Synthesis, Crystal Structure, and Supramolecular Understanding of 1,3,5-Tris(1-phenyl-1H-pyrazol-5-yl)benzenes
Abstract
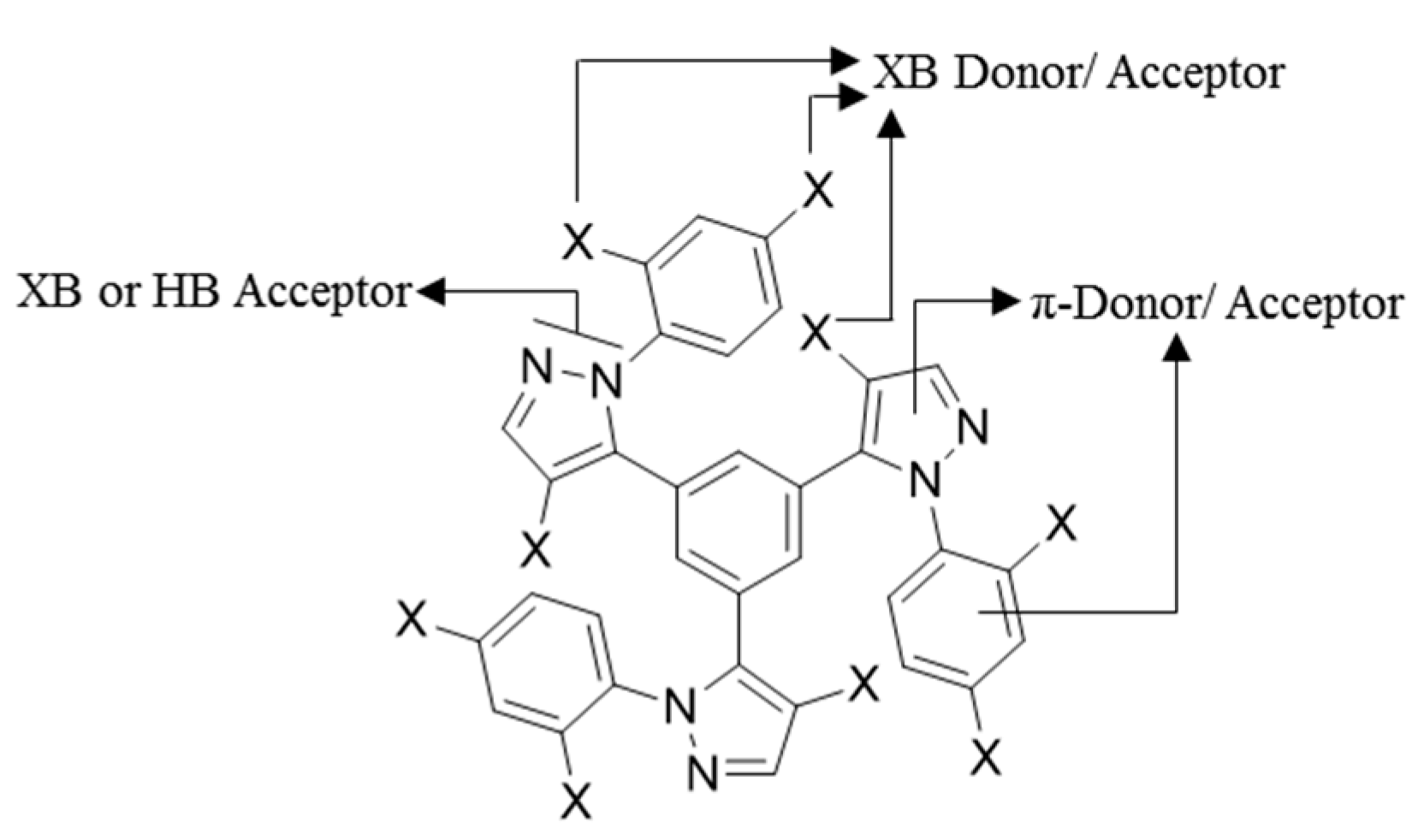
:1. Introduction
2. Results and Discussion
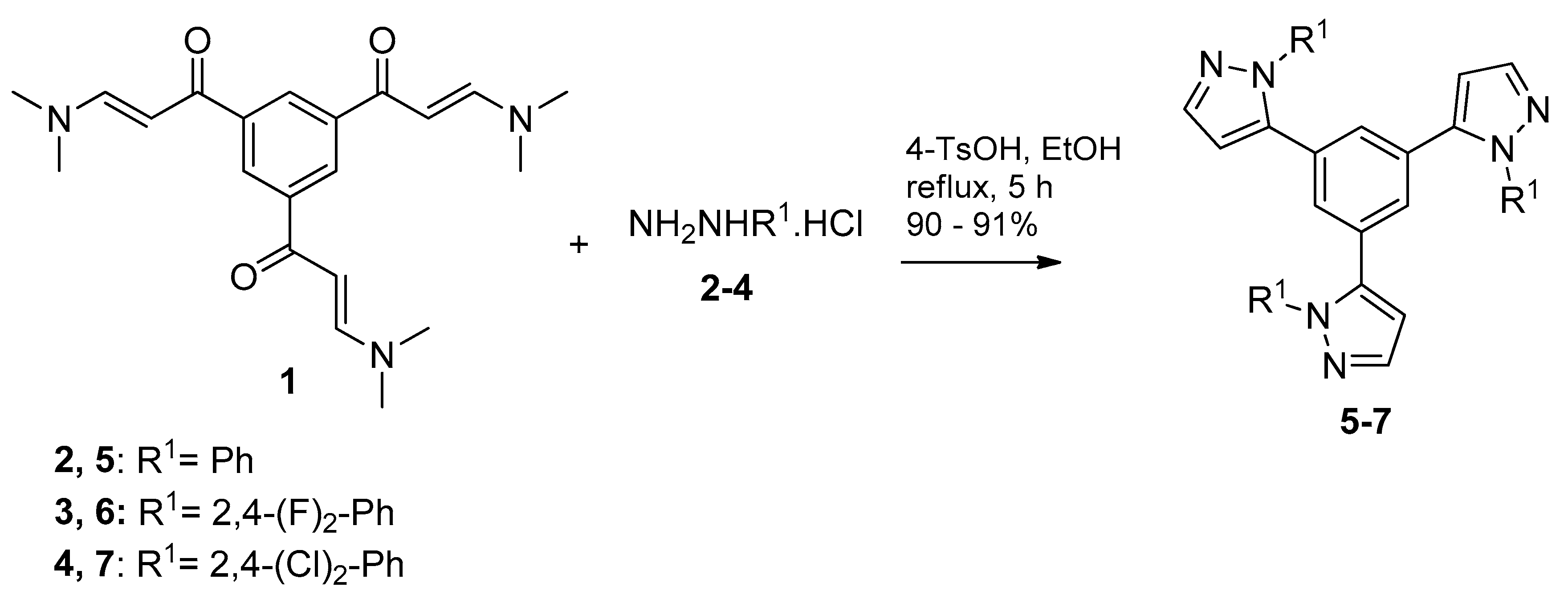
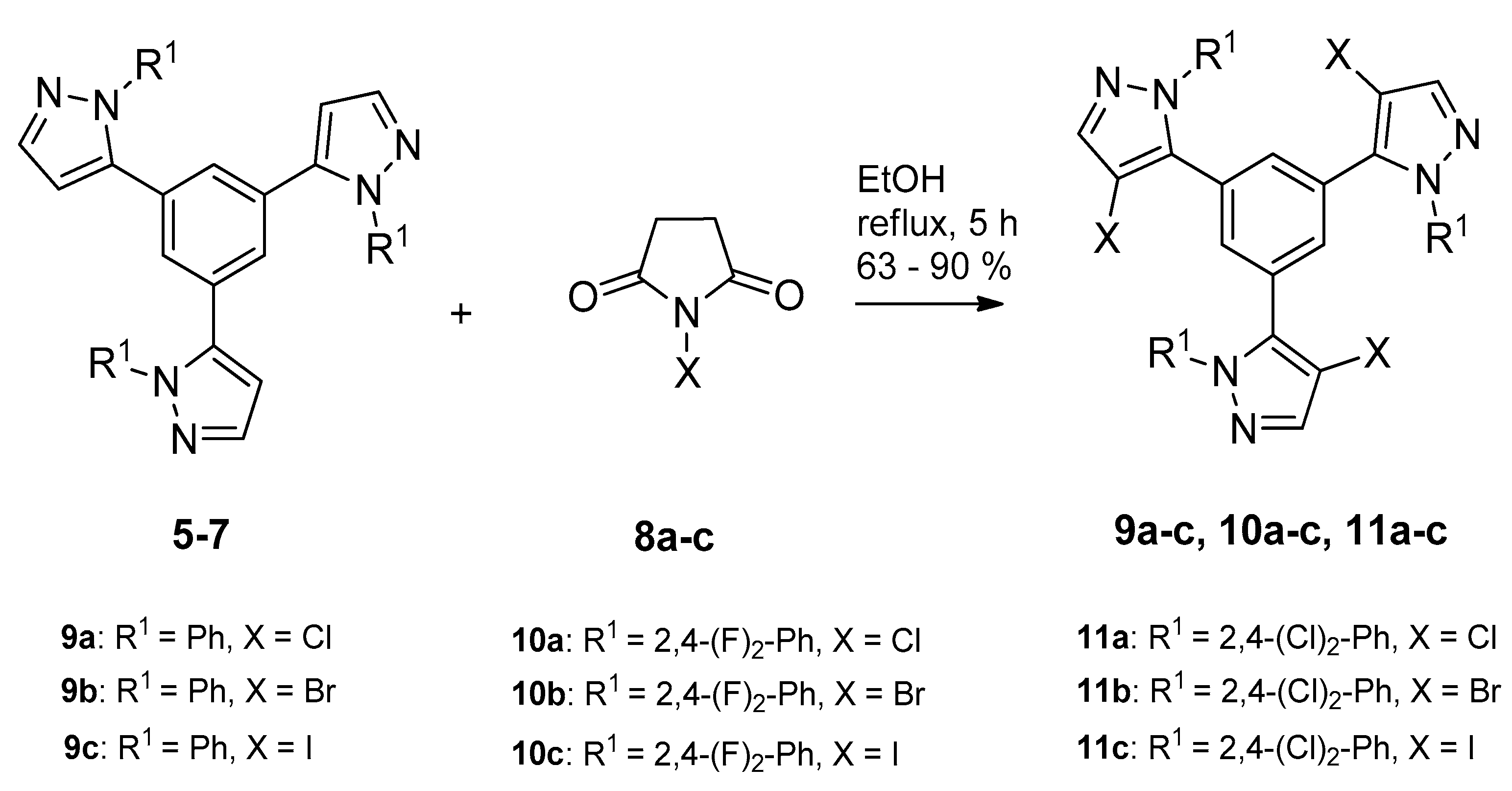
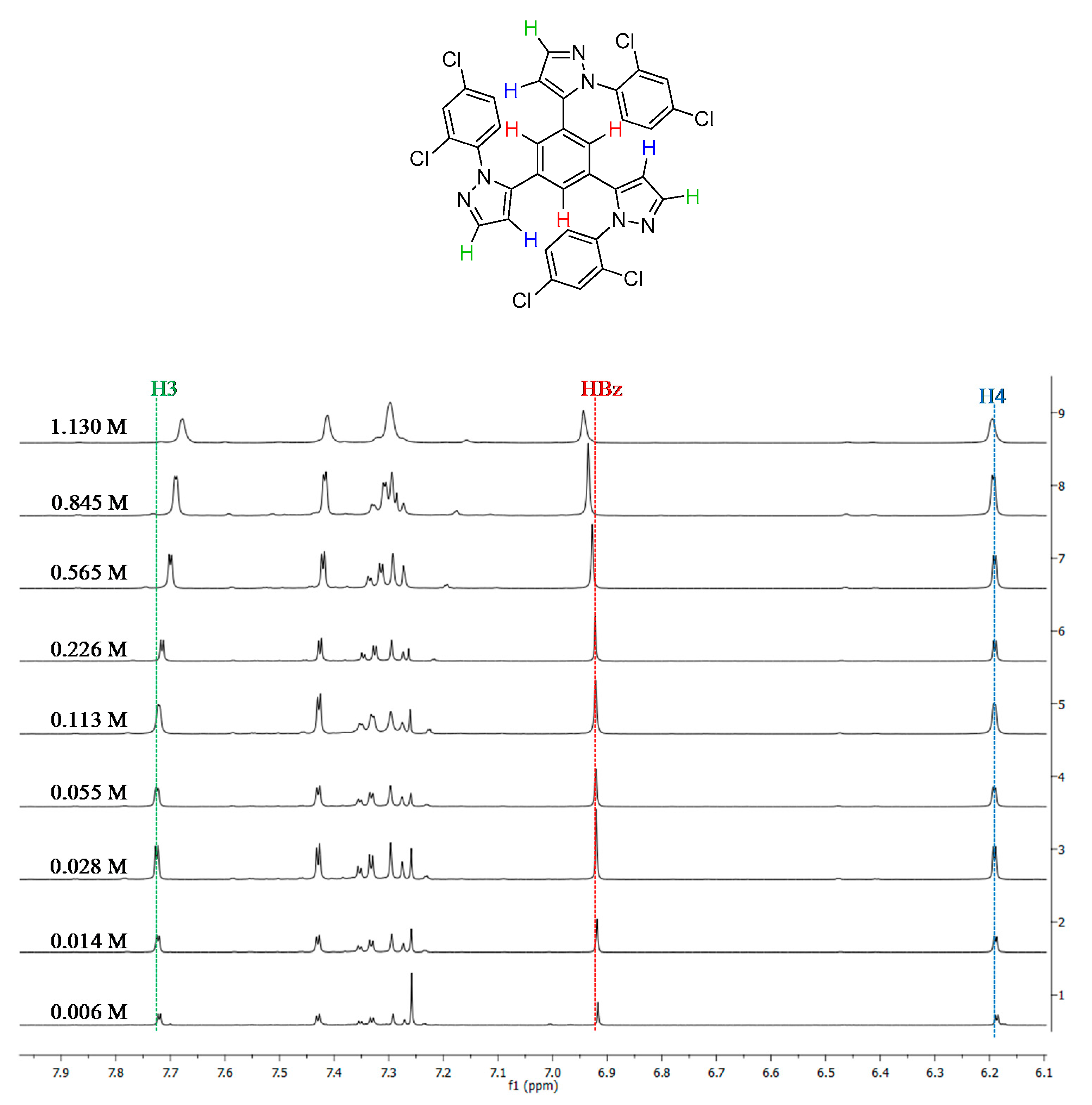
2.1. Synthesis and Characterization
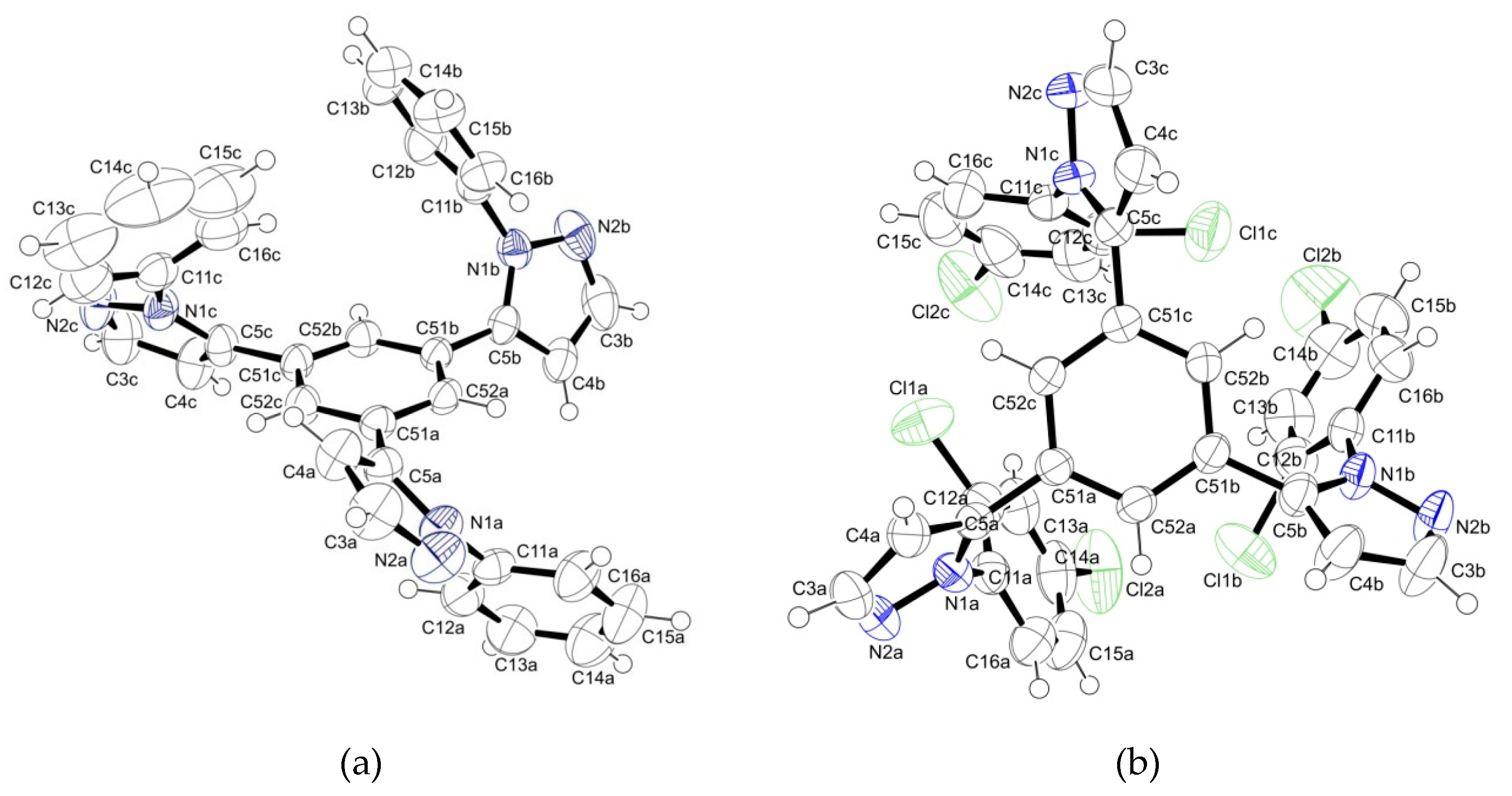
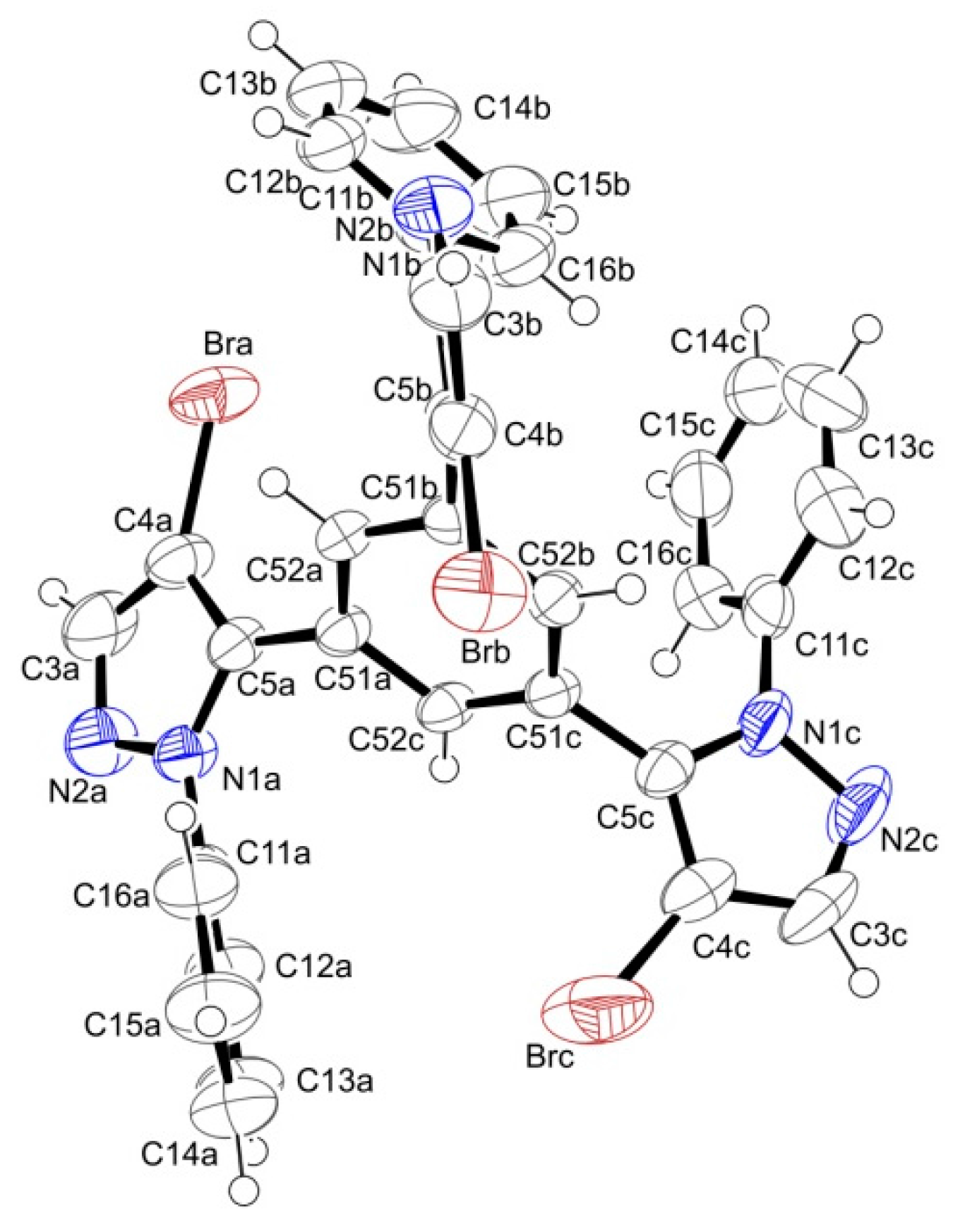
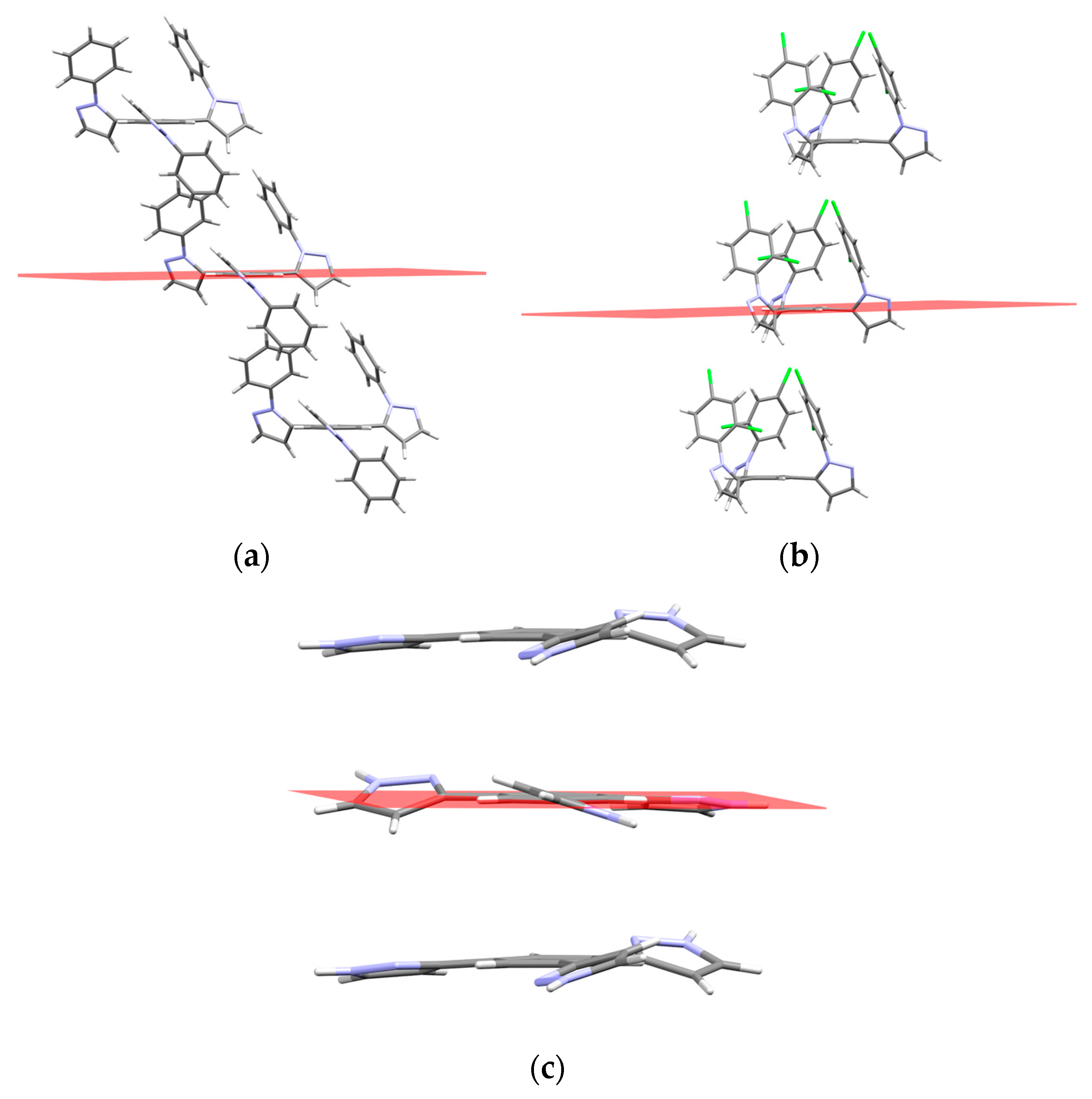
2.2. Supramolecular Arrangement
2.3. QTAIM Analysis
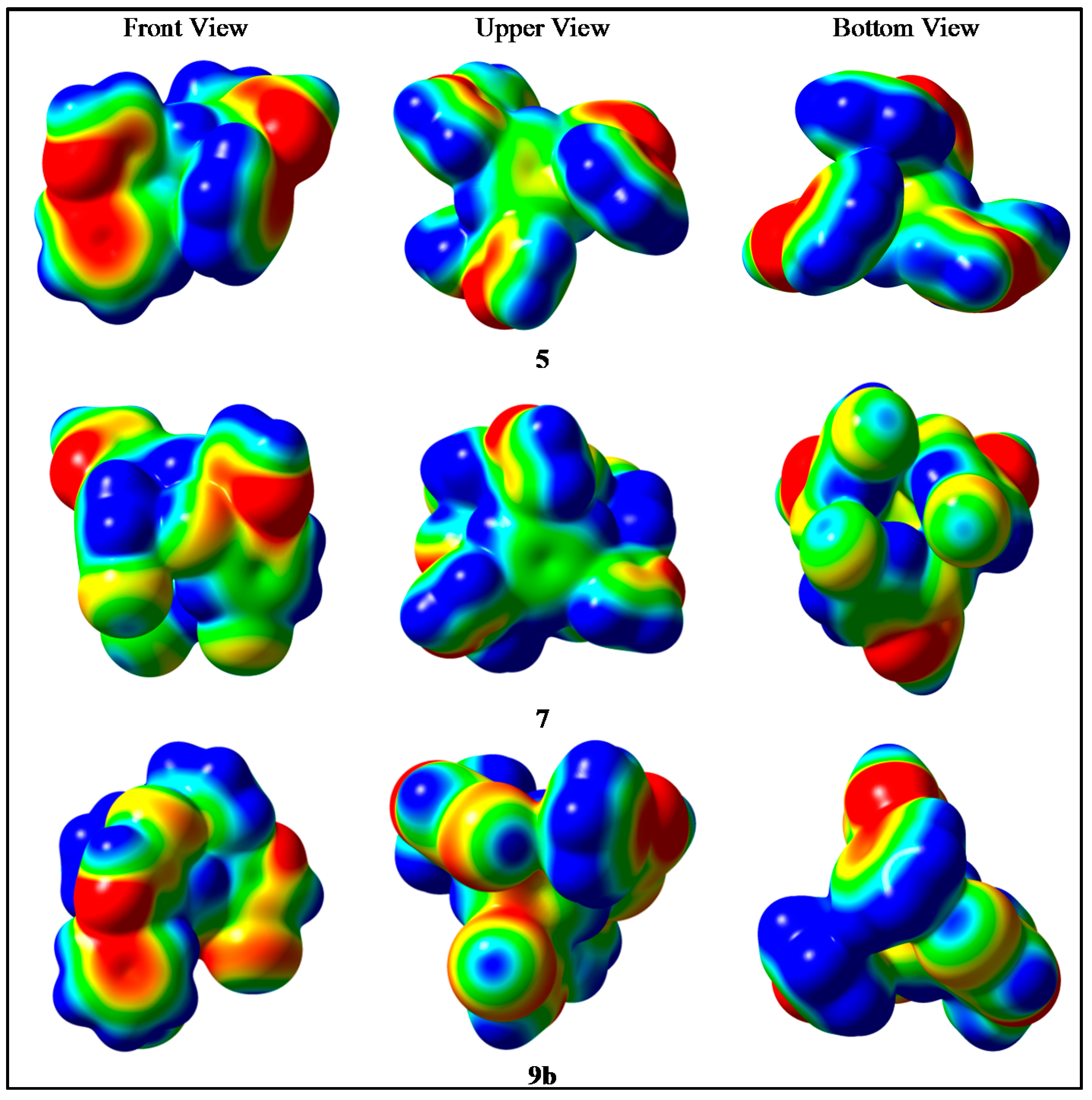
2.4. Molecular Elestrostatic Potential of Compounds 5, 7, and 9b
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.2.1. General Procedure for Preparation of 1,3,5-tris(3-dimethylamino-1-oxoprop-2-en-yl)benzene (1)
3.2.2. General Procedure for Preparation of 1,3,5-tris(pirazolyl)benzenes 5–7
3.2.3. General Procedure for Preparation of 1,3,5-Tris(4-halo-pirazolyl)benzenes 9–11
3.3. X-ray Crystallography
3.4. Quantum Chemical Calculation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martins, M.A.P.; Zimmer, G.C.; Rodrigues, L.V.; Orlando, T.; Buriol, L.; Alajarin, M.; Berna, J.; Frizzo, C.P.; Bonacorso, H.G.; Zanatta, N. Competition between the donor and acceptor hydrogen bonds of the threads in the formation of [2]rotaxanes by clipping reaction. New J. Chem. 2017, 41, 13303–13318. [Google Scholar] [CrossRef]
