Synthesis, In Vitro α-Glucosidase Inhibitory Activity and Molecular Docking Studies of Novel Benzothiazole-Triazole Derivatives
Abstract
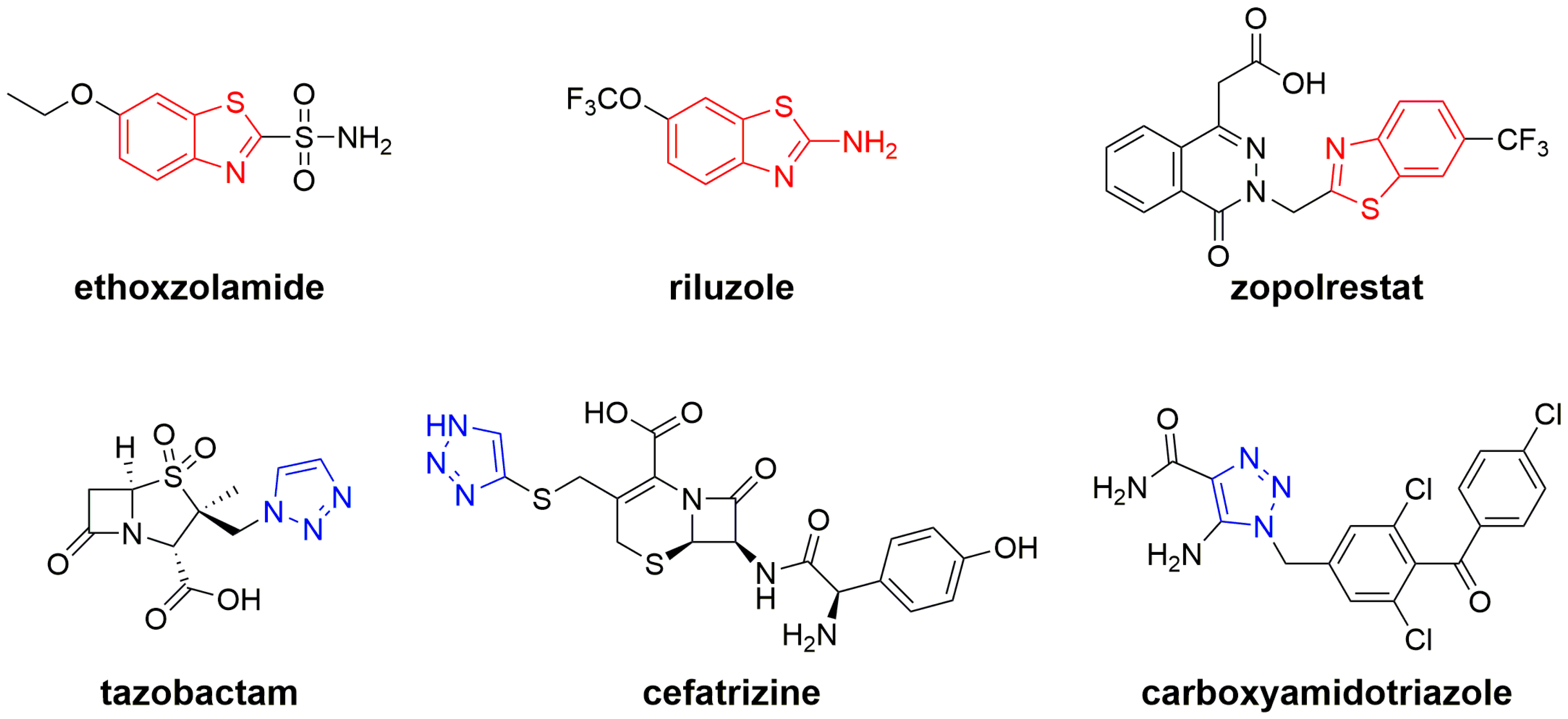
:1. Introduction
2. Results and Discussion
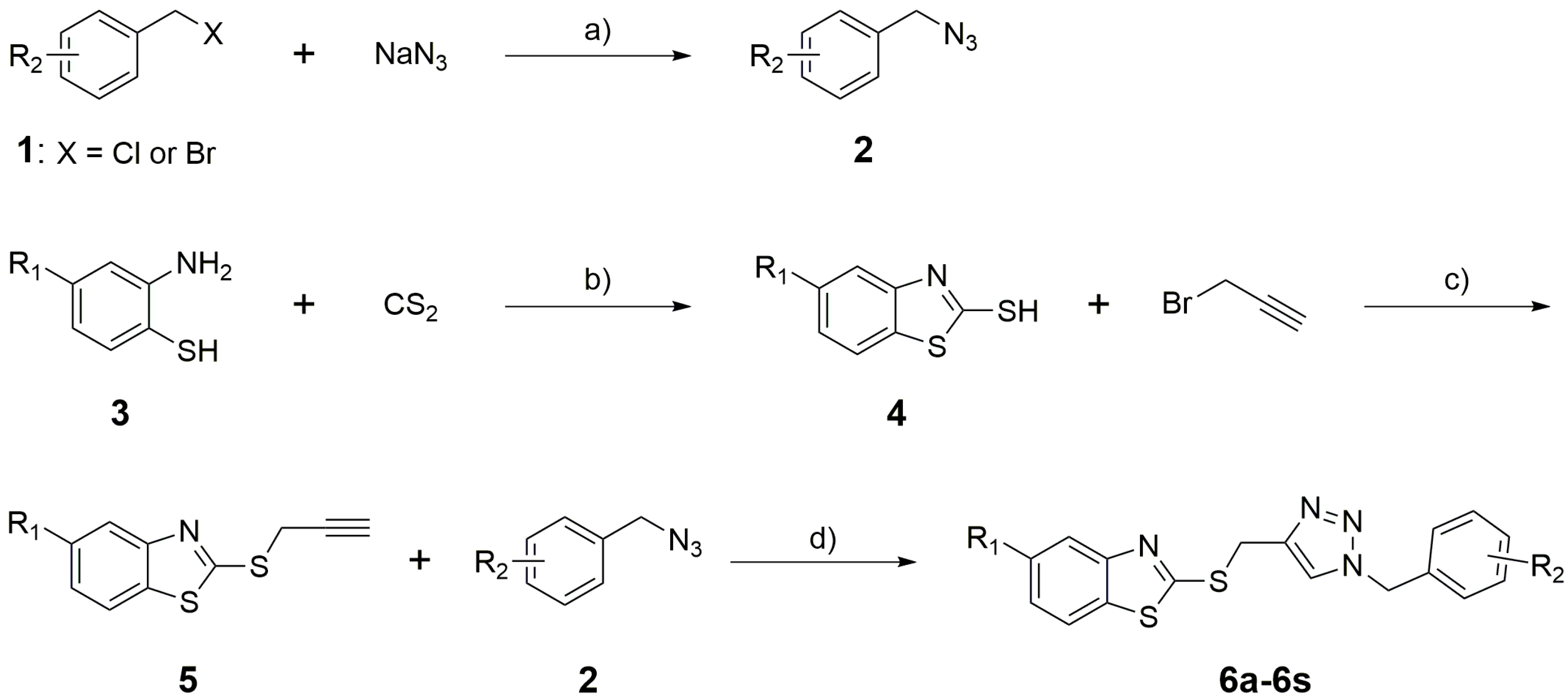
2.1. Chemistry
2.2. α-Glucosidase Inhibition Assay
2.3. Structure-Activity Relationship
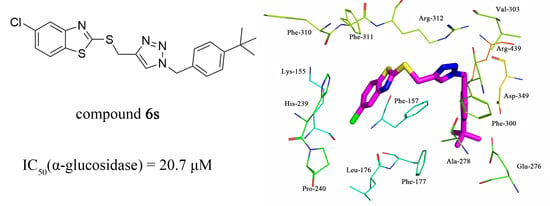
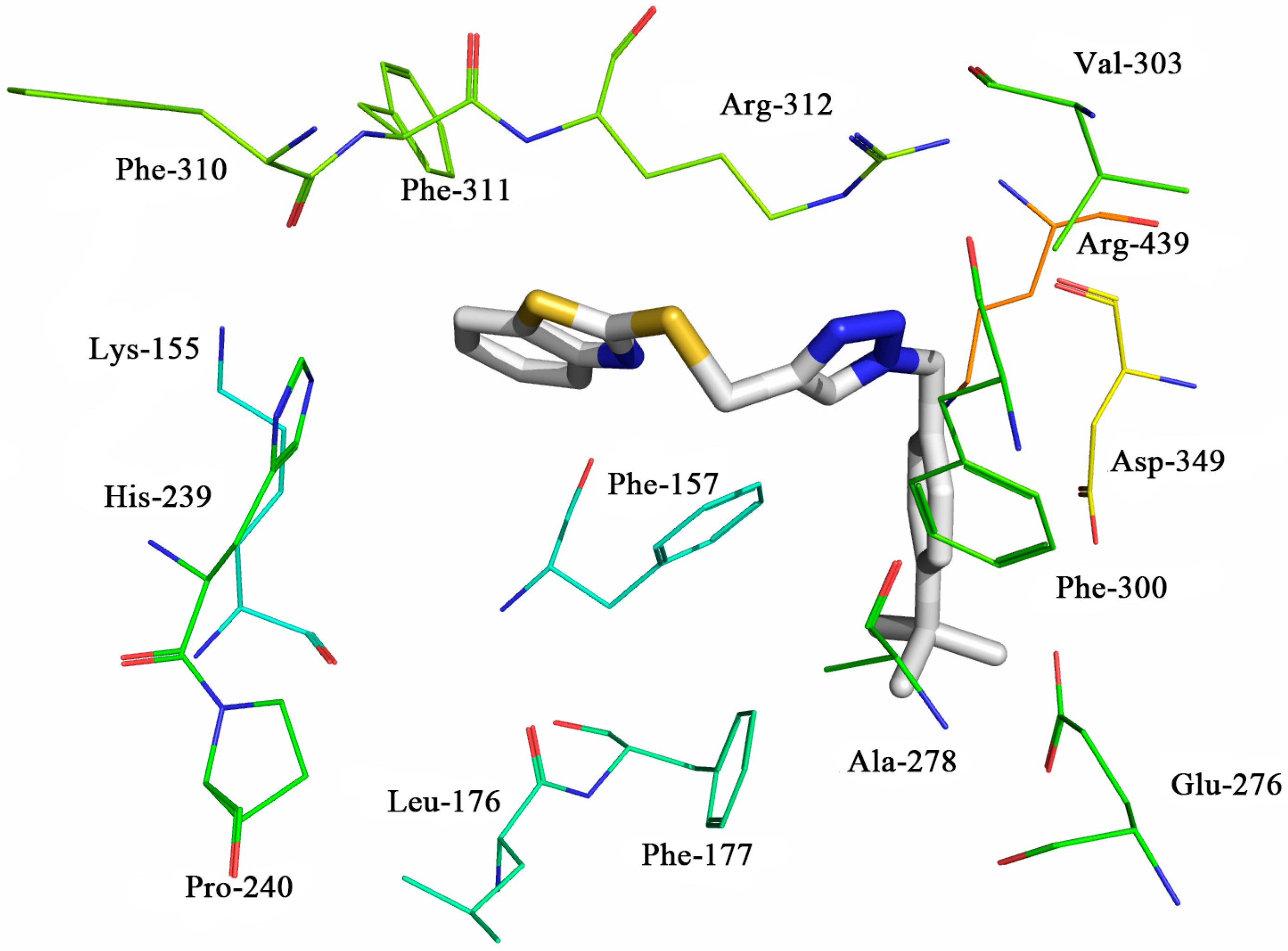
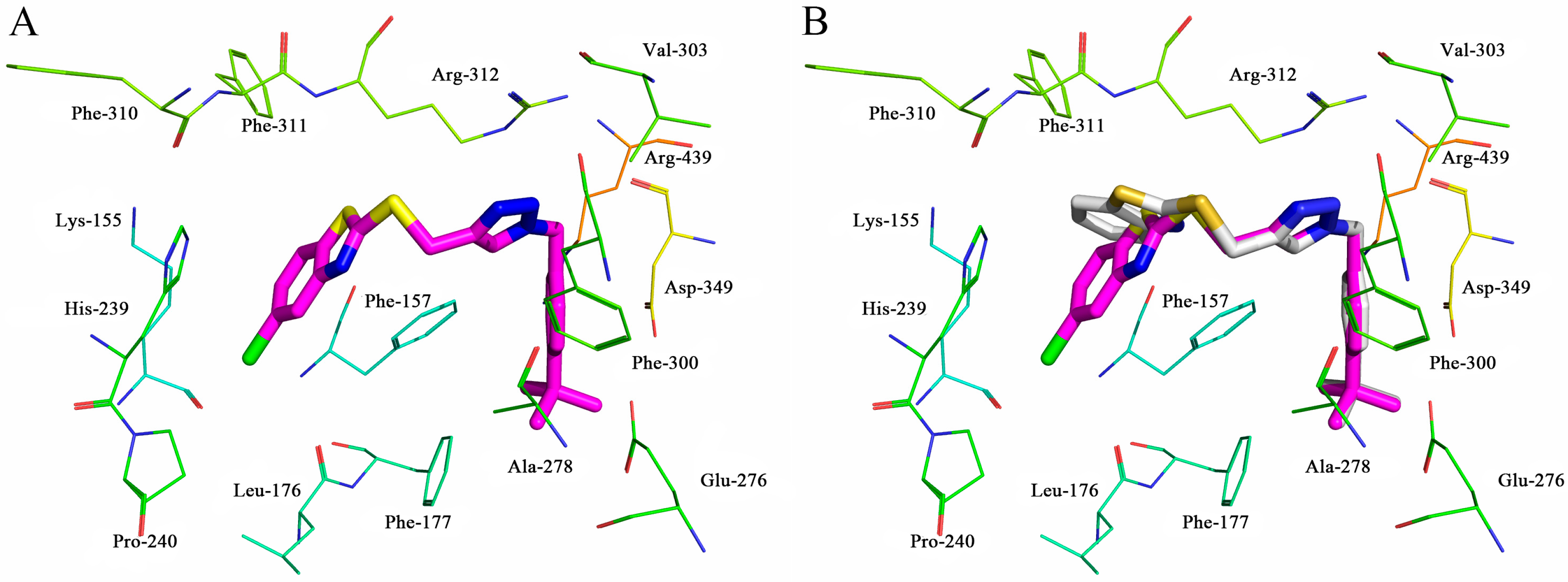
2.4. Molecular Docking
3. Experimental Section
3.1. General
3.2. General Procedure for the Synthesis of 2
3.3. General Procedure for the Synthesis of 4
3.4. General Procedure for the Synthesis of 5
3.5. General Procedure for the Synthesis of Benzothiazole-Triazole Derivatives (6a–6s)
3.6. In Vitro Assay of α-Glucosidase Inhibitory Activity
3.7. Molecular Docking
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ross, S.A.; Gulve, E.A.; Wang, M.H. Chemistry and biochemistry of type 2 diabetes. Chem. Rev. 2004, 104, 1255–1282. [Google Scholar] [CrossRef] [PubMed]
- King, G.L.; Loeken, M.R. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem. Cell Biol. 2004, 122, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, A.J.; Yao, S.Y.M.; Young, J.D.; Cheeseman, C.I. Inhibition of glucose absorption in the rat jejunum: A novel action of alpha-d-glucosidase inhibitors. Gastroenterology 1997, 113, 205–211. [Google Scholar] [CrossRef]
- Joshi, S.R.; Standl, E.; Tong, N.; Shah, P.; Kalra, S.; Rathod, R. Therapeutic potential of alpha-glucosidase inhibitors in type 2 diabetes mellitus: An evidence-based review. Expert Opin. Pharmacother. 2015, 16, 1959–1981. [Google Scholar] [CrossRef] [PubMed]
