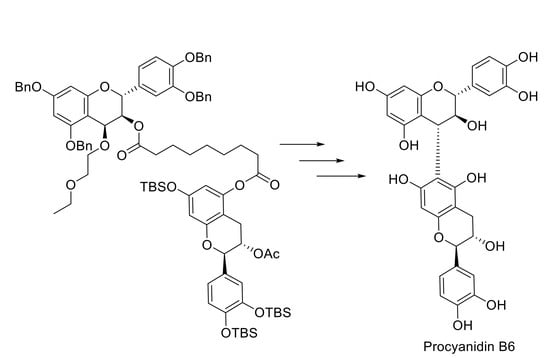
Regioselective Synthesis of Procyanidin B6, A 4-6-Condensed (+)-Catechin Dimer, by Intramolecular Condensation
Abstract
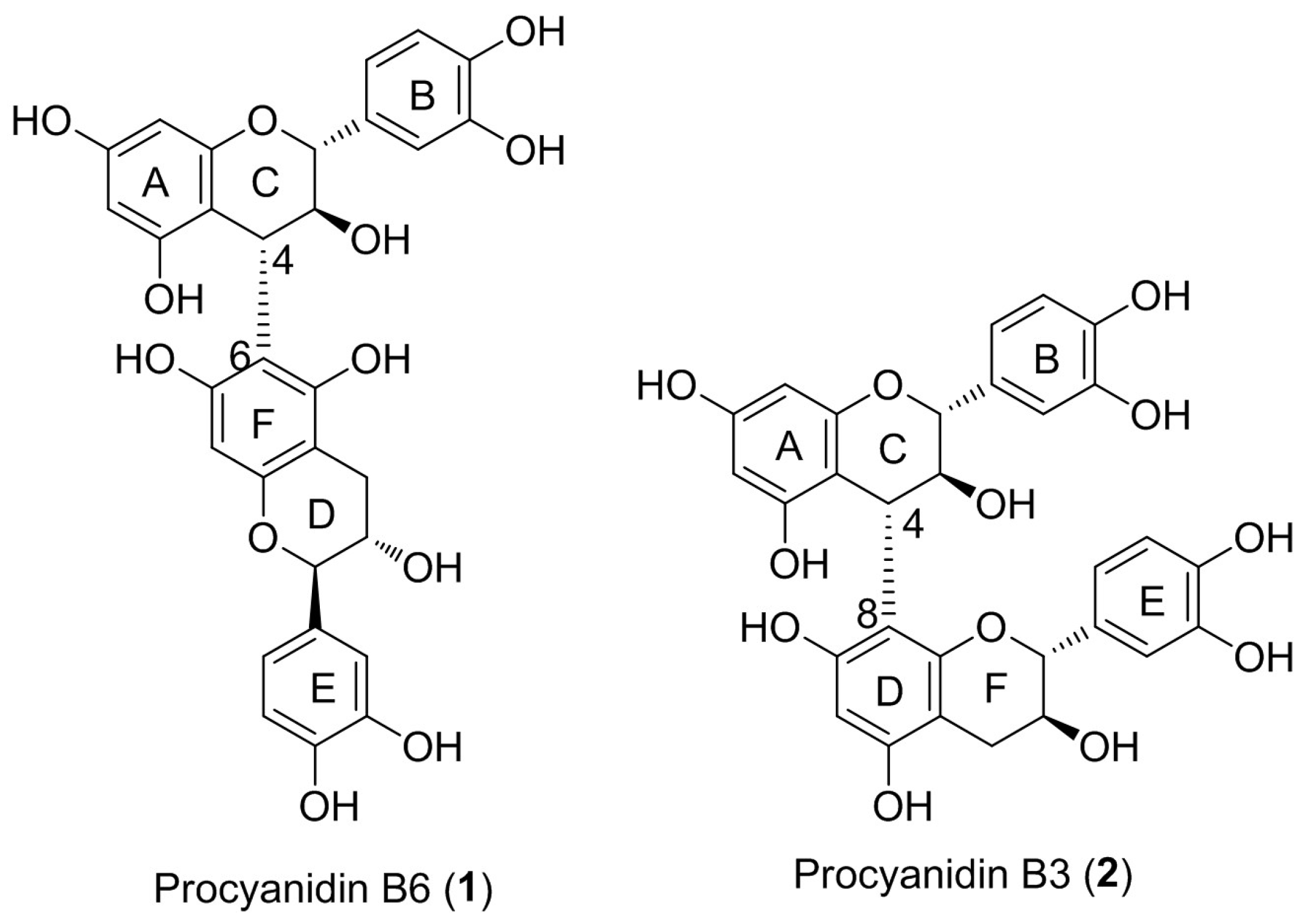
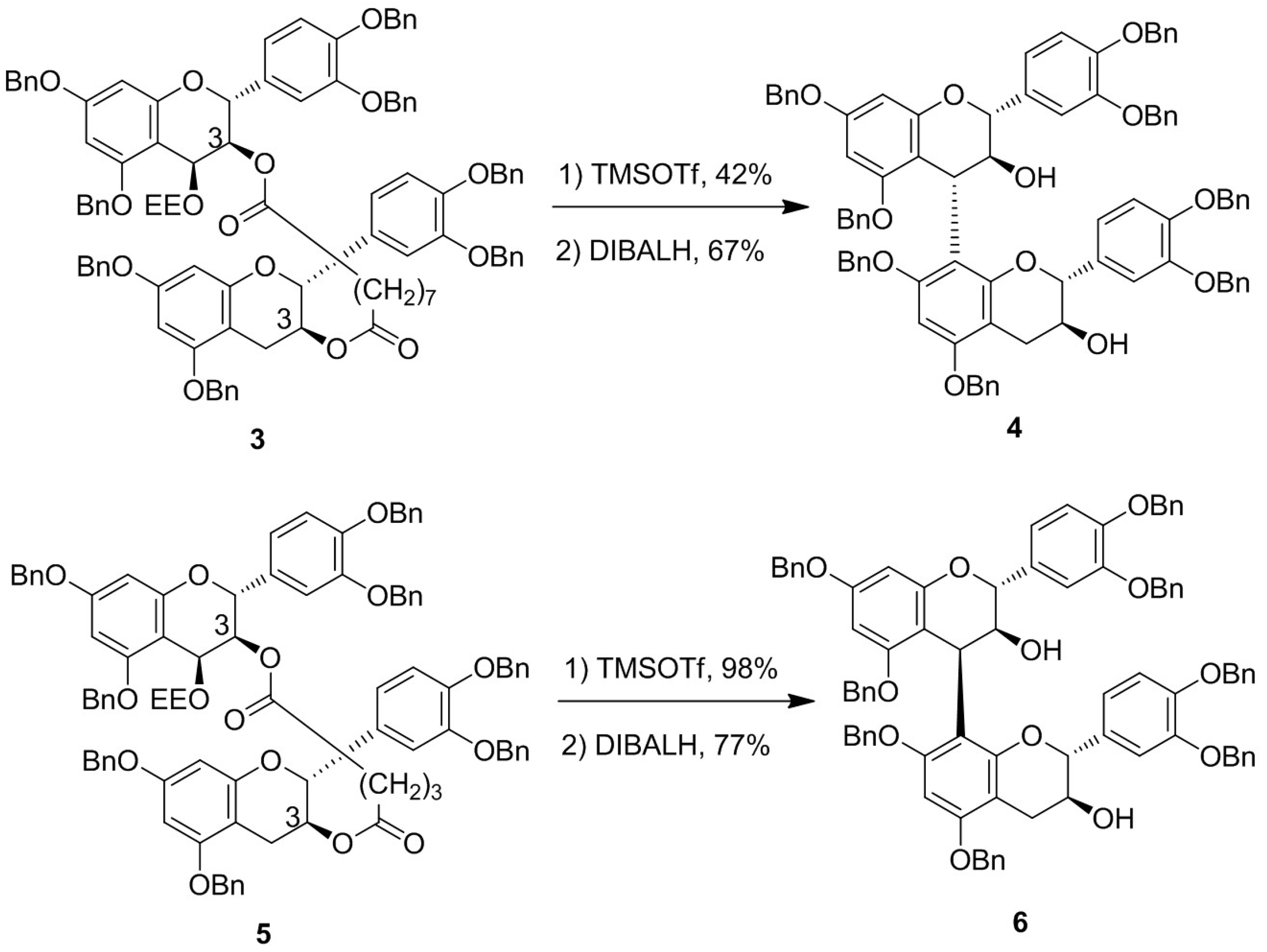
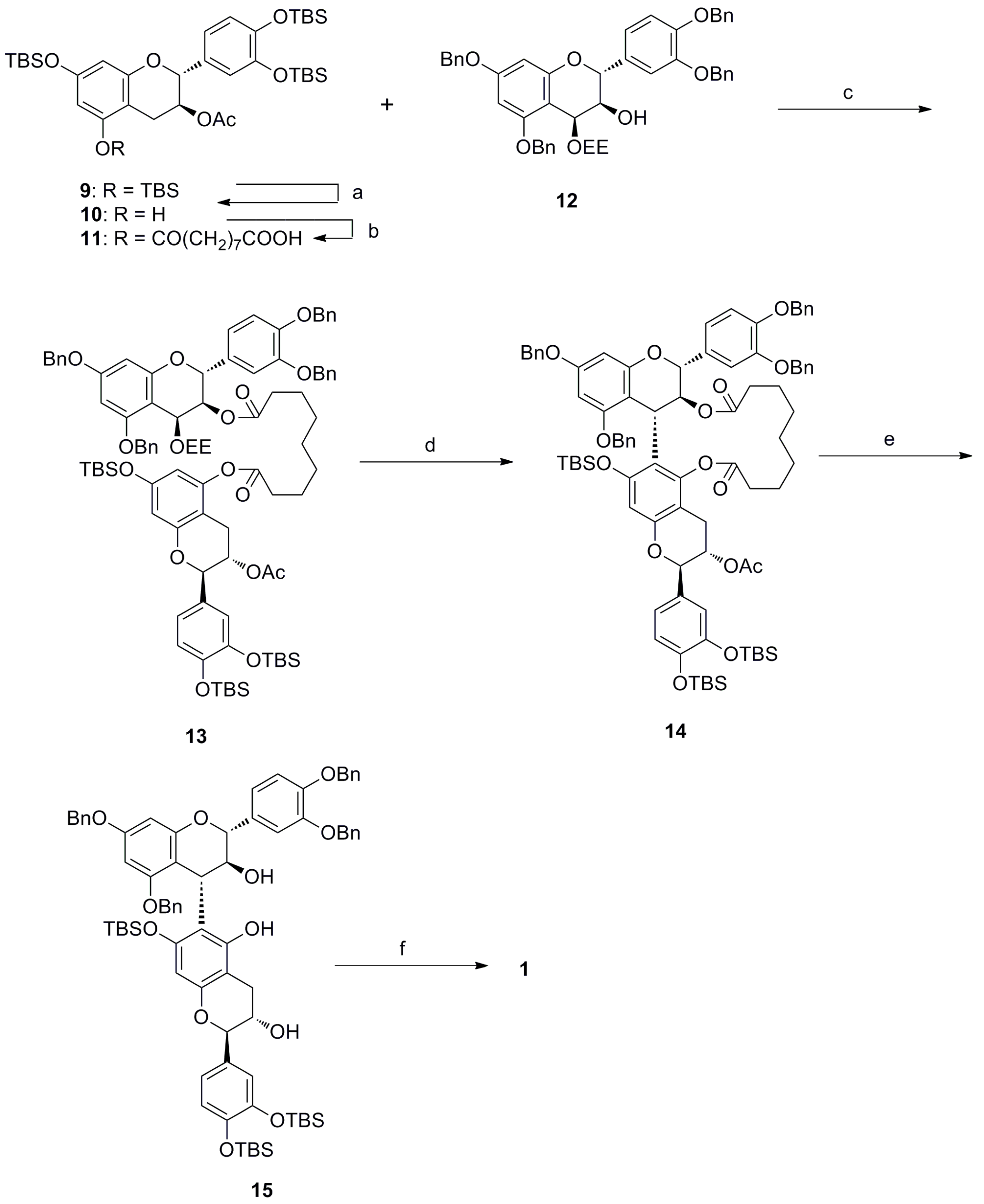
:1. Introduction
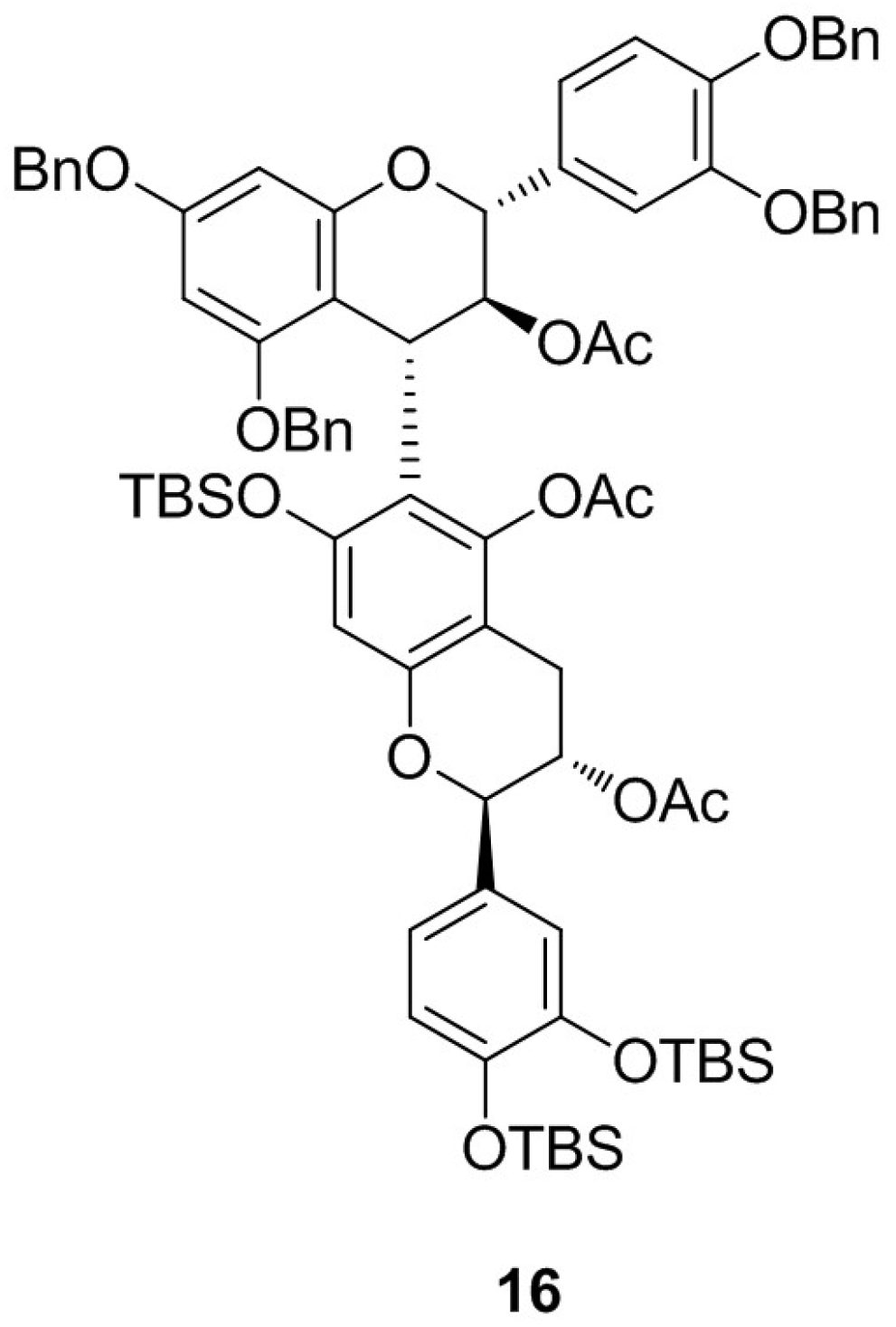
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AcOH | acetic acid |
| Bn | benzyl |
| DCC | N,N′-dicyclohexylcarbodiimide |
| DIBALH | diisobutylaluminum hydrate |
| DMAP | 4-dimethylaminopyridine |
| DMSO | dimethyl sulfoxide |
| EE | ethoxyethyl |
| ESI | electrospray ionization |
| Et3N | trimethylamine |
| EtOAc | ethyl acetate |
| FAB | fast atom bombardment |
| HPLC | high-performance liquid chromatography |
| MeOH | methanol |
| SAR | structure-activity-relationship studies |
| TBAF | tetrabutylammonium fluoride |
| TBDMS and TBS | t-butyldimethylsilyl |
| TFA | trifluoroacetic acid |
| THF | tetrahydrofuran |
| TLC | thin-layer chromatography |
| TMSOTf | trimethylsilyl triflate |
References
- Harborne, J.B. The Flavonoids: Advances in Research from 1986; Chapman and Hall: London, UK, 1993. [Google Scholar]
- Harbone, J.B.; Baxter, H. The Handbook of Natural Flavonoids; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Oracz, J.; Żyżelewicz, D.; Nebesny, E. The content of polyphenolic compounds in cocoa beans (Theobroma cacao L.), depending on variety, growing region and processing operations: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1176–1192. [Google Scholar] [CrossRef] [PubMed]