- Martins, M.A.P.; Salbego, P.R.S.; de Moraes, G.A.; Bender, C.R.; Zambiazi, P.J.; Orlando, T.; Pagliari, A.B.; Frizzo, C.P.; Hörner, M. Understanding the crystalline formation of triazene N-oxides and the role of halogen…π interactions. CrystEngComm 2018. [Google Scholar] [CrossRef]
- Martins, M.A.P.; Frizzo, C.P.; Martins, A.C.L.; Tier, A.Z.; Gindri, I.M.; Meyer, A.R.; Bonacorso, H.G.; Zanatta, N. Energetic and topological approach for characterization of supramolecular clusters in organic crystals. RSC Adv. 2014, 4, 44337–44349. [Google Scholar] [CrossRef]
- Martins, M.A.P.; Hörner, M.; Beck, J.; Tier, A.Z.; Belladona, A.L.; Meyer, A.R.; Zanatta, N.; Bonacorso, H.G.; Frizzo, C.P. Polymorphism in an 18-membered macrocycle: An energetic and topological approach to understand the supramolecular structure. CrystEngComm 2016, 18, 3866–3876. [Google Scholar] [CrossRef]
- Gemming, S.; Schreiber, M.; Thiel, W.; Heine, T.; Seifert, G.; De Abreu, H.A.; Duarte, H.A. Tunable discotic building blocks for liquid crystalline displays. J. Lumin. 2004, 108, 143–147. [Google Scholar] [CrossRef]
- Zhao, W.-X.; Gao, Y.-X.; Dong, S.-F.; Li, Y.; Zhang, W.-P. 1,3,5-Tris(1H-pyrazol-3-yl)benzene. Acta Crystallogr. Sect. C 2007, E63, o3448. [Google Scholar] [CrossRef]
- Martins, M.A.P.; Meyer, A.R.; Tier, A.Z.; Longhi, K.; Ducati, L.C.; Bonacorso, H.G.; Zanatta, N.; Frizzo, C.P. Proposal for crystallization of 3-amino-4-halo-5-methylisoxazoles: An energetic and topological approach. CrystEngComm 2015, 17, 7381–7391. [Google Scholar] [CrossRef]
