kinases in Regulating Kinase Activity in Arabidopsis thaliana and Brassica oleracea
Abstract
:1. Introduction
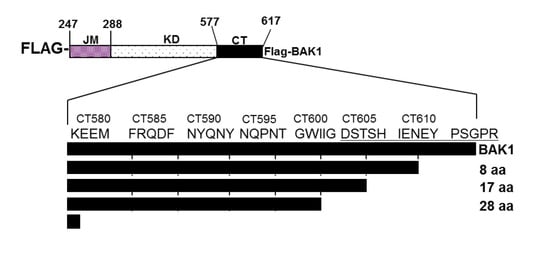
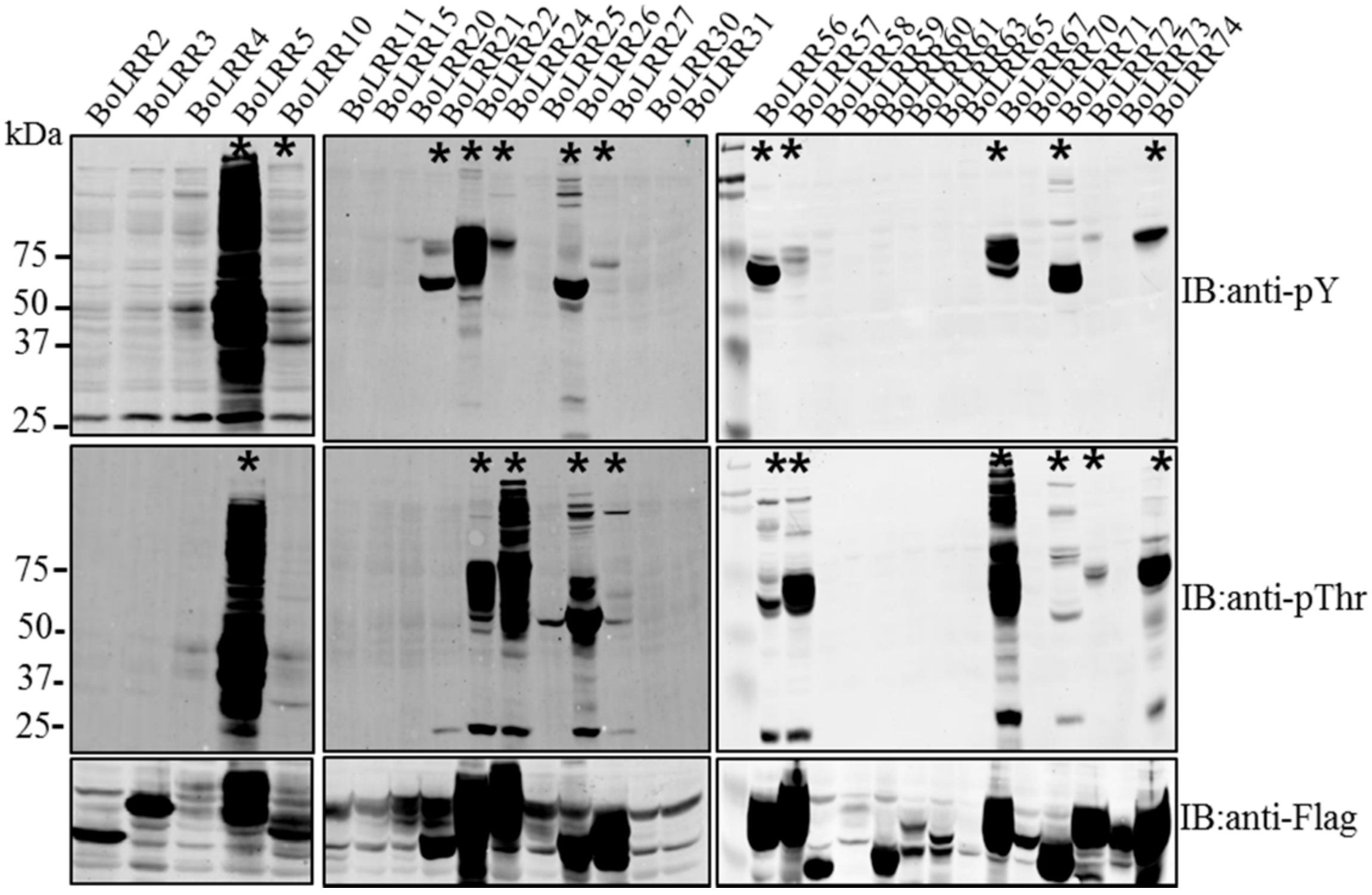
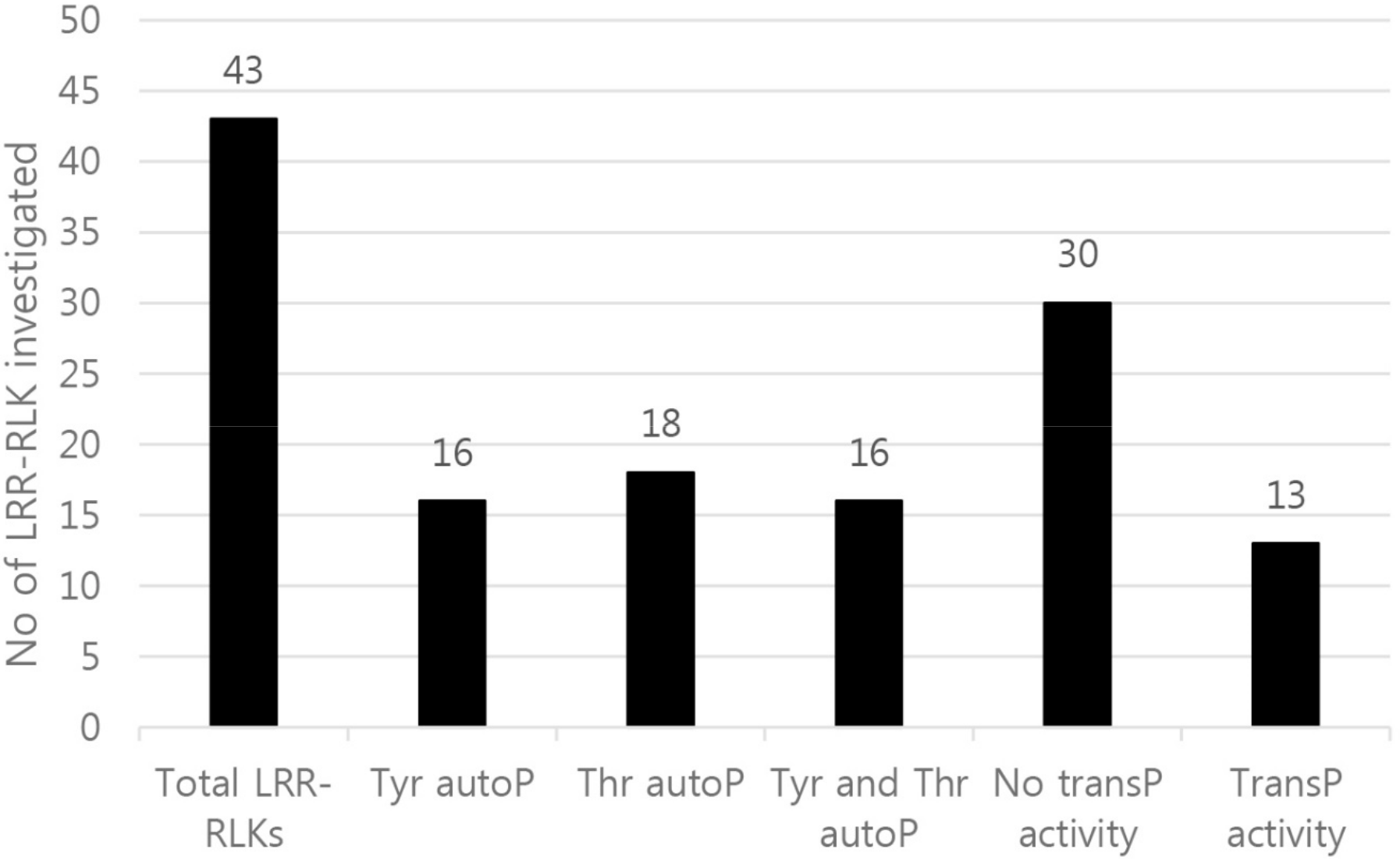
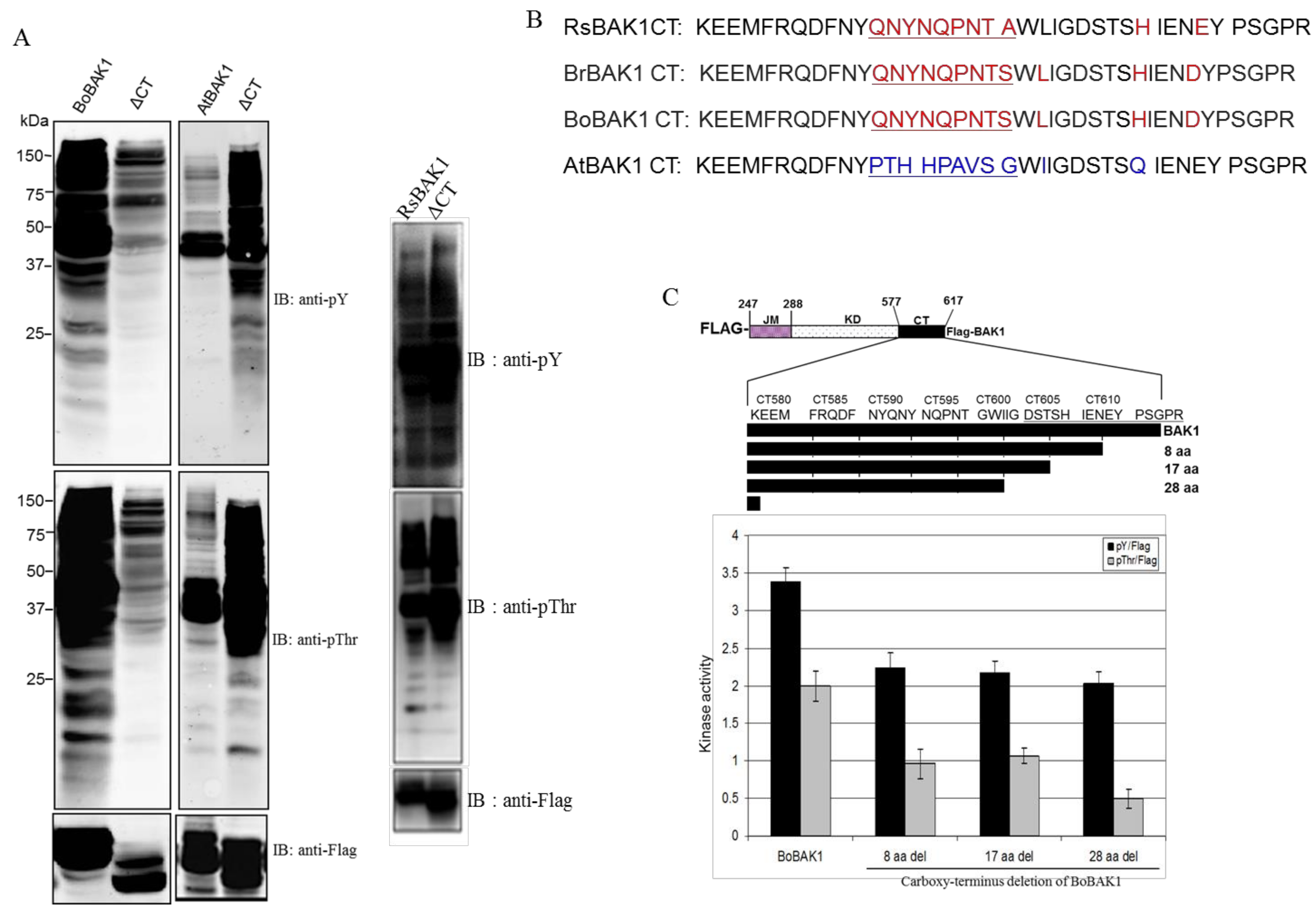
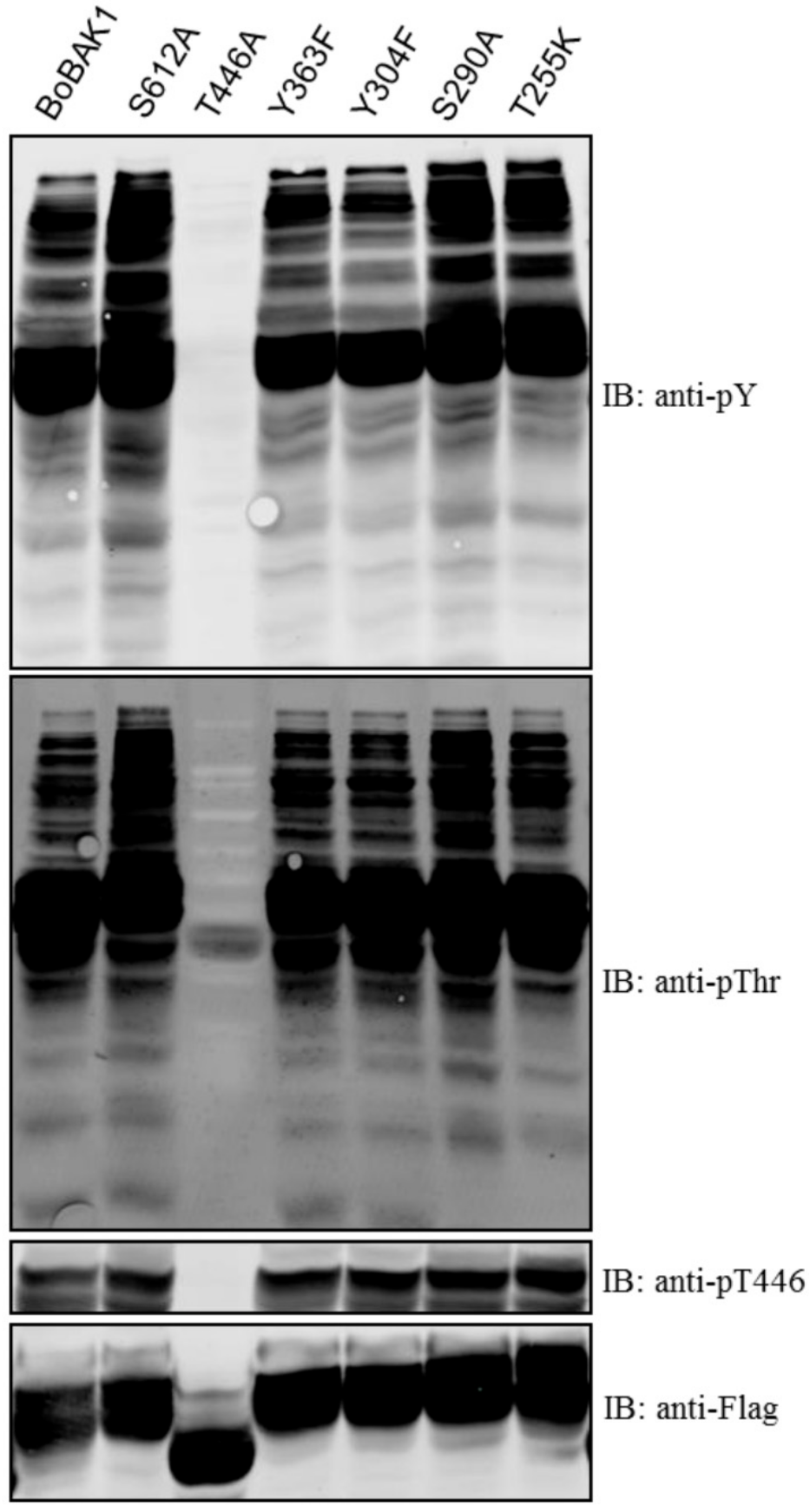
2. Results and Discussion
3. Material and Methods
3.1. Isolation of Total RNA and Cloning of LRR-RLKs
3.2. Recombinant Protein Production and Purification
3.3. Electrophoresis and Immunoblotting
3.4. Phylogenetic Tree Construction
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haruta, M.; Sussman, M.R. Ligand receptor-mediated regulation of growth in plants. Curr. Top. Dev. Biol. 2017, 123, 331–363. [Google Scholar] [PubMed]
- Becraft, P.W. Receptor kinase signaling in plant development. Annu. Rev. Cell Dev. Biol. 2002, 18, 163–192. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, L.; Merchan, F.; Laporte, P.; Thompson, R.; Clarke, J.; Sousa, C.; Crespi, M. A novel plant leucine-rich repeat receptor kinase regulates