Mechano-Enzymatic Deconstruction with a New Enzymatic Cocktail to Enhance Enzymatic Hydrolysis and Bioethanol Fermentation of Two Macroalgae Species
Abstract
:1. Introduction
- (i)
- Explore the efficiency of a new enzymatic cocktail to hydrolyze polysaccharides of two macroalgae species (red and green sp.)
- (ii)
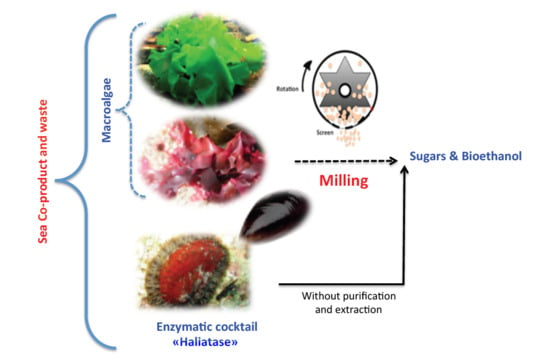
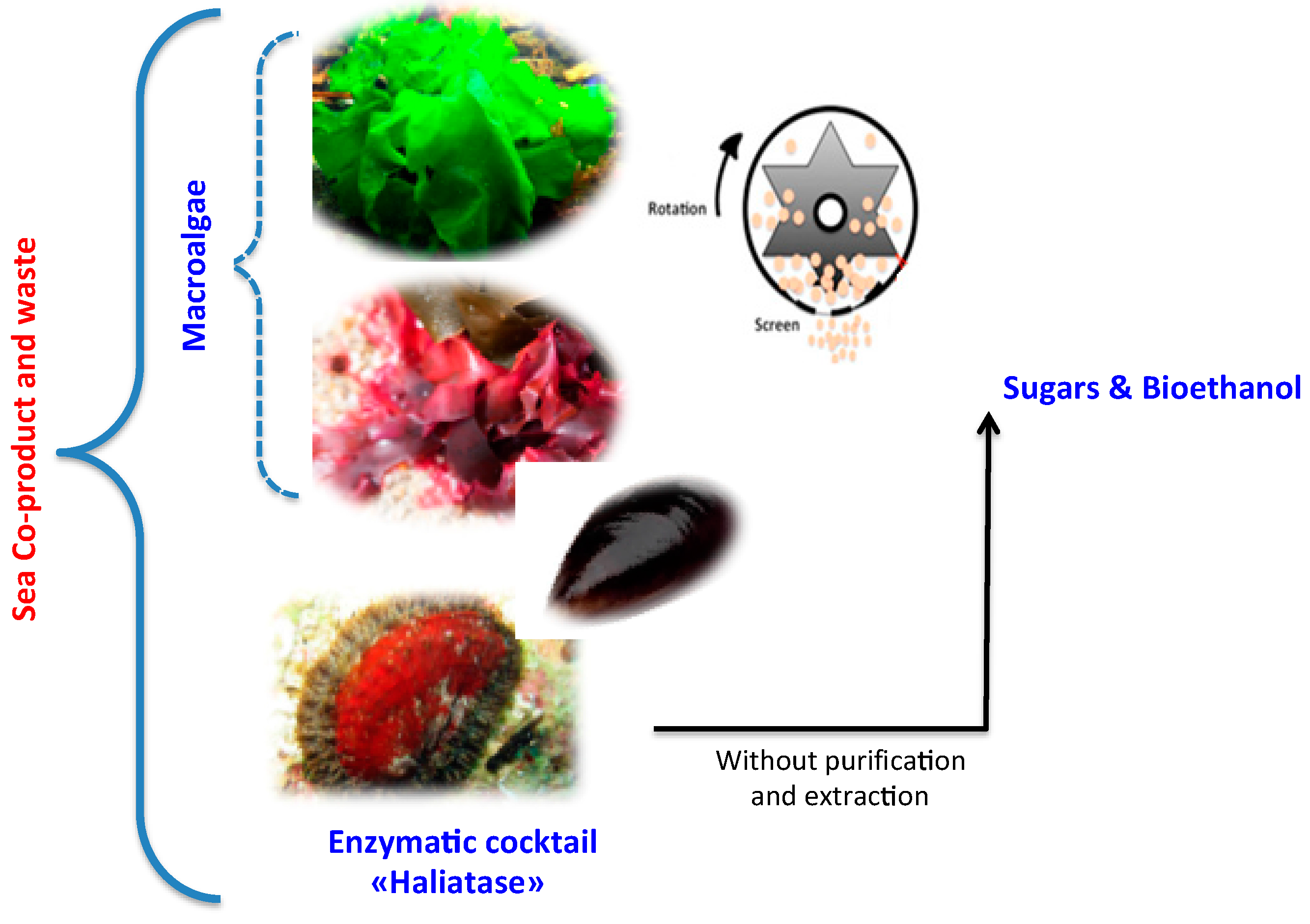
- Study the effect of two mechanical pretreatments, centrifugal milling (CM) and vibro ball milling (VBM) on enzymatic hydrolysis and bioethanol fermentation of the two-macroalgae species (red G. sesquipedale and green U. lactuca) (Figure 1).