- Lucarelli, C.; Galli, S.; Maspero, A.; Cimino, A.; Bandinelli, C.; Lolli, A.; Velasquez Ochoa, J.; Vaccari, A.; Cavani, F.; Albonetti, S. Adsorbent–Adsorbate Interactions in the Oxidation of HMF Catalyzed by Ni-Based MOFs: A DRIFT and FT-IR Insight. J. Phys. Chem. C 2016, 120, 15310–15321. [Google Scholar] [CrossRef]
- Martins, M.A.P.; Frizzo, C.P.; Tier, A.Z.; Moreira, D.N.; Zanatta, N.; Bonacorso, H.G. Update 1 of: Ionic Liquids in Heterocyclic Synthesis. Chem. Rev. 2014, 114, PR1–PR70. [Google Scholar] [CrossRef] [PubMed]
- Elassar, A.Z.A.; El-Khair, A.A. Recent developments in the chemistry of enaminones. Tetrahedron 2003, 59, 8463–8480. [Google Scholar] [CrossRef]
- Pleier, A.-K.; Holger Glas, B.; Manja Grosche, B.; Peter Sirsch, B.; Werner, R. Thie Microwave Assisted Synthesis of 1-Aryl-3-dimethylaminoprop-2-enones: A Simple and Rapid Access to 3-Arylpyrazoles. Synthesis 2001, 2001, 0055–0062. [Google Scholar] [CrossRef]
- Al-Zaydi, K.M.; Nhari, L.M.; Borik, R.M.; Elnagdi, M.H. Green technologies in organic synthesis: Self-condensation of enamines, enaminones and enaminoesters under microwave irradiation in ionic liquid. Green Chem. Lett. Rev. 2010, 3, 93–99. [Google Scholar] [CrossRef]
- Abdel-khalik, M. M.; Elnagdi, M.H. Enaminones in Organic Synthesis : A Novel Synthesis of 1,3,5-Trisubstituted Benzene Derivatives and of 2-Substituted-5-Aroylpyridines. Synth. Commun. 2002, 32, 159–164. [Google Scholar] [CrossRef]
- Kiss, L.E.; Ferreira, H.S.; Torrão, L.; Bonifácio, M. J.; Palma, P.N.; Soares-Da-Silva, P.; Learmonth, D.A. Discovery of a long-acting, peripherally selective inhibitor of catechol-O-methyltransferase. J. Med. Chem. 2010, 53, 3396–3411. [Google Scholar] [CrossRef] [PubMed]