the response of Medicago truncatula roots to salt stress. Plant Cell 2009, 21, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Tör, M.; Lotze, M.T.; Holton, N. Receptor-mediated signalling in plants: Molecular patterns and programmes. J. Exp. Bot. 2009, 60, 3645–3654. [Google Scholar] [CrossRef] [PubMed]
- Torii, S.; Kusakabe, M.; Yamamoto, T.; Maekawa, M.; Nishida, E. SEF is a spatial regulator for Ras/MAP kinase signaling. Dev. Cell 2004, 7, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Clouse, S.D. Brassinosteroid Signal Transduction: Clarifying the pathway from ligand perception to gene expression. Mol. Cell 2002, 10, 973–982. [Google Scholar] [CrossRef]
- Wang, X.; Kota, U.; He, K.; Blackburn, K.; Li, J.; Goshe, M.B.; Huber, S.C.; Clouse, S.D. Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev. Cell 2008, 15, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.-H.; Wang, X.; Kota, U.; Goshe, M.B.; Clouse, S.D.; Huber, S.C. Tyrosine phosphorylation of the BRI1 receptor kinase emerges as a novel component of brassinosteroid signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.-H.; Ray, W.K.; Huber, S.C.; Asara, J.M.; Gage, D.A.; Clouse, S.D. Recombinant brassinosteroid insensitive 1 receptor-like kinase autophosphorylates on serine and threonine residues and phosphorylates a conserved peptide motif in vitro. Plant Physiol. 2000, 124, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.-H.; Clouse, S.D.; Huber, S.C. Tyrosine phosphorylation of the BRI1 receptor kinase occurs via a posttranslational modification and is activated by the juxtamembrane domain. Front. Plant Sci. 2012, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Akter, S.; Jacques, S.; Huang, J.; Messens, J.; Van Breusegem, F. Oxidative post-translational modifications of cysteine residues in plant signal transduction. J. Exp. Bot. 2015, 66, 2923–2934. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, N.; Nakagami, H.; Mochida, K.; Daudi, A.; Tomita, M.; Shirasu, K.; Ishihama, Y. Large-scale phosphorylation mapping reveals the extent of tyrosine phosphorylation in Arabidopsis. Mol. Syst. Biol. 2008, 4, 193. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, H.; Sugiyama, N.; Mochida, K.; Daudi, A.; Yoshida, Y.; Toyoda, T.; Tomita, M.; Ishihama, Y.; Shirasu, K. Large-scale comparative phosphoproteomics identifies conserved phosphorylation sites in plants. Plant Physiol. 2010, 153, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Rameneni, J.J.; Lee, Y.; Dhandapani, V.; Yu, X.; Choi, S.R.; Oh, M.H.; Lim, Y.P. Genomic and Post-Translational Modification Analysis of Leucine-Rich-Repeat Receptor-Like Kinases in Brassica rapa. PLoS ONE 2015, 10, e0142255. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Saijo, Y.; Nakagami, H.; Takano, Y. Regulation of sugar transporter activity for antibacterial defense in Arabidopsis. Science 2016, 16, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Dai, C.; Lee, M.M.; Kwak, J.M.; Nam, K.H. BRI1-associated receptor kinase 1 regulates guard cell ABA signaling mediated by Open Stomata 1 in Arabidopsis. Mol. Plant 2016, 9, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Shinohara, H.; Tanaka, M.; Baba, K.; Ogawa-Ohnishi, M.; Matsubayashi, Y. A peptide hormone required for Casparian strip diffusion barrier formation in Arabidopsis roots. Science 2017, 355, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Goshe, M.B.; Soderblom, E.J.; Phinney, B.S.; Kuchar, J.A.; Li, J.; Asami, T.; Yoshida, S.; Huber, S.C.; Clouse, S.D. Identification and functional analysis of in vivo phosphorylation sites of the Arabidopsis BRASSINOSTEROID-INSENSITIVE1 receptor kinase. Plant Cell 2005, 17, 1685–1703. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, D.; Zipfel, C.; Robatzek, S.; Kemmerling, B.; Nürnberger, T.; Jones, J.D.; Felix, G.; Boller, T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 2007, 448, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Ntoukakis, V.; Schwessinger, B.; Segonzac, C.; Zipfel, C. Cautionary notes on the use of C-terminal BAK1 fusion proteins for functional studies. Plant Cell 2011, 23, 3871–3878. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Mackey, K. Short technical report. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide and proteoglycan-rich sources. Biotechniques 1995, 19, 942–945. [Google Scholar] [PubMed]