- Żyżelewicz, D.; Zakłos-Szyda, M.; Juśkiewicz, J.; Bojczuk, M.; Oracz, J.; Budryn, G.; Miśkiewicz, K.; Krysiak, W.; Zduńczyk, Z.; Jurgoński, A. Cocoa bean (Theobroma cacao L.) phenolic extracts as PTP1B inhibitors, hepatic HepG2 and pancreatic β-TC3 cell cytoprotective agents and their influence on oxidative stress in rats. Food Res. Int. 2016, 89, 946–957. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, D.; Thajuddin, N.; Panneerselvam, A. Fungicides for Plant and Animal Diseases; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Tsukuda, S.; Watashi, K.; Hojima, T.; Isogawa, M.; Iwamoto, M.; Omagari, K.; Suzuki, R.; Aizaki, H.; Kojima, S.; Sugiyama, M.; et al. A New class of hepatitis B and D virus entry inhibitors, proanthocyanidin and its analogs, that directly act on the viral large surface proteins. Hepatology 2017, 65, 1104–1116. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Domínguez-Perles, R.; Gironés-Vilaplana, A.; Baenas, N.; García-Viguera, C.; Villaño, D. Flavan-3-ols, anthocyanins, and inflammation. IUBMB Life 2014, 66, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Du, G.J.; Zhang, Z. Epigallocatechin gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Mehendale, S.; Calway, T.; Yuan, C.S. Botanical flavonoids on coronary heart disease. Am. J. Chin. Med. 2011, 39, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Matsuo, Y.; Kouno, I. Chemistry of secondary polyphenols produced during processing of tea and selected foods. Int. J. Mol. Sci. 2009, 11, 14–40. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.C.; Thomas, A.L.; Greenlief, C.M. Impact of frozen storage on the anthocyanin and polyphenol content of American elderberry fruit juice. J. Agric. Food Chem. 2015, 63, 5653–5659. [Google Scholar] [CrossRef] [PubMed]
- Delcour, J.A.; Tuytens, G.M. Structure elucidation of three demeric proanthocyanidins isolated from a commercial Belgan pilsner beer. J. Inst. Brew. 1984, 90, 153–161. [Google Scholar] [CrossRef]
- Tückmantel, W.; Kozikowski, A.P.; Romanczyk, L.J. Studies in polyphenol chemistry and bioactivity. 1. Preparation of building blocks from (+)-catechin. Procyanidin formation. Synthesis of the cancer cell growth inhibitor, 3-O-galloyl-(2R,3R)-epicatechin-4β,8-[3-O-galloyl-(2R,3R)-epicatechin]. J. Am. Chem. Soc. 1999, 121, 12073–12081. [Google Scholar] [CrossRef]
- Kozikowski, A.P.; Tückmantel, W.; George, C. Studies in polyphenol chemistry and bioactivity. 2. Establishment of interflavan linkage regio- and stereochemistry by oxidative degradation of an O-alkylated derivative of procyanidin B2 to (R)-(−)-2,4-diphenylbutyric acid. J. Org. Chem. 2000, 65, 5371–5381. [Google Scholar] [CrossRef] [PubMed]
- Kozikowski, A.P.; Tückmantel, W.; Böttcher, G.; Romanczyk, L.J., Jr. Studies in polyphenol chemistry and bioactivity. 4. Synthesis of trimeric, tetrameric, pentameric, and higher oligomeric epicatechin-derived procyanidins having all-4β,8-interflavan connectivity and their inhibition of cancer cell growth through cell cycle arrest. J. Org. Chem. 2003, 68, 1641–1658. [Google Scholar] [PubMed]