- Pili, R.; Chang, J.; Partis, R.A.; Mueller, R.A.; Chrest, F.J.; Passaniti, A. The alpha-glucosidase I inhibitor castanospermine alters endothelial cell glycosylation, prevents angiogenesis, and inhibits tumor growth. Cancer Res. 1995, 55, 2920–2926. [Google Scholar] [PubMed]
- Rawlings, A.J.; Lomas, H.; Pilling, A.W.; Lee, M.J.R.; Alonzi, D.S.; Rountree, J.S.S.; Jenkinson, S.F.; Fleet, G.W.J.; Dwek, R.A.; Jones, J.H.; et al. Synthesis and Biological Characterisation of Novel N-Alkyl-Deoxynojirimycin alpha-Glucosidase Inhibitors. ChemBioChem 2009, 10, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, N.; Mehta, A.S.; Carrouée, S.; Butters, T.D.; Platt, F.M.; McCauley, J.; Blumberg, B.S.; Dwek, R.A.; Block, T.M. Imino sugars inhibit the formation and secretion of bovine viral diarrhea virus, a pestivirus model of hepatitis C virus: Implications for the development of broad spectrum anti-hepatitis virus agents. Proc. Natl. Acad. Sci. USA 1999, 96, 11878–11882. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Patil, M.R.; Patil, S.A.; Budagumpi, S. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur. J. Med. Chem. 2015, 89, 207–251. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Rana, A.; Khan, S.A.; Bhat, M.A.; Haque, S.E. Synthesis of benzothiazole semicarbazones as novel anticonvulsants—The role of hydrophobic domain. Bioorg. Med. Chem. Lett. 2007, 17, 4178–4182. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, C.; Alam, M.S.; Hamid, H.; Javed, K.; Bano, S.; Dhulap, A.; Ali, Y.; Nazreen, S.; Haider, S. Synthesis and evaluation of pyrazolines bearing benzothiazole as anti-inflammatory agents. Bioorg. Med. Chem. 2014, 22, 5804–5812. [Google Scholar] [CrossRef] [PubMed]