2. Results and Discussion
2.1. Chemical Composition
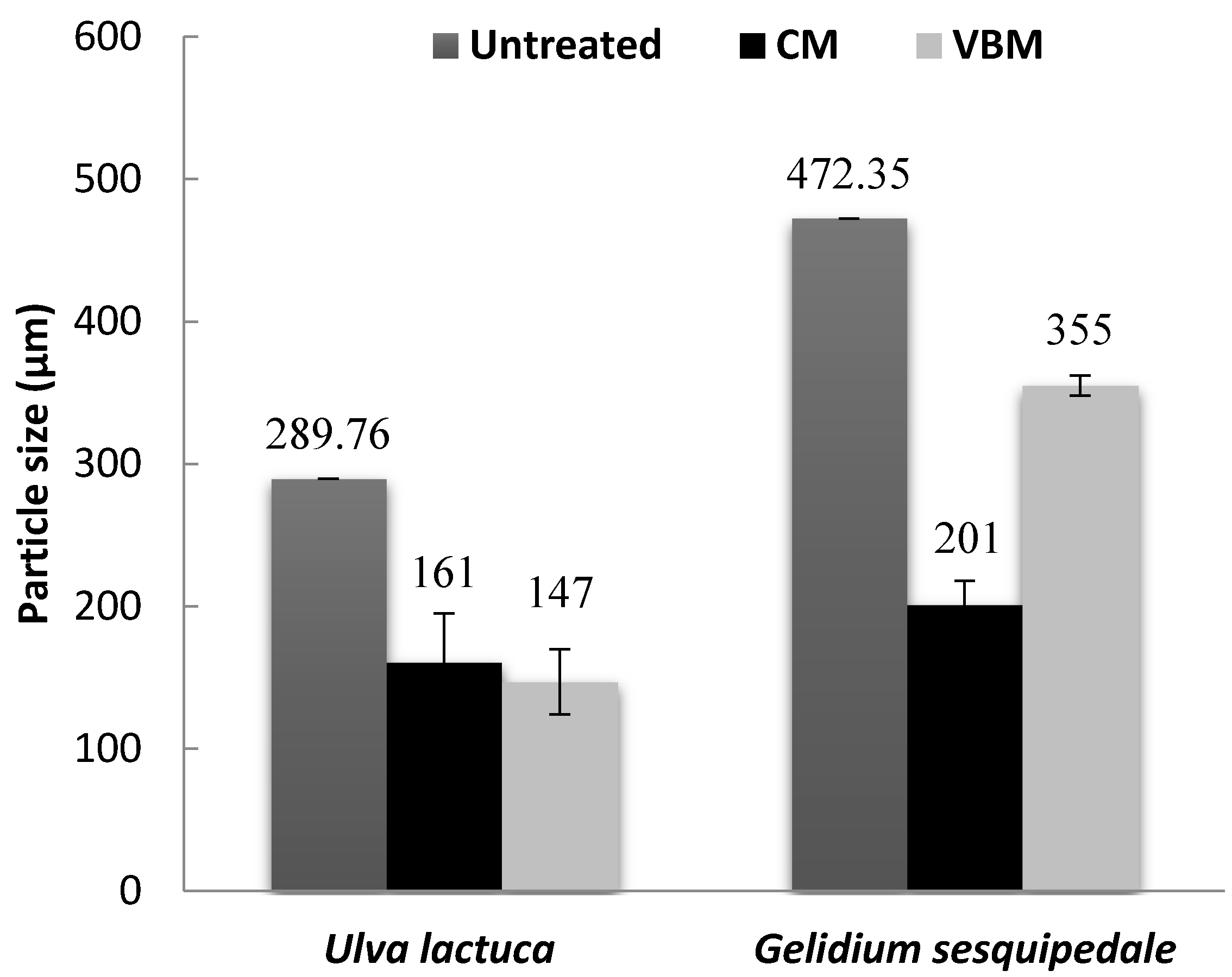
2.2. Particle Size of Macroalgae
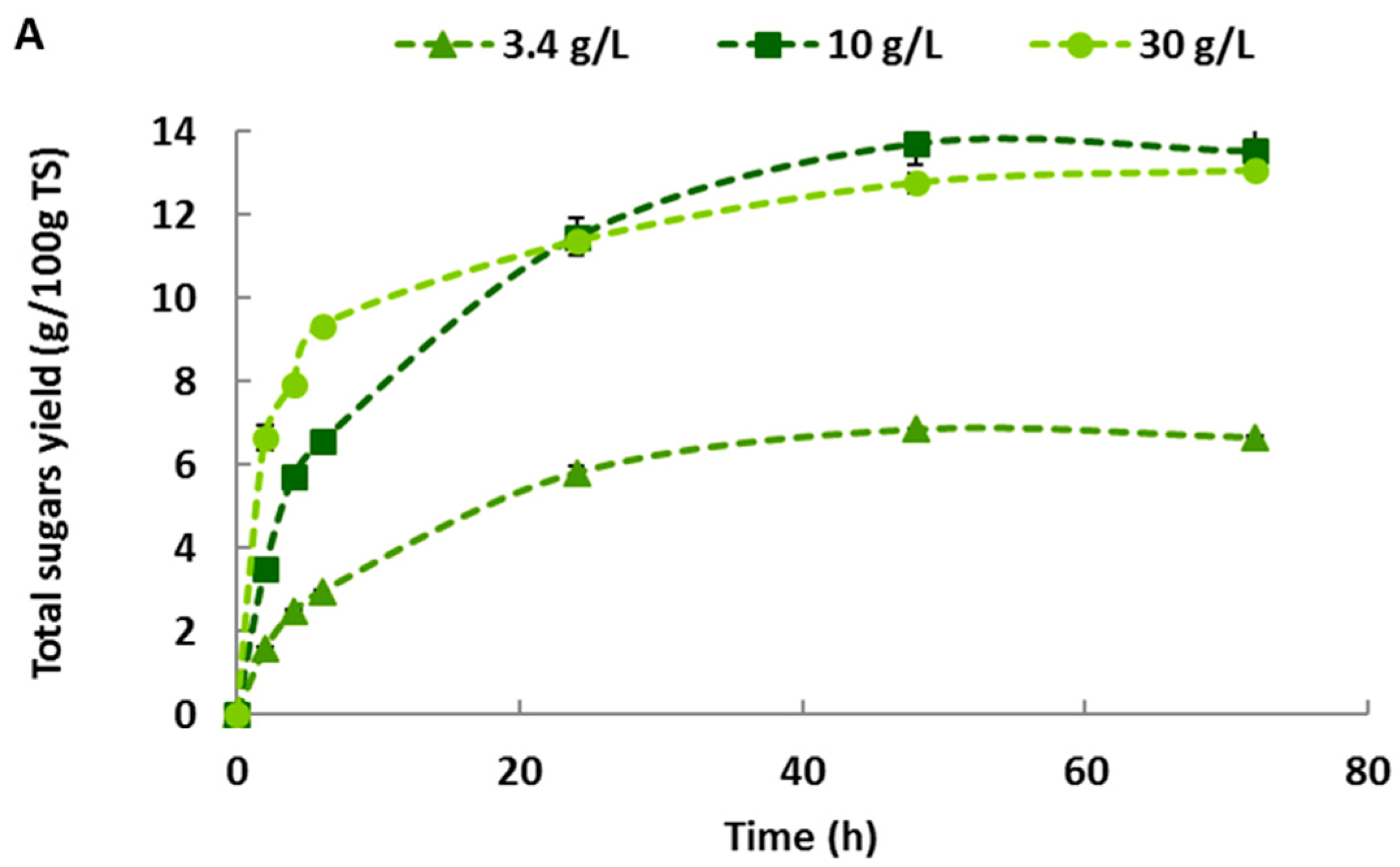
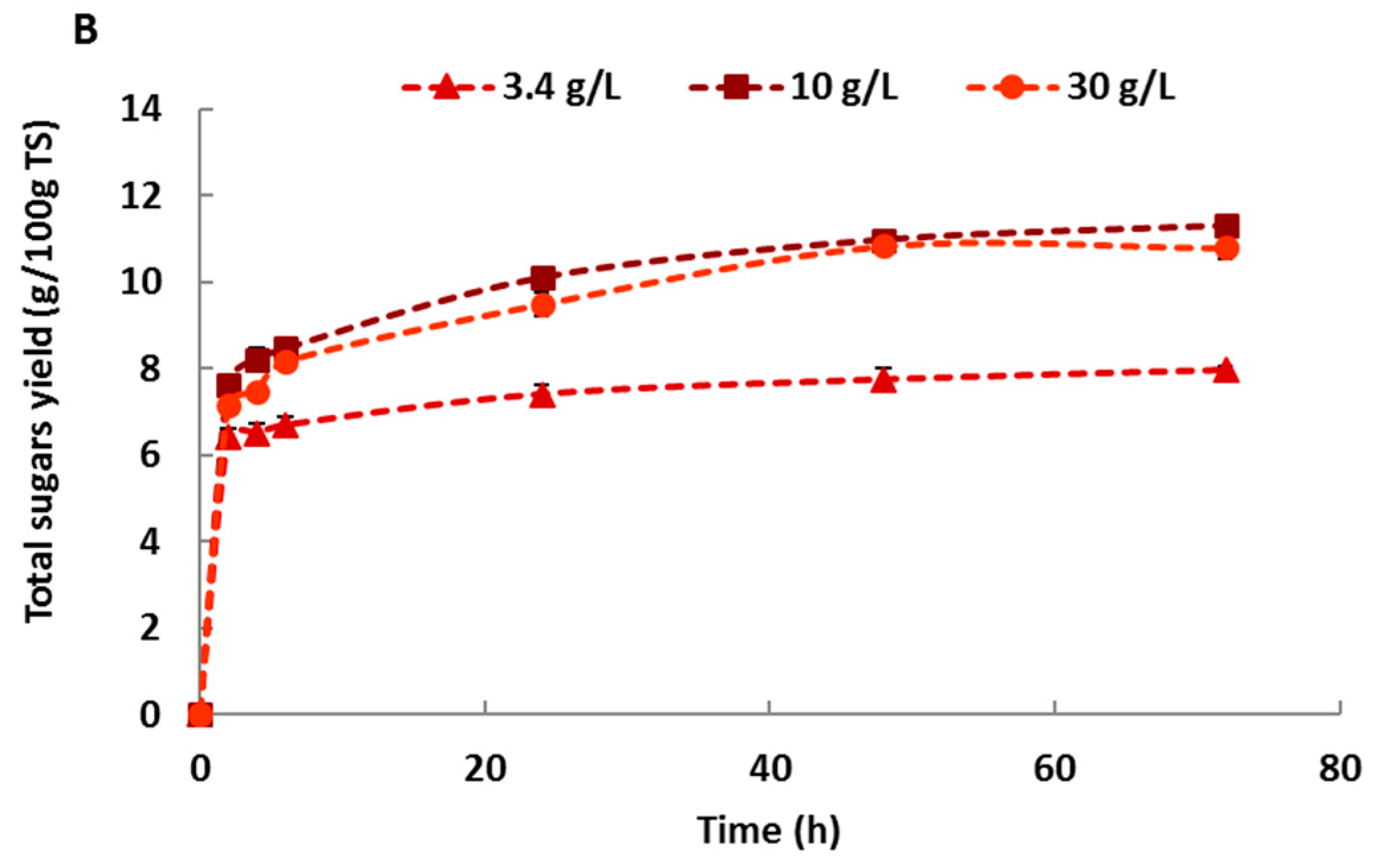
2.3. Effect of Haliatase Cocktail Activity on Sugars Yield of Untreated Macroalgae
2.4. Effect of Mechanical Pretreatments on Enzymatic Hydrolysis of Macroalgae
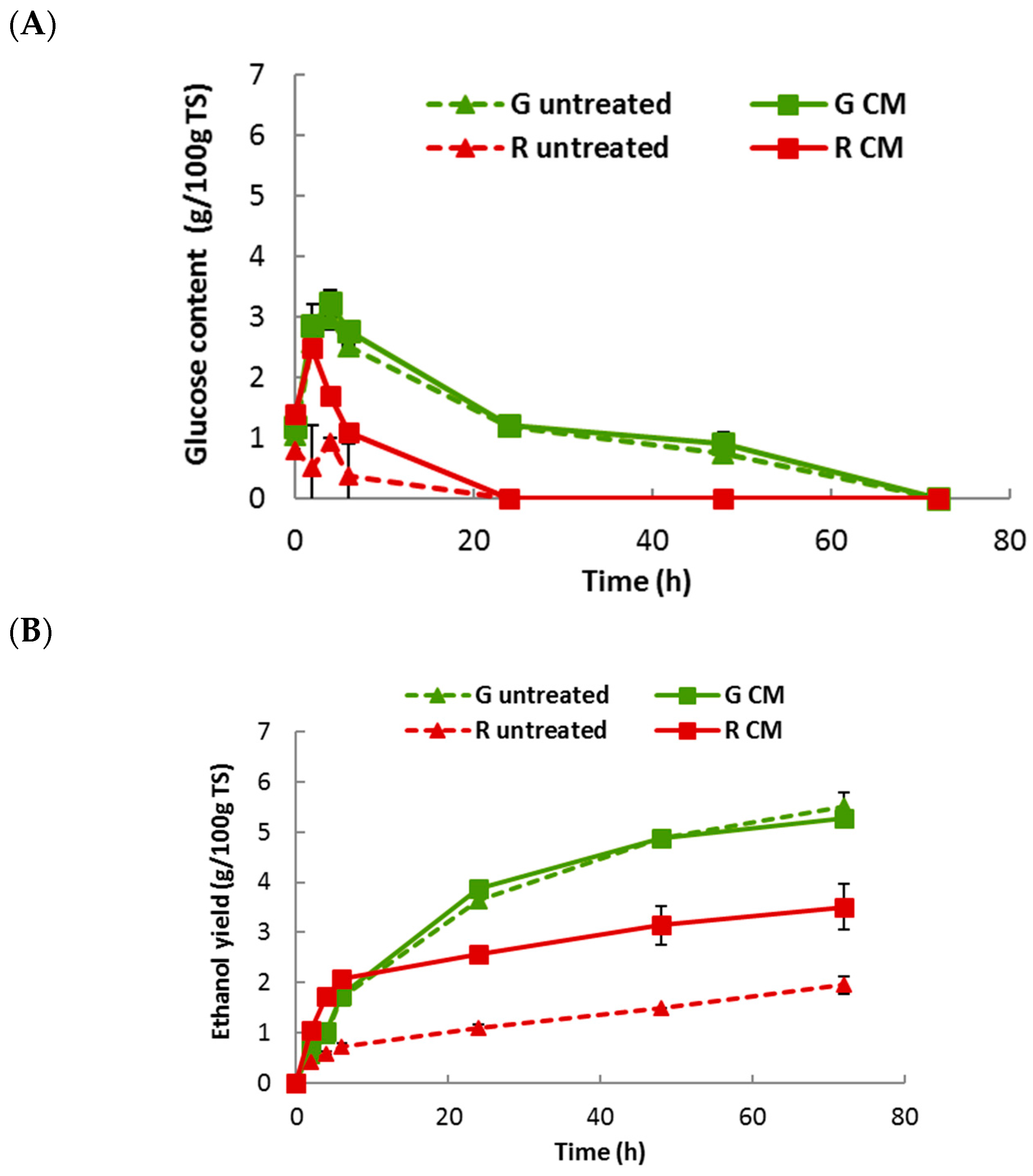
2.5. Bioethanol Fermentation of U. lactuca and G. sesquipedale
3. Materials and Methods
3.1. Macroalgae
3.2. Enzymatic Cocktail
3.3. Enzymatic Hydrolysis
3.4. Bioethanol Fermentation
3.5. Analytical Determinations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| VBM | Vibro-Ball Milling |
| CM | Centrifugal Milling |
| TS | Total Solids |
| VS | Volatile Solids |
| SSF | Simultaneous Saccharification Fermentation |
References
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Micro and macroalgal biomass: A renewable source for bioethanol. Bioresour. Technol. 2011, 102, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Borines, M.G.; de Leon, R.L.; Cuello, J.L. Bioethanol production from the macroalgae Sargassum spp. Bioresour. Technol. 2013, 138, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.; Um, Y.; Yoon, H.H. Pretreatment of macroalgae for volatile fatty acid production. Bioresour. Technol. 