- Larkin, MA.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Experimental resources including LRR-RLK genes are available from the authors. |

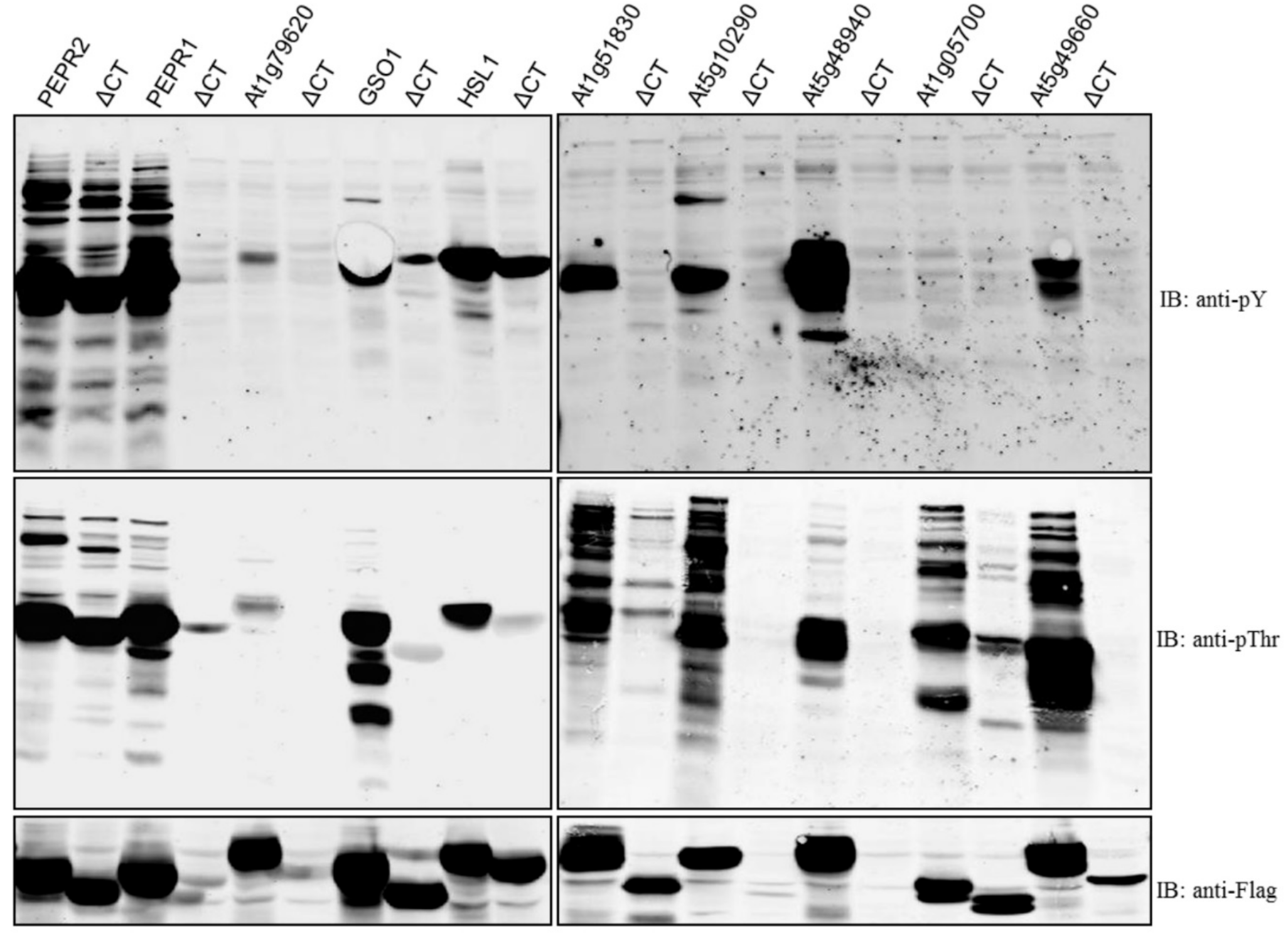
| Gene Locus | Symbol | Total Amino Acids | Amino Acids of CT | CD autoP | ΔCT autoP |
|---|---|---|---|---|---|
| At1g17750 | PEPR2 | 1088 | 8 | pThr/pY | pThr/pY |
| At1g73080 | PEPR1 | 1123 | 8 | pThr/pY | poor expression |
| At1g79620 | 971 | 59 | pThr/pY | poor expression | |
| At4g20140 | GSO1 | 1249 | 9 | pThr/pY | ND |
| At1g28440 | HSL1 | 996 | 34 | pThr/pY | pY only |
| At1g51830 | 882 | 34 | pThr/pY | ND | |
| At5g10290 | 613 | 44 | pThr/pY | poor expression | |
| At5g48940 | 1135 | 69 | pThr/pY | poor expression | |
| At1g05700 | 852 | 7 | pThr | Reduced pThr | |
