Valorization of By-Products from Commercial Fish Species: Extraction and Chemical Properties of Skin Gelatins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Process and Chemical Composition
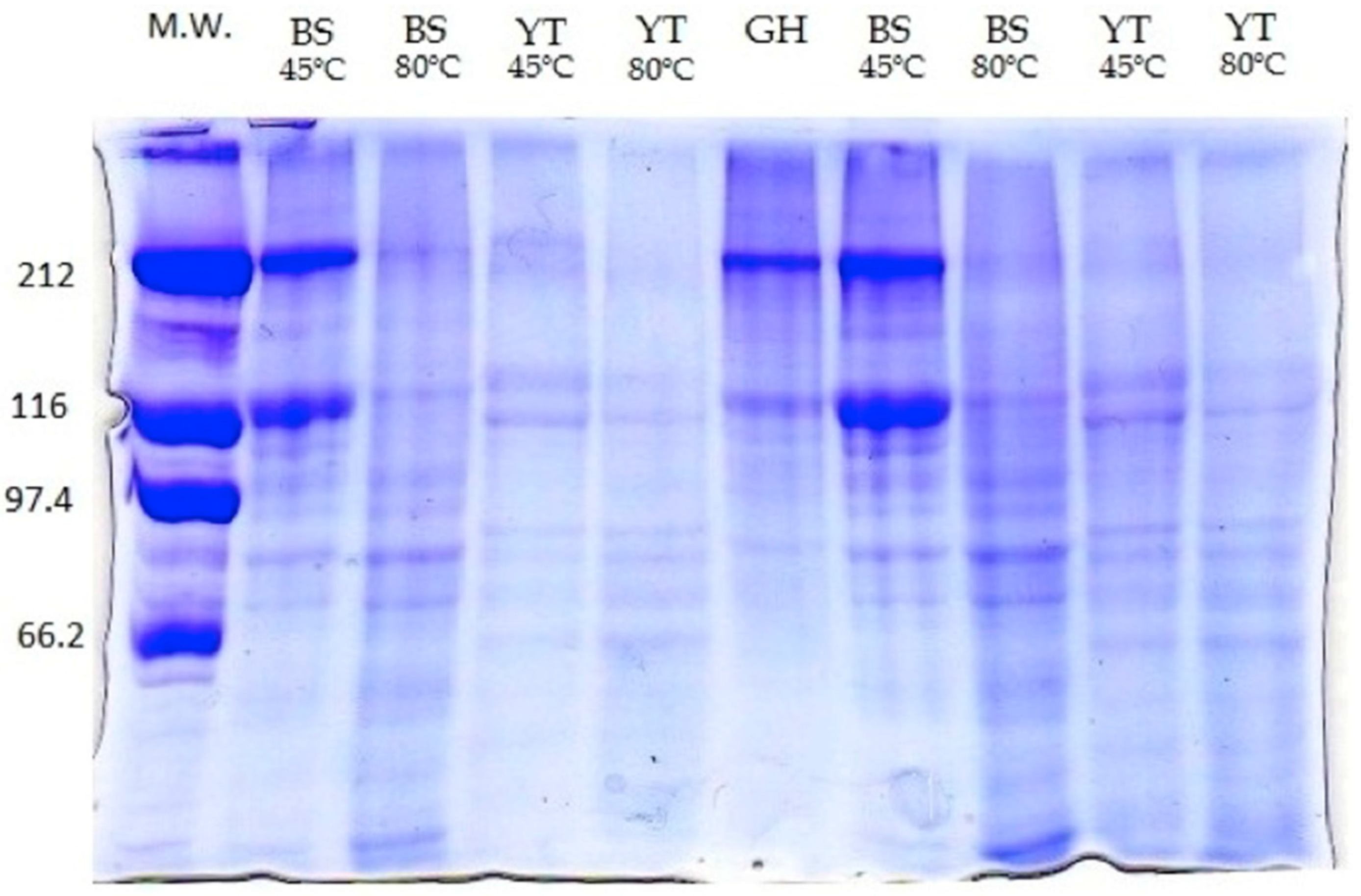
2.2. Molecular Weight
2.3. Amino Acid Composition
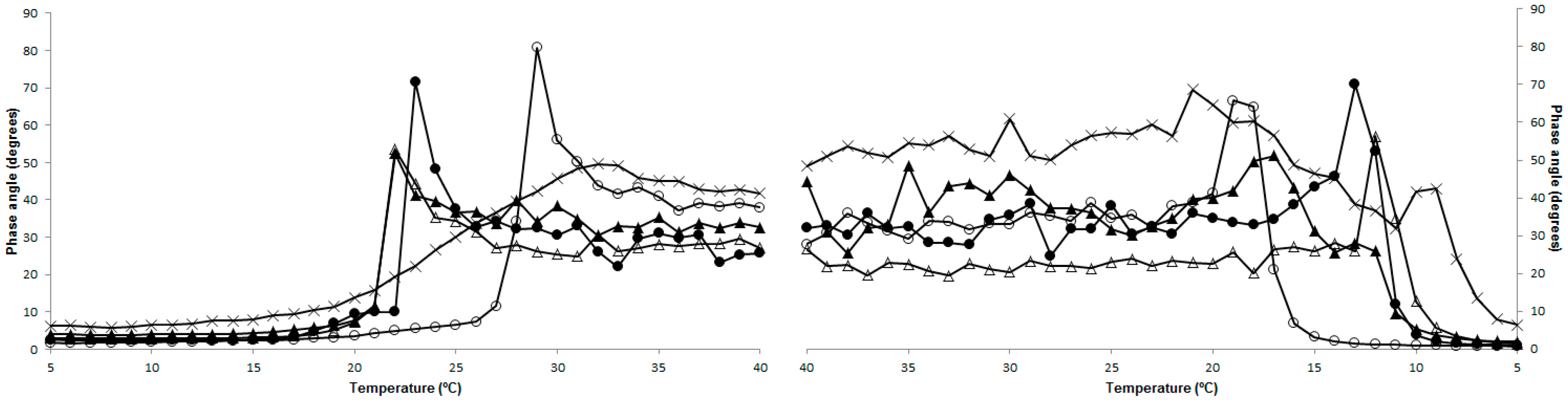
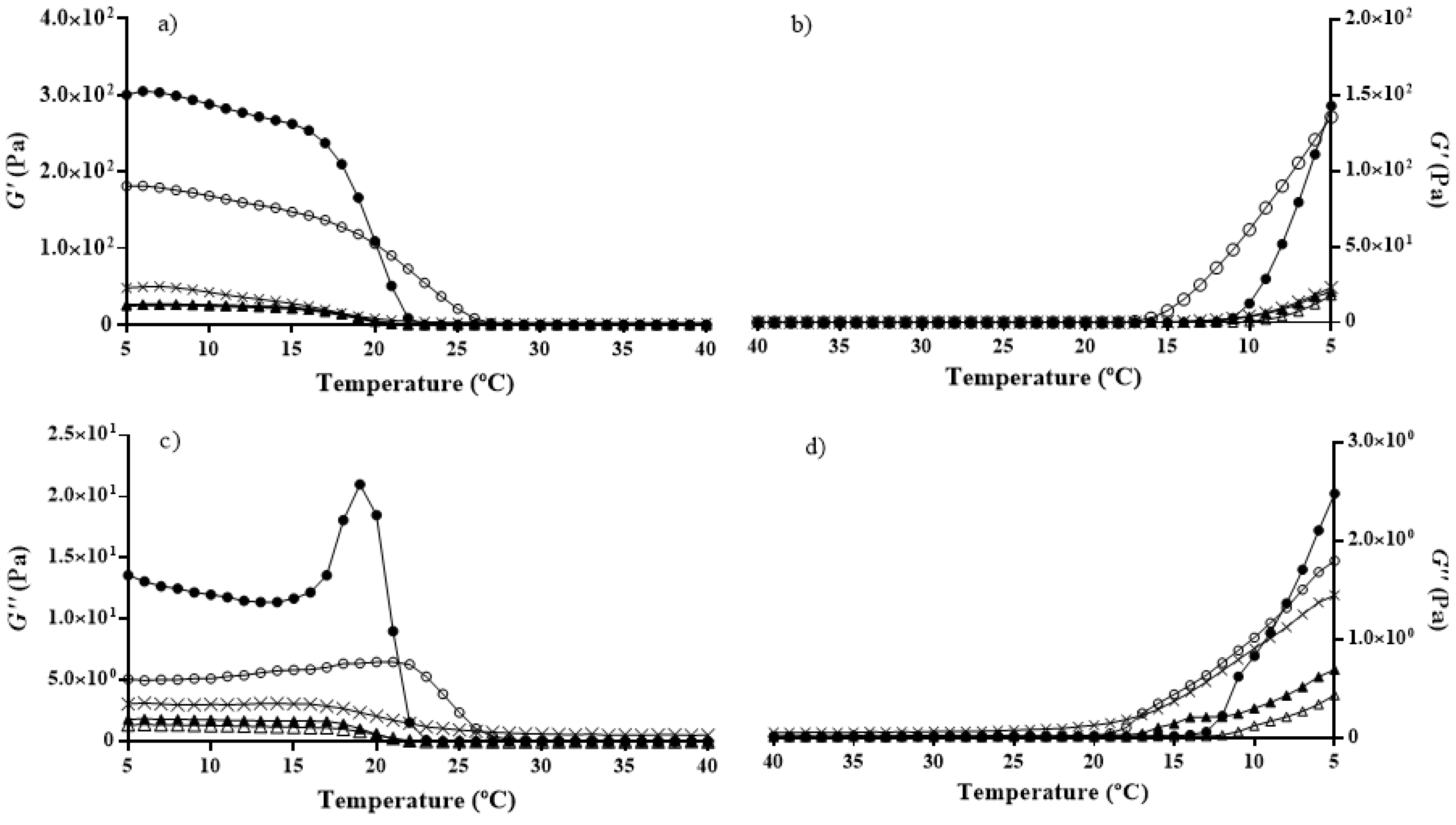
2.4. Rheology
2.5. Textural Analysis
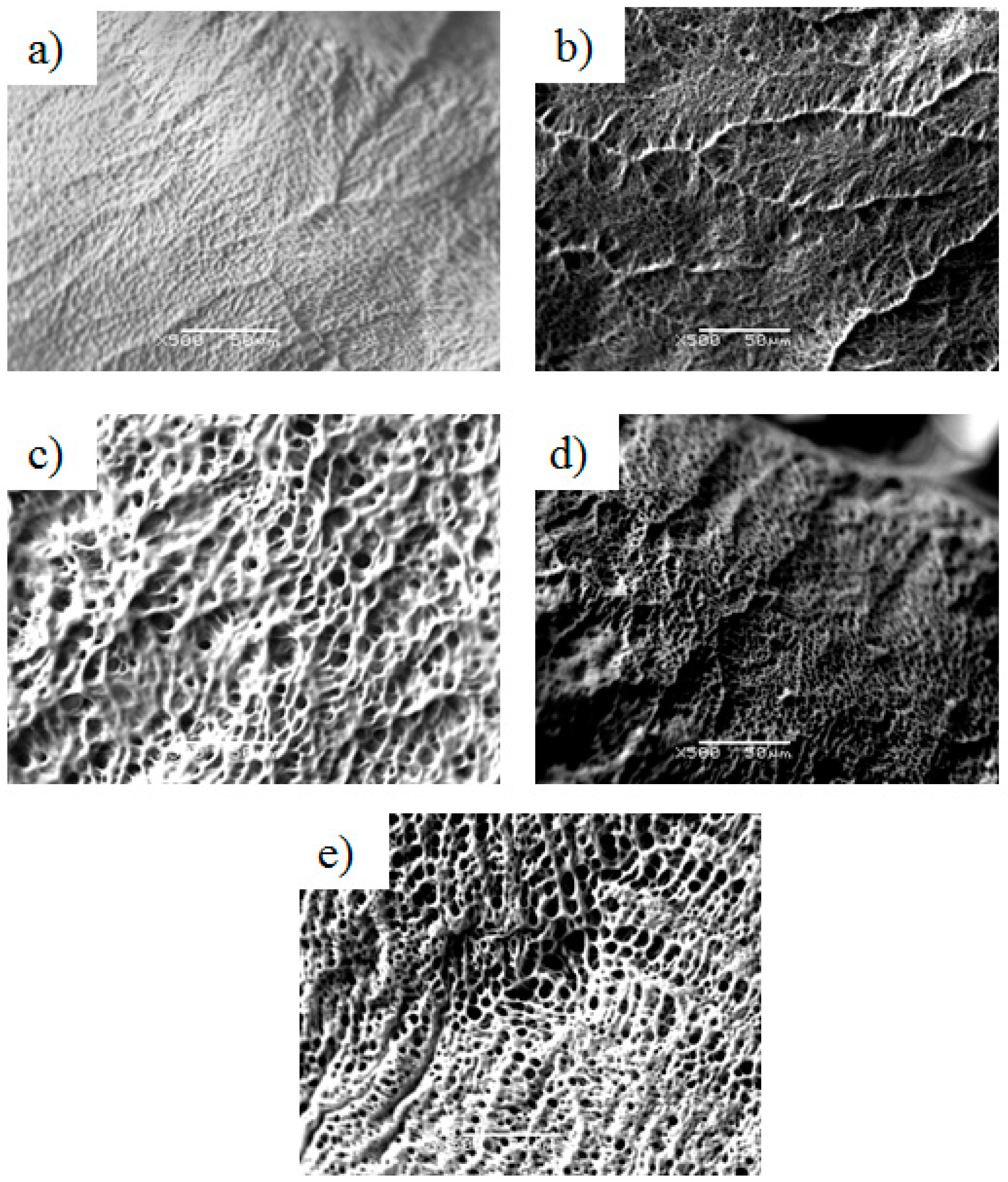
2.6. Structure
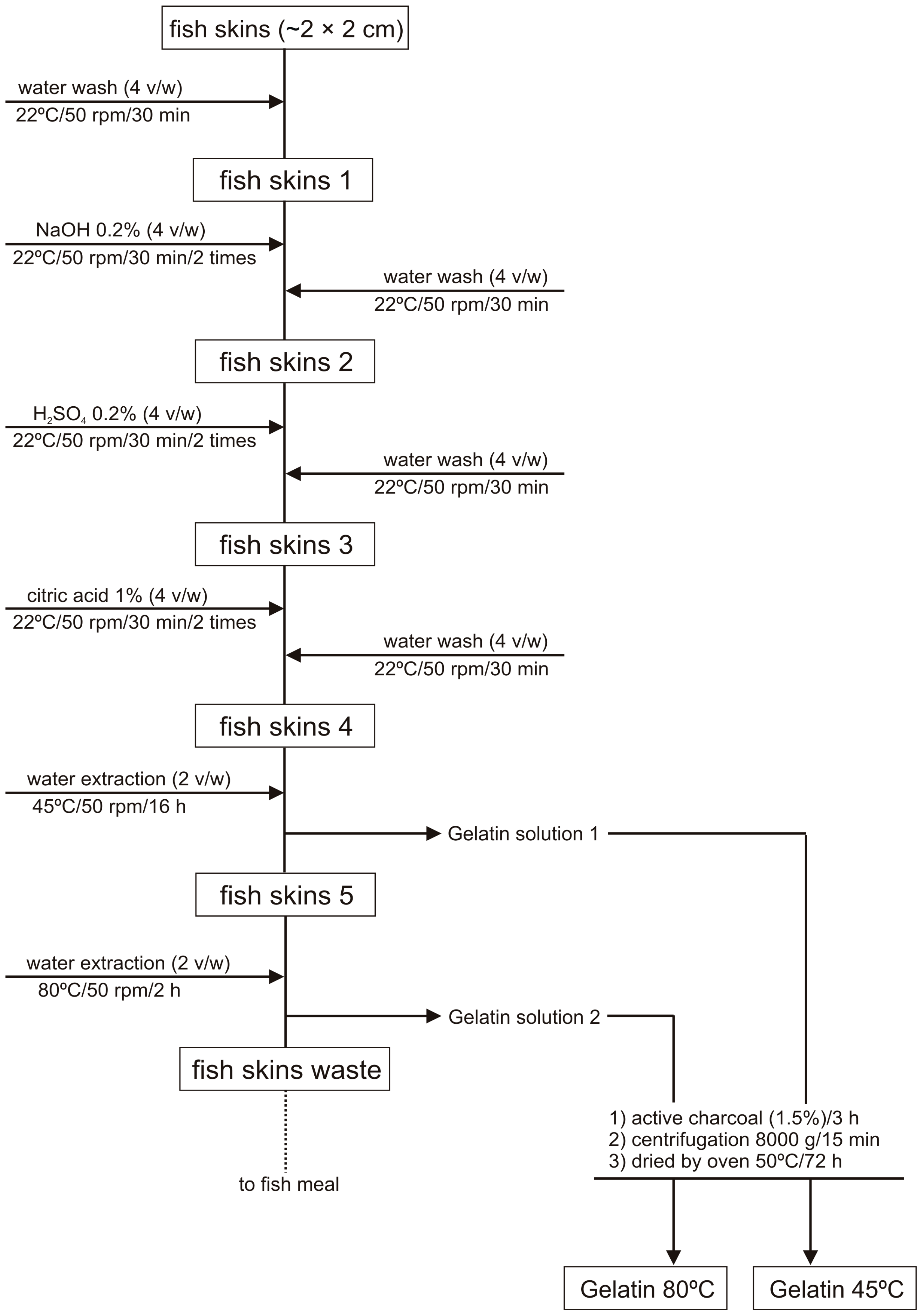
3. Materials and Methods
3.1. Samples
3.2. Gelatin Extraction
3.3. Molecular Weight
3.4. Chemical Composition
3.5. Rheology
3.6. Texture
3.7. Structure
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eysturskarð, J.; Haug, J.; Elharfaoui, N.; Djabourov, M.; Draget, I. Structural and mechanical properties of fish gelatin as a function of extraction conditions. Food Hydrocoll. 2009, 23, 1702–1711. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Thitipramote, N.; Benjakul, S. Preparation and functional characterisation of fish skin gelatin and comparison with commercial gelatin. Int. J. Food Sci. Technol. 2013, 48, 1093–1102. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Chandra, M.; Shamasundar, B. Rheological properties of gelatin prepared from the swim bladders of freshwater fish Catla catla. Food Hydrocoll. 