2013, 146, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Li, P.; Lee, J.; Ryu, H.J.; Oh, K.K. Ethanol production from Saccharina japonica using an optimized extremely low acid pretreatment followed by simultaneous saccharification and fermentation. Bioresour. Technol. 2013, 127, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Hong, J.-Y.; Jang, H.C.; Oh, S.G.; Kim, S.-H.; Yoon, J.-J.; Kim, Y.J. Use of Gelidium amansii as a promising resource for bioethanol: A practical approach for continuous dilute-acid hydrolysis and fermentation. Bioresour. Technol. 2012, 108, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Sambusiti, C.; Bellucci, M.; Zabaniotou, A.; Beneduce, L.; Monlau, F. Algae as promising feedstocks for fermentative biohydrogen production according to a biorefinery approach: A comprehensive review. Renew. Sustain. Energy Rev. 2015, 44, 20–36. [Google Scholar] [CrossRef]
- Jung, K.A.; Lim, S.-R.; Kim, Y.; Park, J.M. Potentials of macroalgae as feedstocks for biorefinery. Bioresour. Technol. 2013, 135, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Meinita, M.D.N.; Hong, Y.-K.; Jeong, G.-T. Comparison of sulfuric and hydrochloric acids as catalysts in hydrolysis of Kappaphycus alvarezii (cottonii). Bioprocess Biosyst. Eng. 2012, 35, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Wang, G. Two-stage