- Chugunova, E.; Boga, C.; Sazykin, I.; Cino, S.; Micheletti, G.; Mazzanti, A.; Sazykina, M.; Burilov, A.; Khmelevtsova, L.; Kostina, N. Synthesis and antimicrobial activity of novel structural hybrids of benzofuroxan and benzothiazole derivatives. Eur. J. Med. Chem. 2015, 93, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Gurdal, E.E.; Buclulgan, E.; Durmaz, I.; Cetin-Atalay, R.; Yarim, M. Synthesis and Anticancer Activity Evaluation of Some Benzothiazole-Piperazine Derivatives. Anticancer Agents Med. Chem. 2015, 15, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Puranik, N.V.; Puntambekar, H.M.; Srivastava, P. Antidiabetic potential and enzyme kinetics of benzothiazole derivatives and their non-bonded interactions with alpha-glucosidase and alpha-amylase. Med. Chem. Res. 2016, 25, 805–816. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Lalani, S.; Fatmi, M.Q.; Atia-tul-Wahab; Siddiqui, S.; Khan, K.M.; Imran, S.; Choudhary, M.I. Synthesis of novel inhibitors of alpha-glucosidase based on the benzothiazole skeleton containing benzohydrazide moiety and their molecular docking studies. Eur. J. Med. Chem. 2015, 92, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click Chemistry: 1,2,3-Triazoles as Pharmacophores. Chem. Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Liu, X.; Shen, J.; Zhao, G.; Wang, P.G. The impact of click chemistry in medicinal chemistry. Expert Opin. Drug Discov. 2012, 7, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Saraei, M.; Ghasemi, Z.; Dehghan, G.; Hormati, M.; Ojaghi, K. Synthesis of some novel 1,2,3-triazole derivatives containing kojic acid moiety and evaluation for their antioxidant activity. Monatsh. Chem. 2017, 148, 917–923. [Google Scholar] [CrossRef]