2015, 48, 47–54. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kishimura, H. Molecular characteristics and properties of gelatin from skin of seabass with different sizes. Int. J. Biol. Macromol. 2015, 73, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, I.; Skierka, E.; Sadowska, M.; Kołodziejski, W.; Niecikowska, C. Effect of extracting time and temperature on yield of gelatin from different fish offal. Food Chem. 2008, 107, 700–706. [Google Scholar] [CrossRef]
- Fustier, P.; Achouri, A.; Taherian, A.R.; Britten, M.; Pelletier, M.; Sabik, H.; Villeneuve, S.; Mondor, M. Protein-protein multilayer oil-in-water emulsions for the microencapsulation of flaxseed oil: Effect of whey and fish gelatin concentration. J. Agric. Food Chem. 2015, 63, 9239–9250. [Google Scholar] [CrossRef] [PubMed]
- Binsi, P.; Shamasundar, B.; Dileep, A.; Badii, F.; Howell, N. Rheological and functional properties of gelatin from the skin of Bigeye snapper (Priacanthus hamrur) fish: Influence of gelatin on the gel-forming ability of fish mince. Food Hydrocoll. 2009, 23, 132–145. [Google Scholar] [CrossRef] [Green Version]
- Jamilah, B.; Tan, K.; Hartina, M.U.; Azizah, A. Gelatins from three cultured freshwater fish skins obtained by liming process. Food Hydrocoll. 2011, 25, 1256–1260. [Google Scholar] [CrossRef]
- Liu, H.; Li, D.; Guo, S. Rheological properties of channel catfish (Ictalurus punctaus) gelatine from fish skins preserved by different methods. LWT-Food Sci. Technol. 2008, 41, 1425–1430. [Google Scholar] [CrossRef]
- Sow, L.C.; Yang, H. Effects of salt and sugar addition on the physicochemical properties and nanostructure of fish gelatin. Food Hydrocoll. 2015, 45, 72–82. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, S.; Wang, Z. Pre-treatment optimization and properties of gelatin from freshwater fish scales. Food Bioprod. Proc. 2011, 89, 185–193. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Gelatin alternatives for the food industry: Recent developments, challenges and prospects. Trends Food Sci. Technol. 2008, 19, 644–656. [Google Scholar] [CrossRef]
- Grossman, S.; Bergman, M. Process for the Production of Gelatin from Fish Skins. U.S. Patent No. 5093474, 3 March 1992. [Google Scholar]
- Gómez-Guillén, M.; Turnay, J.; Fernández-Dıaz, M.; Ulmo, N.; Lizarbe, M.; Montero, P. Structural and physical properties of gelatin extracted from different marine species: A comparative study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef]