Hydrolysis of Invasive Algal Feedstock for Ethanol FermentationF. J. Integr. Plant Biol. 2011, 53, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Schultz-Jensen, N.; Thygesen, A.; Leipold, F.; Thomsen, S.T.; Roslander, C.; Lilholt, H.; Bjerre, A.B. Pretreatment of the macroalgae Chaetomorpha linum for the production of bioethanol—Comparison of five pretreatment technologies. Bioresour. Technol. 2013, 140, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Monlau, F.; Sambusiti, C.; Antoniou, N.; Zabaniotou, A.; Solhy, A.; Barakat, A. Pyrochars from bioenergy residue as novel bio-adsorbents for lignocellulosic hydrolysate detoxification. Bioresour. Technol. 2015, 187, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Delgenes, J.P.; Moletta, R.; Navarro, J.M. Effects of lignocellulose degradation products on ethanol fermentations of glucose and xylose by Saccharomyces cerevisiae, Zymomonas mobilis, Pichia stipitis, and Candida shehatae. Enzym. Microb. Technol. 1996, 19, 220–225. [Google Scholar] [CrossRef]
- Motte, J.C.; Delenne, J.Y.; Rouau, X.; Mayer-Laigle, C. Mineral–vegetal co-milling: An effective process to improve lignocellulosic biomass fine milling and to increase interweaving between mixed particles. Bioresour. Technol. 