- Ohmori, K.; Ushimaru, N.; Suzuki, K. Oligomeric catechins: An enabling synthetic strategy by orthogonal activation and C(8) protection. Proc. Natl. Acad. Sci. USA 2004, 101, 12002–12007. [Google Scholar] [CrossRef] [PubMed]
- Tarascou, I.; Barathieu, K.; Andre, Y.; Pianet, I.; Dufourc, E.; Fouquet, E. An improved synthesis of procyanidin dimers: Regio- and stereocontrol of the interflavan bond. Eur. J. Org. Chem. 2006, 23, 5367–5377. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kolchinski, A.; Shea, H.A.; Nair, J.J.; Gou, Y.; Romanczyk, L.J.; Schmitz, H.H. Scale-up syntheses of two naturally occurring procyanidins: (−)-epicatechin-(4β,8)-(+)-catechin and (−)-epicatechin-3-O-galloyl-(4β,8)-(−)-epicatechin-3-O-gallate. Org. Process Res. Dev. 2007, 11, 422–430. [Google Scholar] [CrossRef]
- Mohri, Y.; Sagehashi, M.; Yamada, T.; Hattori, Y.; Morimura, K.; Kamo, T.; Hirota, M.; Makabe, H. An efficient synthesis of procyanidins. Rare earth metal Lewis acid catalyzed equimolar condensation of catechin and epicatechin. Tetrahedron Lett. 2007, 48, 5891–5894. [Google Scholar] [CrossRef]
- Oyama, K.; Kuwano, M.; Ito, M.; Yoshida, K.; Kondo, T. Synthesis of procyanidins by stepwise- and self-condensation using 3,4-cis-4-acetoxy-3-O-acetyl-4-dehydro-5,7,3,4-tetra-O-benzyl-(+)-catechin and (−)-epicatechin as a key building monomer. Tetrahedron Lett. 2008, 49, 3176–3180. [Google Scholar] [CrossRef]