- Thomas, K.D.; Adhikari, A.V.; Shetty, N.S. Design, synthesis and antimicrobial activities of some new quinoline derivatives carrying 1,2,3-triazole moiety. Eur. J. Med. Chem. 2010, 45, 3803–3810. [Google Scholar] [CrossRef] [PubMed]
- Boechat, N.; Ferreira, V.F.; Ferreira, S.B.; Ferreira, M.D.L.G.; da Silva, F.D.C.; Bastos, M.M.; Costa, M.D.S.; Lourenco, M.C.S.; Pinto, A.C.; Krettli, A.U.; et al. Novel 1,2,3-Triazole Derivatives for Use against Mycobacterium tuberculosis H37Rv (ATCC 27294) Strain. J. Med. Chem. 2011, 54, 5988–5999. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Shankaraiah, N.; Devaiah, V.; Reddy, K.L.; Juvekar, A.; Sen, S.; Kurian, N.; Zingde, S. Synthesis of 1,2,3-triazole-linked pyrrolobenzodiazepine conjugates employing ‘click’ chemistry: DNA-binding affinity and anticancer activity. Bioorg. Med. Chem. Lett. 2008, 18, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Chinthala, Y.; Domatti, A.K.; Sarfaraz, A.; Singh, S.P.; Arigari, N.K.; Gupta, N.; Satya, S.K.V.N.; Kumar, J.K.; Khan, F.; Tiwari, A.K.; et al. Synthesis, biological evaluation and molecular modeling studies of some novel thiazolidinediones with triazole ring. Eur. J. Med. Chem. 2013, 70, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Chinthala, Y.; Thakur, S.; Tirunagari, S.; Chinde, S.; Domatti, A.K.; Arigari, N.K.; Srinivas, K.V.N.S.; Alam, S.; Jonnala, K.K.; Khan, F.; et al. Synthesis, docking and ADMET studies of novel chalcone triazoles for anti-cancer and anti-diabetic activity. Eur. J. Med. Chem. 2015, 93, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, F.; Shehzadi, S.A.; Fatmi, M.Q.; Shaheen, S.; Iqbal, L.; Afza, N.; Panda, S.S.; Ansari, F.L. Synthesis, in vitro and computational studies of 1,4-disubstituted 1,2,3-triazoles as potential alpha-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Peng, Z.; Wang, J.; Li, J.; Li, X. Synthesis and biological evaluation of novel 2,4,5-triarylimidazole-1,2,3-triazole derivatives via click chemistry as α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 5719–5723. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Peng, Z.; Wang, J.; Li, X.; Li, J. Synthesis, in vitro evaluation and molecular docking studies of novel triazine-triazole derivatives as potential α-glucosidase inhibitors. Eur. J. Med. Chem. 2017, 125, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.; He, D.; Li, X.; Li, J.; Peng, Z. Synthesis and biological evaluation of novel 1,2,4-triazine derivatives bearing carbazole moiety as potent α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 2806–2809. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, M.; Wang, J.; Peng, Y.; Li, L.; Xie, Z.; Deng, B.; Chen, S.; Li, W. Synthesis, biological evaluation and molecular docking studies of chromone hydrazone derivatives as α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 2957–2961. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Peng, Z.; Wang, J.; Li, J.; Li, X. Synthesis, biological evaluation and molecular docking study of N-arylbenzo[d]oxazol-2-amines as potential α-glucosidase inhibitors. Bioorg. Med. Chem. 2016, 24, 5374–5379. [Google Scholar] [CrossRef] [PubMed]