2015, 192, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-J.; Li, H.; Jung, K.; Chang, H.N.; Lee, P.C. Ethanol production from marine algal hydrolysates using Escherichia coli KO11. Bioresour. Technol. 2011, 102, 7466–7469. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, N.; Gupta, V.; Reddy, C.R.K.; Jha, B. Enzymatic hydrolysis and production of bioethanol from common macrophytic green alga Ulva fasciata Delile. Bioresour. Technol. 2013, 150, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Jard, G.; Marfaing, H.; Carrère, H.; Delgenes, J.P.; Steyer, J.P.; Dumas, C. French Brittany macroalgae screening: Composition and methane potential for potential alternative sources of energy and products. Bioresour. Technol. 2013, 144, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Ra, C.-H.; Kim, S.-K. Ethanol Production from the Seaweed Gelidium Amansii, Using Specific Sugar Acclimated Yeasts. J. Microbiol. Biotechnol. 2014, 24, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Motte, J.-C.; Sambusiti, C.; Dumas, C.; Barakat, A. Combination of dry dark fermentation and mechanical pretreatment for lignocellulosic deconstruction: An innovative strategy for biofuels and volatile fatty acids recovery. Appl. Energy 2015, 147, 67–73. [Google Scholar] [CrossRef]
- Barakat, A.; De Vries, H.; Rouau, X. Dry fractionation process as an important step in current and future lignocellulose biorefineries: A review. Bioresour. Technol. 2013, 134, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Chuetor, S.; Monlau, F.; Solhy, A.; Rouau, X. Eco-friendly dry chemo-mechanical pretreatments of lignocellulosic biomass: Impact on energy and yield of the enzymatic hydrolysis. Appl. Energy 2014, 113, 97–105. [Google Scholar] [CrossRef]
- Berlowska, J.; Pielech-Przybylska, K.; Balcerek, M.; Cieciura, W.; Borowski, S.; Kregiel, D. Integrated Bioethanol Fermentation/Anaerobic Digestion for Valorization of Sugar Beet Pulp. Energies 2017, 10, 1255. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Wychen, V.; Laurens, L.M.L. Determination of Total Carbohydrates in Algal Biomass; Laboratory Analytical Pro-Cedure (LAP): Golden, CO, USA, 2013. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

| Composition | G. sesquipedale | U. lactuca |
|---|---|---|
| TS (g/100 g FM) | 91 ± 0 | 90 ± 0 |
| VS (g/100 g TS) | 78 ± 0.1 | 62 ± 0.7 |
| Ash (g/100 g TS) | 11 ± 0.3 | 32 ± 0.7 |
| C (g/100 g TS) | 34.7 ± 0.5 | 26.8 ± 1.5 |
| N (g/100 g TS) | 2.5 ± 0.1 | 2.3 ± 0.4 |
| H (g/100 g TS) | 5.7 ± 0.05 | 4.5 ± 0.6 |
| S (g/100 g TS) | 2.1 ± 0.02 | 3.7 ± 0.12 |
| Proteins (g/100 g TS) | 19.9 ± 0.82 | 15.9 ± 1.91 |
| Total Sugars (g/100 g TS) | 30.9 | 25.8 |
| Monomeric sugars * | ||
| Glucose (g/100 g TS) | 9.6 ± 0.06 | 15.2 ± 1.01 |