- Achilonu, M.C.; Bonnet, S.L.; van der Westhuizen, J.H. Synthesis of proanthocyanidins. Part 1. The first oxidative formation of the interflavanyl bond in procyanidins. Org. Lett. 2008, 10, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Mohri, Y.; Sagehashi, M.; Yamada, T.; Hattori, Y.; Morimura, K. An efficient synthesis of procyanidins using equimolar condensation of catechin and/or epicatechin catalyzed by ytterbium triflate. Heterocycles 2009, 79, 549–563. [Google Scholar] [CrossRef]
- Watanabe, G.; Ohmori, K.; Suzuki, K. First regiocontrolled synthesis of procyanidin B6, a catechin dimer with rare connectivity: A halo-capping strategy for formation of 4,6-interflavan bonds. Chem. Commun. 2013, 49, 5210–5216. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Nakajima, N.; Tanaka, A.; Ubukata, M. Synthetic studies of proanthocyanidins. Highly stereoselective synthesis of catechin dimer, procyanidin-B3. Biosci. Biotechnol. Biochem. 2002, 66, 1764–1767. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Nakajima, N.; Tanaka, A.; Ubukata, M. Synthetic studies of proanthocyanidins. Part 2: Stereoselective gram-scale synthesis of procyanidin-B3. Tetrahedron 2002, 58, 7829–7837. [Google Scholar] [CrossRef]
- Saito, A.; Nakajima, N.; Tanaka, A.; Ubukata, M. Synthetic studies of proanthocyanidins. Part 3: Stereoselective 3,4-cis catechin and catechin condensation by TMSOTf-catalyzed intramolecular coupling method. Tetrahedron Lett. 2003, 44, 5449–5452. [Google Scholar] [CrossRef]
- Saito, A.; Nakajima, N.; Tanaka, A.; Ubukata, M. Synthetic studies of proanthocyanidins. Part 4. The synthesis of procyanidin B1 and B4. TMSOTf-catalyzed cyclization of catechin and epicatechin condensation. Heterocycles 2003, 61, 287–298. [Google Scholar]