- Gudmundsson, M.; Hafsteinsson, H. Gelatin from cod skins as affected by chemical treatments. J. Food Sci. 1997, 62, 37–39. [Google Scholar] [CrossRef]
- Limpisophon, K.; Tanaka, M.; Weng, W.; Abe, S.; Osako, K. Characterization of gelatin films prepared from under-utilized blue shark (Prionace glauca) skin. Food Hydrocoll. 2009, 23, 1993–2000. [Google Scholar] [CrossRef]
- Sai-Ut, S.; Jongjareonrak, A.; Rawdkuen, S. Re-extraction, recovery and characteristics of skin gelatin from farmed giant catfish. Food Biopr. Technol. 2012, 5, 1197–1205. [Google Scholar] [CrossRef]
- Jamilah, B.; Harvinder, K.G. Properties of gelatins from skins of fish-black tilapia (Oreochromis mossambicus) and red tilapia (Oreochromis nilotica). Food Chem. 2002, 77, 81–84. [Google Scholar] [CrossRef]
- Muyonga, J.; Cole, C.; Duodu, K. Extraction and physico-chemical characterisation of Nile perch (Lates niloticus) skin and bone gelatin. Food Hydrocoll. 2004, 18, 581–592. [Google Scholar] [CrossRef]
- Cho, S.-H.; Jahncke, L.; Chin, K.-B.; Eun, J.-B. The effect of processing conditions on the properties of gelatin from skate (Raja kenojei) skins. Food Hydrocoll. 2006, 20, 810–816. [Google Scholar] [CrossRef]