- Balbi, A.; Anzaldi, M.; MacCi, C.; Aiello, C.; Mazzei, M.; Gangemi, R.; Castagnola, P.; Miele, M.; Rosano, C.; Viale, M. Synthesis and biological evaluation of novel pyrazole derivatives with anticancer activity. Eur. J. Med. Chem. 2011, 46, 5293–5309. [Google Scholar] [CrossRef] [PubMed]
- Reidlinger, C.; Dworczak, R.; Junek, H. Cyanoacetophenone as a synthon for 1,4,5-substituted pyrazoles. Monatshefte Chem. 1998, 1211, 1207–1211. [Google Scholar] [CrossRef]
- Hernández, S.; Moreno, I.; SanMartin, R.; Herrero, M.T.; Domínguez, E. An straightforward entry to new pyrazolo-fused dibenzo[1,4]diazepines. Org. Biomol. Chem. 2011, 9, 2251–2257. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.; Cayir, M.; Nieger, M.; Thiel, W.R.; Bräse, S. Roadmap towards N-heterocyclic [2.2]paracyclophanes and their application in asymmetric catalysis. Eur. J. Org. Chem. 2013, 6108–6123. [Google Scholar] [CrossRef]
- Moreira, D.N.; Longhi, K.; Frizzo, C.P.; Bonacorso, H.G.; Zanatta, N.; Martins, M.A.P. Ionic liquid promoted cyclocondensation reactions to the formation of isoxazoles, pyrazoles and pyrimidines. Catal. Commun. 2010, 11, 476–479. [Google Scholar] [CrossRef]
- Janjic, M.; Prebil, R.; Kralj, D.; Golobic, A.; Stare, K.; Dahmann, G.; Stanovnik, B.; Svete, J. A Simple Synthesis of 5-(2-Aminophenyl )-1H-pyrazoles. Helv. Chim. Acta 2011, 94, 1703–1717. [Google Scholar] [CrossRef]
- Ceccarelli, S.M.; Jagasia, R.; Jakob-Roetne, R.; Wichmann, J. Benzisoxazole Modulators of Neurogenesis. PCT Int. Application WO2014016267 (A1), 30 January 2014. [Google Scholar]