- Saito, A.; Nakajima, N.; Matsuura, M.; Tanaka, A.; Ubukata, M. Synthetic studies of proanthocyanidins. Part 5. Highly stereoselective synthesis and inhibitory activity of Maillard reaction of 3,4-trans catechin and epicatechin dimers, procyanidin B1, B2, B3, B4 and their acetates. Heterocycles 2004, 62, 479–489. [Google Scholar]
- Saito, A.; Tanaka, A.; Ubukata, M.; Nakajima, N. Efficient stereoselective synthesis of proanthocyanidin trimers with TMSOTf-catalyzed intermolecular condensation. Synlett 2004, 2014, 1069–1073. [Google Scholar] [CrossRef]
- Saito, A.; Tanaka, A.; Ubukata, M.; Nakajima, N. Stereoselection of 3,4-cis and 3,4-trans catechin and catechin condensation under intramolecular coupling method. Synlett 2004, 2014, 2040–2042. [Google Scholar] [CrossRef]
- Saito, A.; Doi, Y.; Matsuura, N.; Tanaka, A.; Ubukata, M.; Nakajima, N. Systematic synthesis of four epicatechin series procyanidin trimers and their inhibitory activity on the Maillard reaction and antioxidant activity. Bioorg. Med. Chem. 2004, 12, 4783–4790. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Enomoto, M.; Tanaka, A.; Doi, Y.; Shoji, K.; Mizushina, Y.; Ikawa, H.; Yoshida, H.; Matsuura, N.; Nakajima, N. Stereoselective synthesis of procyanidin B3-3-O-gallate and 3,3″-di-O-gallate, and their abilities as antioxidant and DNA polymerase inhibitor. Tetrahedron 2004, 60, 12043–12049. [Google Scholar] [CrossRef]