- Karayannakidis, P.D.; Zotos, A. Fish processing by-products as a potential source of gelatin: A Review. J. Aquatic Food Prod. Technol. 2016, 25, 65–92. [Google Scholar] [CrossRef]
- Fernández-Dı́, M.; Montero, P.; Gómez-Guillén, M. Effect of freezing fish skins on molecular and rheological properties of extracted gelatin. Food Hydrocoll. 2003, 17, 281–286. [Google Scholar] [CrossRef]
- Kasankala, L.M.; Xue, Y.; Weilong, Y.; Hong, S.D.; He, Q. Optimization of gelatine extraction from grass carp (Catenopharyngodon idella) fish skin by response surface methodology. Bioresour. Technol. 2007, 98, 3338–3343. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Gu, Y.; Kim, S. Extracting optimization and physical properties of yellowfin tuna (Thunnus albacares) skin gelatin compared to mammalian gelatins. Food Hydrocoll. 2005, 19, 221–229. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.; Montero, P. Extraction of gelatin from megrim (Lepidorhombus boscii) skins with several organic acids. J. Food Sci. 2001, 62, 213–216. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Spackman, D.H.; Stein, W.H. Chromatography of amino acids on sulfonated polystyrene resins. An improved system. Anal. Chem. 1958, 30, 1185–1190. [Google Scholar] [CrossRef]