| Galactose (g/100 g TS) | 20.3 ± 0.78 | n.d. |
| Arabinose (g/100 g TS) | 0.9 ± 0.06 | n.d. |
| Xylose (g/100 g TS) | n.d. | 3.1 ± 0.18 |
| Rhamnose (g/100 g TS) | n.d. | 7.5 ± 0.13 |
| Fucose (g/100 g TS) | n.d. | 0.5 ± 0.04 |
| Glucuronic acid (g/100 g TS) | 0.3 ± 0.03 | 3.86 ± 0.01 |
| Galacturonic acid (g/100 g TS) | 3.0 ± 0.06 | 1.15 ± 0.00 |
| Samples | Enzyme Loading | 3.4 g/L | 10 g/L | 30 g/L |
|---|---|---|---|---|
| Green alga: Ulva lactuca | Untreated | 6.66 ± 0.04 | 13.52 ± 0.51 | 13.05 ± 0.16 |
| Centrifugal milling | 6.48 ± 0.40 | 13.46 ± 0.61 | 13.24 ± 0.37 | |
| Vibro ball milling | 6.70 ± 0.35 | 13.33 ± 0.37 | 12.49 ± 0.20 | |
| Red alga: Gelidium sesquipedale | Untreated | 7.96 ± 0.09 | 11.30 ± 0.13 | 10.79 ± 0.26 |
| Centrifugal milling | 10.28 ± 0.06 | 13.28 ± 0.19 | 13.09 ± 0.48 | |
| Vibro ball milling | 10.03 ± 0.20 | 12.70 ± 0.11 | 13.59 ± 0.34 |
| Samples | Enzyme Loading | 3.4 g/L | 10 g/L | 30 g/L |
|---|---|---|---|---|
| Green alga: Ulva lactuca | Untreated | 5.78 ± 0.00 | 12.45 ± 0.47 | 10.49 ± 0.10 |
| Centrifugal milling | 5.79 ± 0.52 | 12.59 ± 0.50 | 10.71 ± 0.13 | |
| Vibro ball milling | 6.01 ± 0.60 | 12.63 ± 0.46 | 10.21 ± 0.08 | |
| Red alga: Gelidium sesquipedale | Untreated | 1.48 ± 0.04 | 4.07 ± 0.22 | 4.12 ± 0.13 |
| Centrifugal milling | 3.87 ± 0.17 | 7.09 ± 0.22 | 6.68 ± 0.70 | |