- Christou, S.; Edwards, A.C.; Pritchard, R.G.; Quayle, P.; Song, Y.; Stratford, I.J.; Williams, K.F.; Whitehead, R.C. Synthesis of hybrid natural product analogues with anti-tumour properties. Tetrahedron 2016, 72, 5433–5443. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 6a–6s are available from the authors. |
| Compound | R1 | R2 | IC50 (μM) |
|---|---|---|---|
| 6a | H | 4-Br | 28.7 |
| 6b | H | 2-Cl | >100 |
| 6c | H | 2-Br | 37.4 |
| 6d | H | 3-F | 61.1 |
| 6e | H | 4-Cl | 27.4 |
| 6f | H | 2-F | >100 |
| 6g | H | H | >100 |
| 6h | H | 3,5-Cl2 | 41.0 |
| 6i | H | 4-tBu | 29.4 |
| 6j | Cl | 2-F | 45.9 |
| 6k | Cl | 2-Br | 48.4 |
| 6l | Cl | 2-Cl | 33.6 |
| 6m | Cl | 4-Cl | 28.2 |
| 6n | Cl | 4-Br | 28.0 |
| 6o | Cl | 3,5-Cl2 | 22.3 |
| 6p | Cl | 3-F | 38.6 |
| 6q | Cl | 4-F | 53.1 |
| 6r | Cl | H | 44.4 |
| 6s | Cl | 4-tBu | 20.7 |
| Acarbose | 817.38 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Z.; Peng, Y.; Qiu, J.; Cao, A.; Wang, G.; Peng, Z. Synthesis, In Vitro α-Glucosidase Inhibitory Activity and Molecular Docking Studies of Novel Benzothiazole-Triazole Derivatives. Molecules 2017, 22, 1555. https://doi.org/10.3390/molecules22091555
Gong Z, Peng Y, Qiu J, Cao A, Wang G, Peng Z. Synthesis, In Vitro α-Glucosidase Inhibitory Activity and Molecular Docking Studies of Novel Benzothiazole-Triazole Derivatives. Molecules. 2017; 22(9):1555. https://doi.org/10.3390/molecules22091555
Chicago/Turabian StyleGong, Zipeng, Yaping Peng, Jie Qiu, Anbai Cao, Guangcheng Wang, and Zhiyun Peng. 2017. "Synthesis, In Vitro α-Glucosidase Inhibitory Activity and Molecular Docking Studies of Novel Benzothiazole-Triazole Derivatives" Molecules 22, no. 9: 1555. https://doi.org/10.3390/molecules22091555