- Saito, A.; Mizushina, Y.; Ikawa, H.; Yoshida, H.; Doi, Y.; Tanaka, A.; Nakajima, N. Systematic synthesis of galloyl-substituted procyanidin B1 and B2, and their ability of DPPH radical scavenging activity and inhibitory activity of DNA polymerases. Bioorg. Med. Chem. 2005, 13, 2759–2771. [Google Scholar] [CrossRef] [PubMed]
- Sakuda, H.; Saito, A.; Mizushina, Y.; Ikawa, H.; Yoshida, H.; Nakajima, N. Synthesis of galloyl-substituted procyanidin B4 series, and their DPPH radical scavenging activity and DNA polymerase inhibitory activity. Heterocycles 2006, 67, 175–188. [Google Scholar]
- Saito, A.; Mizushina, Y.; Tanaka, A.; Nakajima, N. Versatile synthesis of epicatechin series procyanidin oligomers, and their antioxidant and DNA polymerase inhibitory activity. Tetrahedron 2009, 65, 7422–7428. [Google Scholar] [CrossRef]
- Ishihara, S.; Doi, S.; Harui, K.; Okamoto, T.; Okamoto, S. Development of a new synthetic strategy for procyanidin dimer condensation using peracetylated electrophiles. Heterocycles 2014, 88, 1595–1602. [Google Scholar]
- Okamoto, S.; Ishihara, S.; Okamoto, T.; Doi, S.; Harui, K.; Higashino, Y.; Kawasaki, T.; Nakajima, N.; Saito, A. Inhibitory activity of synthesized acetylated procyanidin B1 analogues against HeLa S3 cells proliferation. Molecules 2014, 19, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Takano, S.; Ayano, Y.; Tokunaga, M.; Koashi, T.; Hamada, M.; Nakajima, N.; Saito, A. Structure—Activity relationship of oligomeric flavan-3-ols: Importance of upper-unit B-ring hydroxyl groups in the dimeric structure for strong activities. Molecules 2015, 20, 18870–18885. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Ayano, Y.; Hamada, Y.; Hojima, T.; Tanaka, R.; Higashino, Y.; Izuno, M.; Okamoto, T.; Kawasaki, T.; Hamada, M.; Nakajima, N.; Saito, A. Role of 2,3-cis structure of (–)-epicatechin-3,5-O-digallate in inhibition of HeLa S3 cell proliferation. Nat. Prod. Chem. Res. 2015, 3, 172. [Google Scholar]
- Flecher, A.C.; Porter, L.J.; Haslam, E.; Gupta, R.K. Plant proanthocyanidins. Part 3. Conformational and configurational studies of natural procyanidins. J. Chem. Soc. Perkin Trans. 1 1977, 14, 1628–1637. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1 and 2 are available from the authors. |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higashino, Y.; Okamoto, T.; Mori, K.; Kawasaki, T.; Hamada, M.; Nakajima, N.; Saito, A. Regioselective Synthesis of Procyanidin B6, A 4-6-Condensed (+)-Catechin Dimer, by Intramolecular Condensation. Molecules 2018, 23, 205. https://doi.org/10.3390/molecules23010205
Higashino Y, Okamoto T, Mori K, Kawasaki T, Hamada M, Nakajima N, Saito A. Regioselective Synthesis of Procyanidin B6, A 4-6-Condensed (+)-Catechin Dimer, by Intramolecular Condensation. Molecules. 2018; 23(1):205. https://doi.org/10.3390/molecules23010205
Chicago/Turabian StyleHigashino, Yusuke, Taisuke Okamoto, Kazuki Mori, Takashi Kawasaki, Masahiro Hamada, Noriyuki Nakajima, and Akiko Saito. 2018. "Regioselective Synthesis of Procyanidin B6, A 4-6-Condensed (+)-Catechin Dimer, by Intramolecular Condensation" Molecules 23, no. 1: 205. https://doi.org/10.3390/molecules23010205