| Vibro ball milling | 3.17 ± 0.07 | 5.45 ± 0.10 | 6.35 ± 0.58 |
| Ethanol Yield (g/100 g TS) | Ethanol Efficiency (% Theoretical Yield *) | |||
|---|---|---|---|---|
| Samples | Untreated | Centrifugal Milling | Untreated | Centrifugal Milling |
| Ulva lactuca | 5.51 ± 0.29 | 5.27 ± 0.02 | 67.2% | 64.3% |
| Gelidium sesquipedale | 1.95 ± 0.17 | 3.51 ± 0.46 | 38.2% | 68.7% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amamou, S.; Sambusiti, C.; Monlau, F.; Dubreucq, E.; Barakat, A. Mechano-Enzymatic Deconstruction with a New Enzymatic Cocktail to Enhance Enzymatic Hydrolysis and Bioethanol Fermentation of Two Macroalgae Species. Molecules 2018, 23, 174. https://doi.org/10.3390/molecules23010174
Amamou S, Sambusiti C, Monlau F, Dubreucq E, Barakat A. Mechano-Enzymatic Deconstruction with a New Enzymatic Cocktail to Enhance Enzymatic Hydrolysis and Bioethanol Fermentation of Two Macroalgae Species. Molecules. 2018; 23(1):174. https://doi.org/10.3390/molecules23010174
Chicago/Turabian StyleAmamou, Sameh, Cecilia Sambusiti, Florian Monlau, Eric Dubreucq, and Abdellatif Barakat. 2018. "Mechano-Enzymatic Deconstruction with a New Enzymatic Cocktail to Enhance Enzymatic Hydrolysis and Bioethanol Fermentation of Two Macroalgae Species" Molecules 23, no. 1: 174. https://doi.org/10.3390/molecules23010174