| At5g49660 | CEPR1 | 966 | 32 | pThr/pY | ND |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, E.-S.; Lee, Y.; Chae, W.B.; Rameneni, J.J.; Park, Y.-S.; Lim, Y.P.; Oh, M.-H. Biochemical Analysis of the Role of Leucine-Rich Repeat Receptor-Like Kinases and the Carboxy-Terminus of Receptor Kinases in Regulating Kinase Activity in Arabidopsis thaliana and Brassica oleracea. Molecules 2018, 23, 236. https://doi.org/10.3390/molecules23010236
Oh E-S, Lee Y, Chae WB, Rameneni JJ, Park Y-S, Lim YP, Oh M-H. Biochemical Analysis of the Role of Leucine-Rich Repeat Receptor-Like Kinases and the Carboxy-Terminus of Receptor Kinases in Regulating Kinase Activity in Arabidopsis thaliana and Brassica oleracea. Molecules. 2018; 23(1):236. https://doi.org/10.3390/molecules23010236
Chicago/Turabian StyleOh, Eun-Seok, Yeon Lee, Won Byoung Chae, Jana Jeevan Rameneni, Yong-Soon Park, Yong Pyo Lim, and Man-Ho Oh. 2018. "Biochemical Analysis of the Role of Leucine-Rich Repeat Receptor-Like Kinases and the Carboxy-Terminus of Receptor Kinases in Regulating Kinase Activity in Arabidopsis thaliana and Brassica oleracea" Molecules 23, no. 1: 236. https://doi.org/10.3390/molecules23010236