- Khan, T.A.; Kumar, S.; Venkatesh, C.; Ila, H. Regioselective rapid analog synthesis of 1,3-(or 1,5-)diphenyl-4-aryl/heteroaryl-5-(or 3-)(methylthio)pyrazoles via Suzuki cross-coupling. Tetrahedron 2011, 67, 2961–2968. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Z. Halogenation of Pyrazoles Using N-Halosuccinimides in CCl4 and in Water. Synth. Commun. 2007, 37, 137–147. [Google Scholar] [CrossRef]
- Jeon, S.L.; Choi, J.H.; Kim, B.T.; Jeong, I.H. Synthesis of novel 1,4,5-trisubstituted 3-trifluoromethylpyrazoles via microwave-assisted Stille coupling reactions. J. Fluor. Chem. 2007, 128, 1191–1197. [Google Scholar] [CrossRef]
- Bernhammer, J.C.; Huynh, H.V. Correlation of spectroscopically determined ligand donor strength and nucleophilicity of substituted pyrazoles. Dalton Trans. 2012, 41, 8600–8608. [Google Scholar] [CrossRef] [PubMed]
- Hasui, T.; Ohyabu, N.; Ohra, T.; Fuji, K.; Sugimoto, T.; Fujimoto, J.; Asano, K.; Oosawa, M.; Shiotani, S.; Nishigaki, N.; et al. Discovery of 6-[5-(4-fluorophenyl)-3-methyl-pyrazol-4-yl]-benzoxazin-3-one derivatives as novel selective nonsteroidal mineralocorticoid receptor antagonists. Bioorg. Med. Chem. 2014, 22, 5428–5445. [Google Scholar] [CrossRef] [PubMed]