- Havilah, E.; Wallis, D.; Morris, R.; Woolnough, J. A microcolorimetric method for determination of ammonia in Kjeldahl digests with a manual spectrophotometer. Lab. Pract. 1977, 26, 545–547. [Google Scholar]
- Anonymous. Official Method 991.36, Fat (Crude) in meat and meat products. In AOAC—Official Methods of Analysis, 16th ed.; Latimer, J.W., Horwitz, W., Eds.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1999. [Google Scholar]
Sample Availability: Samples of the compounds YT and BS gelatins are available from the authors. |
| Species | M | Ash | Fat | Pr-Nt |
|---|---|---|---|---|
| BS | 760.3 ± 8.3 | 42.4 ± 2.4 | 2.4 ± 0.3 | 227.9 ± 9.7 |
| YT | 625.7 ± 24.0 | 6.7 ± 1.4 | 32.2 ± 7.2 | 323.8 ± 20.4 |
| GH | 554.4 ± 9.8 | 176.3 ± 12.1 | 106.2 ± 9.8 | 159.5 ± 4.2 |
| Gelatins | M | OM | Ash | Fat | Pr-Nt |
|---|---|---|---|---|---|
| BS 45 °C | 96.7 ± 2.0 | 886.6 ± 9.2 | 16.7 ± 1.8 | 8.3 ± 0.6 | 929.0 ± 13.1 |
| BS 80 °C | 97.1 ± 0.9 | 897.9 ± 10.0 | 5.0 ± 0.4 | 26.6 ± 2.7 | 935.0 ± 10.8 |
| YT 45 °C | 99.7 ± 0.8 | 899.2 ± 6.7 | 1.1 ± 0.2 | 28.6 ± 2.9 | 906.0 ± 11.3 |
| YT 80 °C | 74.0 ± 2.1 | 924.3 ± 19.6 | 1.7 ± 0.7 | 25.5 ± 3.9 | 866.0 ± 16.3 |
| GH | 89.6 ± 2.1 | 901.1 ± 9.8 | 9.3 ± 1.2 | 29.3 ± 2.8 | 869.0 ± 14.4 |