- Katoch-Rouse, R.; Pavlova, O.A.; Caulder, T.; Hoffman, A.F.; Mukhin, A.G.; Horti, A.G. Synthesis, structure-activity relationship, and evaluation of SR141716 analogues: Development of central cannabinoid receptor ligands with lower lipophilicity. J. Med. Chem. 2003, 46, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.R.; Katzenellenbogen, J.A. Regioselective Synthesis of 1,3,5-Triaryl-4-alkylpyrazoles via oxidation of pyrazolines. Org. Lett. 2000, 2, 2833–2836. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yan, X.; Han, C.; Huang, F. Characterization of supramolecular gels. Chem. Soc. Rev. 2013, 42, 6697–6722. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, L.; Chen, H.; Wu, W.; Jiang, H. Copper-Catalyzed Aerobic C(sp2)−H Functionalization for C−N Bond Formation: Synthesis of Pyrazoles and Indazoles. J. Org. Chem 2013, 3636–3646. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Hung, M.S.; Song, J.S.; Yeh, T.K.; Chou, M.C.; Chu, C.M.; Jan, J.J.; Hsieh, M.T.; Tseng, S.L.; Chang, C.P.; et al. Discovery of 2-[5-(4-chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-ethyl-1H-pyrazol-3-yl]-1,5,5-trimethyl-1,5-dihydro-imidazol-4-thione (BPR-890) via an active metabolite. A novel, potent and selective cannabinoid-1 receptor inverse agonist with high antiobes. J. Med. Chem. 2009, 52, 4496–4510. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Martins, M.A.P.; Rodrigues, L.V.; Meyer, A.R.; Frizzo, C.P.; Hörner, M.; Zanatta, N.; Bonacorso, H.G.; Berná, J.; Alajarín, M. Density Functional Theory and Quantum Theory of Atoms in Molecules Analysis: Influence of Intramolecular Interactions on Pirouetting Movement in Tetraalkylsuccinamide[2]rotaxanes. Cryst. Growth Des. 2017, 17, 5845–5857. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. The electrostatic potential: An overview. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. Molecular electrostatic potentials and noncovalent interactions. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2017, 7, e1326. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Halogen bonding: An interim discussion. Chem. Phys. Chem. 2013, 14, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Wijethunga, T.K.; Desper, J. Practical crystal engineering using halogen bonding: A hierarchy based on calculated molecular electrostatic potential surfaces. J. Mol. Struct. 2014, 1072, 20–27. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. σ-holes and π-holes: Similarities and differences. J. Comput. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.A.; Castellano, R.K.; Diederich, F. Interactions with Aromatic Rings in Chemical and Biological Recognition. Angew. Chem. Int. Ed. 2003, 42, 1210–1250. [Google Scholar] [CrossRef] [PubMed]
- Gung, B.W.; Amicangelo, J.C. Substituent Effects in C6F6-C6H5X Stacking Interactions. J. Org. Chem. 2006, 71, 9261–9270. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016.
- Keith, T.A. AIMAll, TK Gristmill Software: Overland Park, KS, USA, 2014.
- Dennington, R.; Keith, T.; Millam, J. GaussView, Semichem Inc.: Shawnee Mission, KS, USA, 2009.
Sample Availability: Samples of the compounds are not available from the authors. |
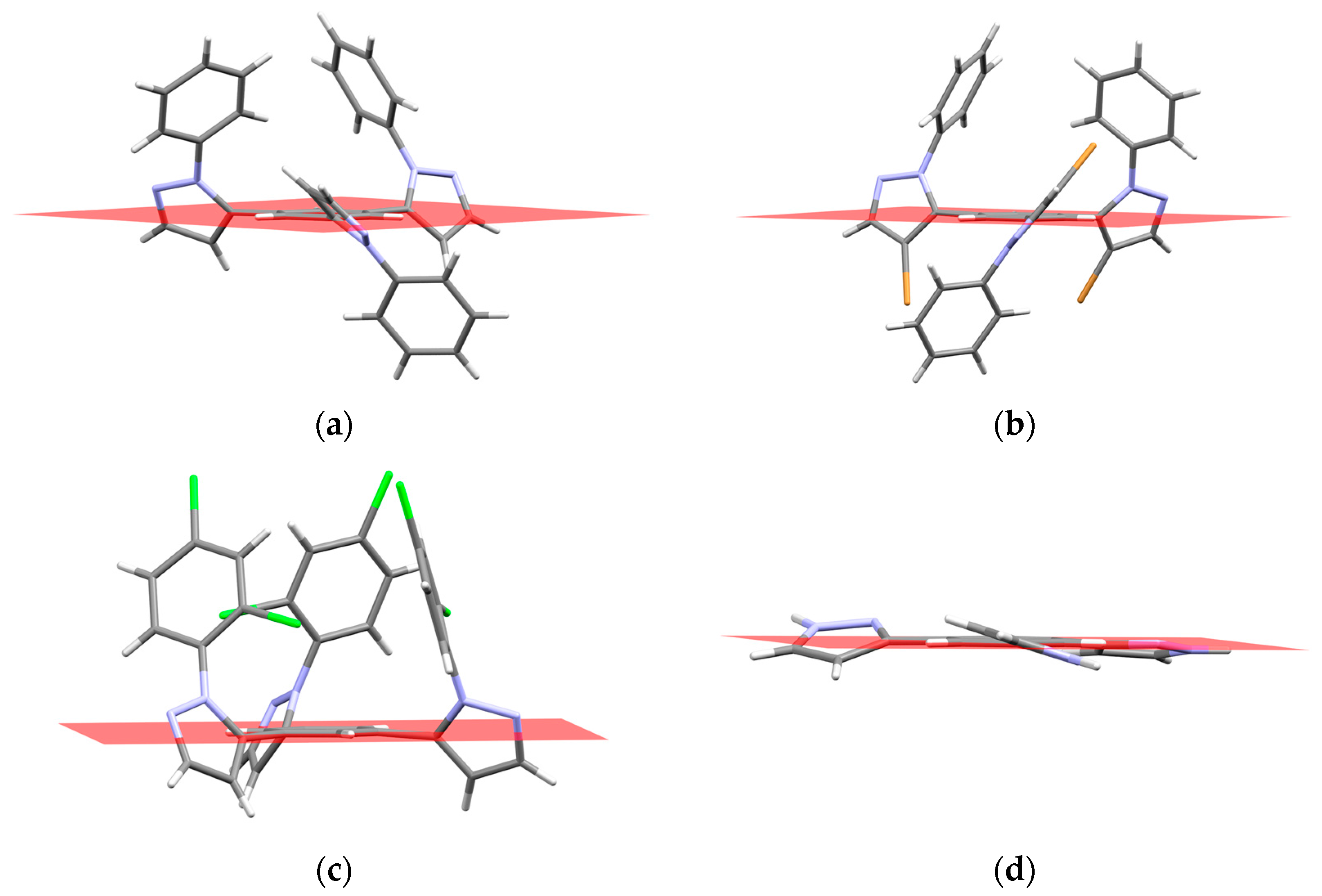
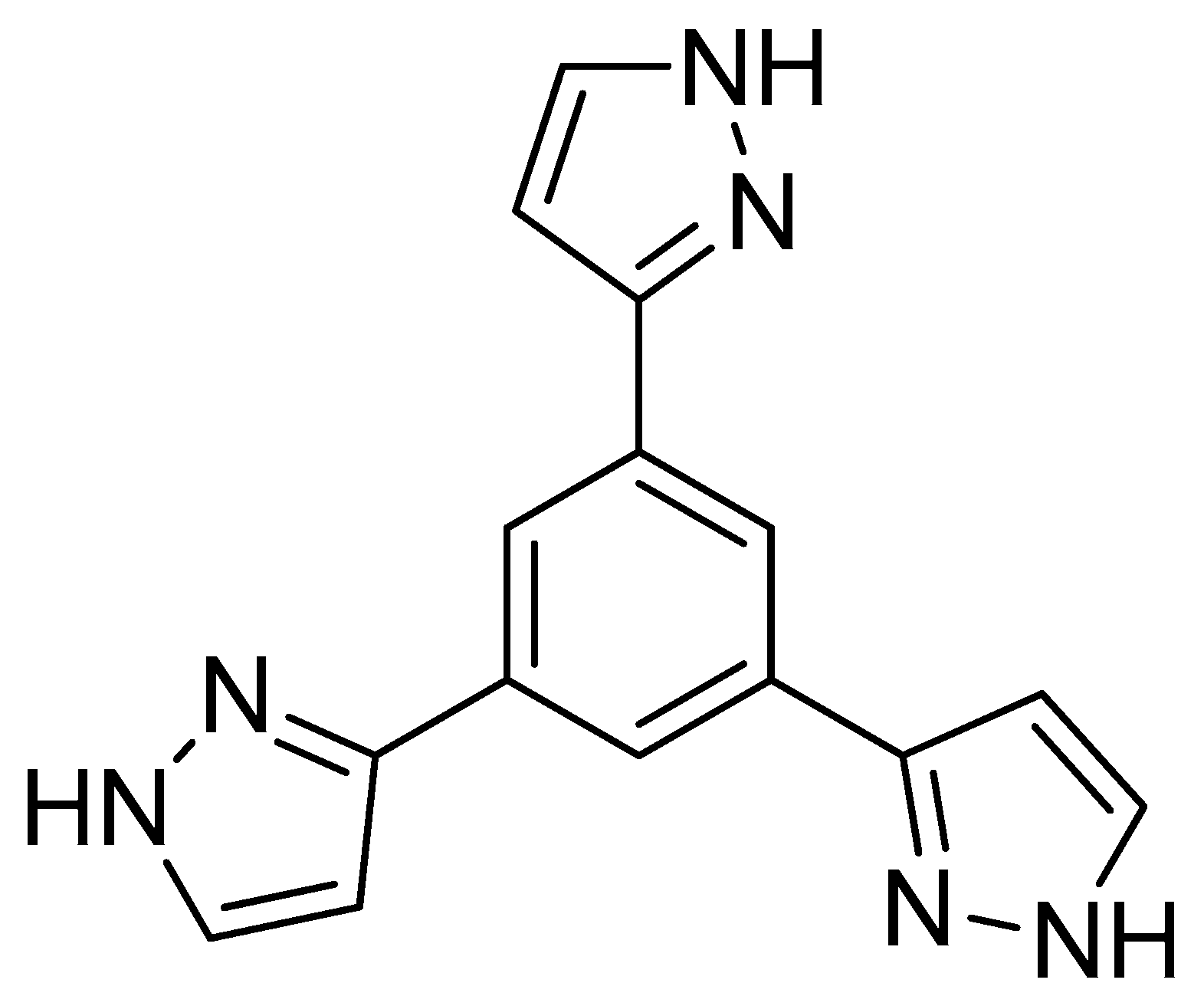
| Compound | Molecular Structure | Intramolecular Interaction | ρ (a.u.) | EINT a (kcal mol−1) |
|---|---|---|---|---|
| 5 |  | I-CHB···πph | 0.0017 | −0.22 |
| II-CHB···πph | 0.0023 | −0.33 | ||
| III-CHB···πbz | 0.0089 | −1.49 | ||
| 7 |  | I-CHA···ClC | 0.0029 | −0.44 |
| II-ClB···πphA | 0.0061 | −0.89 | ||
| III-ClA···πphC | 0.0067 | −0.97 | ||
| IV-ClC···πphB | 0.0071 | −1.08 | ||
| 9b |  | I-BrC···πPhA | 0.0032 | −0.42 |
| II-BrA···πPhB | 0.0042 | −0.61 | ||
| III-CHB···πPhC | 0.0036 | −0.49 | ||
| IV-CHB···πPhC | 0.0026 | −0.35 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, M.A.P.; Meyer, A.R.; Salbego, P.R.S.; Dos Santos, D.M.; De Moraes, G.A.; Bonacorso, H.G.; Zanatta, N.; Frizzo, C.P.; Hörner, M. Synthesis, Crystal Structure, and Supramolecular Understanding of 1,3,5-Tris(1-phenyl-1H-pyrazol-5-yl)benzenes. Molecules 2018, 23, 22. https://doi.org/10.3390/molecules23010022
Martins MAP, Meyer AR, Salbego PRS, Dos Santos DM, De Moraes GA, Bonacorso HG, Zanatta N, Frizzo CP, Hörner M. Synthesis, Crystal Structure, and Supramolecular Understanding of 1,3,5-Tris(1-phenyl-1H-pyrazol-5-yl)benzenes. Molecules. 2018; 23(1):22. https://doi.org/10.3390/molecules23010022
Chicago/Turabian StyleMartins, Marcos A. P., Alexandre R. Meyer, Paulo R. S. Salbego, Daniel M. Dos Santos, Guilherme A. De Moraes, Helio G. Bonacorso, Nilo Zanatta, Clarissa P. Frizzo, and Manfredo Hörner. 2018. "Synthesis, Crystal Structure, and Supramolecular Understanding of 1,3,5-Tris(1-phenyl-1H-pyrazol-5-yl)benzenes" Molecules 23, no. 1: 22. https://doi.org/10.3390/molecules23010022