| Gelatins | Gel Strength (g) | Rupture Strength (g) | Adhesiveness (g) | Brittleness (kg·s−1) |
|---|---|---|---|---|
| BS 45 °C | 189 ± 1 a | 653 ± 11 a | −39 ± 12 a | 6.7 ± 0.2 a |
| BS 80 °C | 64 ± 1 b | 251 ± 14 b | −37 ± 1 a | 2.1 ± 0.2 b |
| YT 45 °C | 107 ± 10 c | 380 ± 34 c | −38 ± 5 a | 3.6 ± 0.1 c |
| YT 80 °C | 97 ± 13 c | 342 ± 72 b,c | −43 ± 7 a | 3.0 ± 0.7 b,c |
| GH 45 °C | 14 ± 0 d | 46 ± 5 d | −2 ± 2 b | 0.6 ± 0.1 d |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, S.C.; Vázquez, J.A.; Pérez-Martín, R.I.; Carvalho, A.P.; Gomes, A.M. Valorization of By-Products from Commercial Fish Species: Extraction and Chemical Properties of Skin Gelatins. Molecules 2017, 22, 1545. https://doi.org/10.3390/molecules22091545
Sousa SC, Vázquez JA, Pérez-Martín RI, Carvalho AP, Gomes AM. Valorization of By-Products from Commercial Fish Species: Extraction and Chemical Properties of Skin Gelatins. Molecules. 2017; 22(9):1545. https://doi.org/10.3390/molecules22091545
Chicago/Turabian StyleSousa, Sérgio C., José A. Vázquez, Ricardo I. Pérez-Martín, Ana P. Carvalho, and Ana M. Gomes. 2017. "Valorization of By-Products from Commercial Fish Species: Extraction and Chemical Properties of Skin Gelatins" Molecules 22, no. 9: 1545. https://doi.org/10.3390/molecules22091545






