3.1. Chemistry
All reagents were purchased from Aldrich (St. Louis, MO, USA) and AppliChem GmbH (Darmstadt, Germany) and were used without further purification. The reaction was monitored by thin-layer chromatography carried out on silica gel plates (60 F254, MERCK, Darmstadt, Germany) as the stationary phase; ethylacetate/n-hexane or MeOH/DCM were used as eluents. Spots were visualized under UV light (245 nm). Nuclear magnetic resonance (1H-NMR, 13C-NMR) spectra were recorded using a Varian MERCURY plus 300 MHz spectrometer (Varian, Palo Alto, CA, USA) in DMSO-d6 at r.t. and at 80 °C. Chemical shifts were reported in parts per million (ppm, d) downfield from TMS (Tetramethylsilane). Splitting patterns are described as singlet (s), doublet (d), triplet (t), quartet (q), multiple (m), broad (br), and doublet of doublet (dd). Infrared (IR) spectra were performed on Perkin-Elmer UATR Two (PerkinElmer Ltd., Beaconsfield, UK) and were recorded as KBr plates. The absorption bands (νmax) are given in wave numbers (cm−1) and the signal strength is given as weak (w), strong (s), or medium (m). All of the HPLC-UV/MS analyses were performed on a chromatographic apparatus consisting of an LC Agilent Infinity System (Agilent Technologies, Santa Clara, CA, USA) equipped with a gradient pump (1290 Bin Pump VL), an automatic injector (1260 HiPals), and a column thermostat (1290 TCC). The LC system was coupled with a photodiode array detector (Infinity 1290 DAD) and a quadrupole time-of-flight mass spectrometer (6520 Accurate Mass Q-TOF LC/MS). Q-TOF was equipped with an electrospray ionization source operated in positive ionization mode. For data acquisition and processing, a computer with Mass Hunter software(version MassHunter Workstation B 04.00, Agilent Technologies, Santa Clara, CA, USA) was used. The HPLC-HRMS analysis was carried out using HILIC column—SeQuant® ZIC®-HILIC, 2.1 × 100 mm, 3.5 µm obtained from Merck KGaA, Darmstadt, Germany. The mobile phases consisted of acetonitrile and 5 mM solution of ammonium acetate pH 5.8 (adjusted with acetic acid). The flow rate of the mobile phase was set at 400 µL/min and 5.0 µL of the sample were injected into the column. The overall time of analysis was 24 min. Gradient elution was used with the column oven temperature set at 35 °C. The following MS parameters were used for all measurements: drying gas temperature 325 °C, drying gas flow 10 L.min-1, nebulizing gas pressure 40 psi, ESI source voltage 3500 V, fragmentor voltage 140 V, collision gas N2.
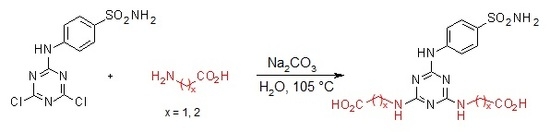
3.2. General Procedure for the Synthesis of Compounds 1–4
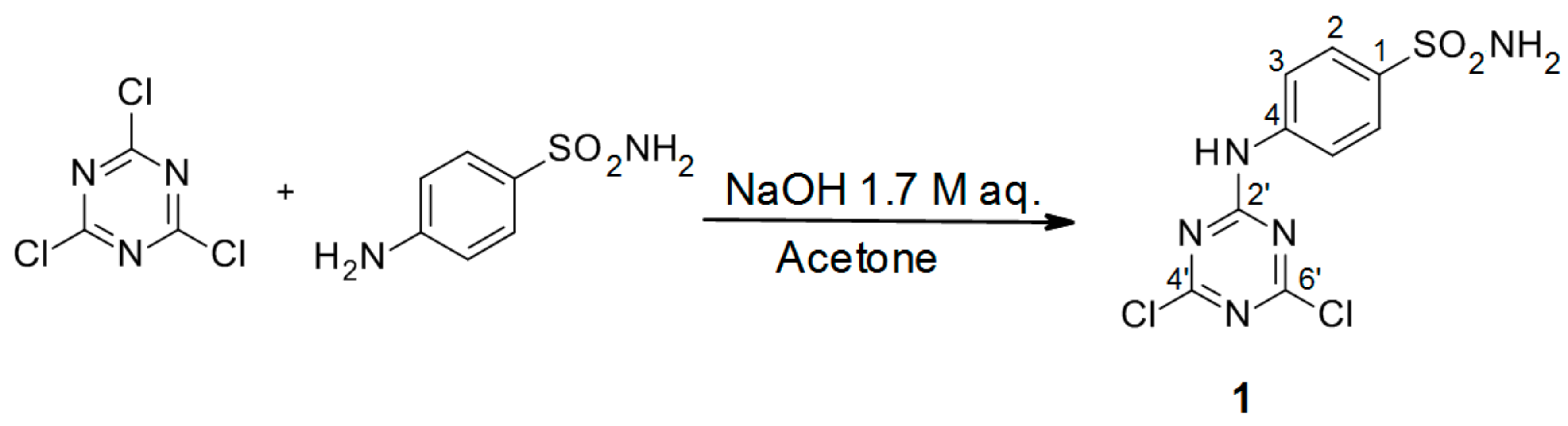
3.2.1. Synthesis of 4-(4′,6′-Dichloro-1′,3′,5′-triazin-2′-ylamino)-benzene-sulfonamide Precursor 1 (Scheme 1)
A 1.0 M solution of 4-aminobenzenesulfanilamide (17.2 g, 0.1 mol, 1.0 equiv) in acetone (100 mL) was added dropwise to a vigorously stirred suspension of cyanuric chloride (18.4 g, 1.0 equiv) in the same solvent (100 mL) at 0 °C. The white slurry was stirred at the same temperature for 30 min. Then, a 1.7 M aqueous solution of NaOH (4.0 g, 1.0 equiv) was added over a period of 20 min. Stirring was continued for 1 h, and the reaction was quenched by the addition of slush (100 mL), after which the solid was filtered off. Crystallization from acetone afforded the title compound
1 as a white solid [
4].
4-(4′,6′-Dichloro-1′,3′,5′-triazin-2′-ylamino)-benzene-sulfonamide (1): The product was obtained as a white solid in 95% yield. IR (KBr): 3294 (s), 3211 (m), 2343 (w), 1621 (s), 1538 (s), 1494 (s), 1380 (m), 1328 (s), 1226 (s), 1164 (s), 1123 (m), 963 (w), 722 (s), 590 (m), 547 (s), 482 (m); 1H-NMR (DMSO-d6) δ: 11.43 (s, 1H, Ar-NH, Notice: The moiety was underlined to indicate the hydrogen to which the signal refers.), 7.86 (d, 2H, J =9.0 Hz, 2 × H-2), 7.79 (d, 2H, J = 9.0 Hz, 2 × H-3), 7.34 (s, 2H, SO2NH2); 13C-NMR (DMSO-d6) δ: 170.25, 169.47, 164.40, 140.44, 140.32, 127.11, 121.55. HRMS (ESI/QTOF) m/z: [M + H]+ Calcd. for [C9H7N5SO2Cl2H]+ 319.9770; Found: 319.9772.
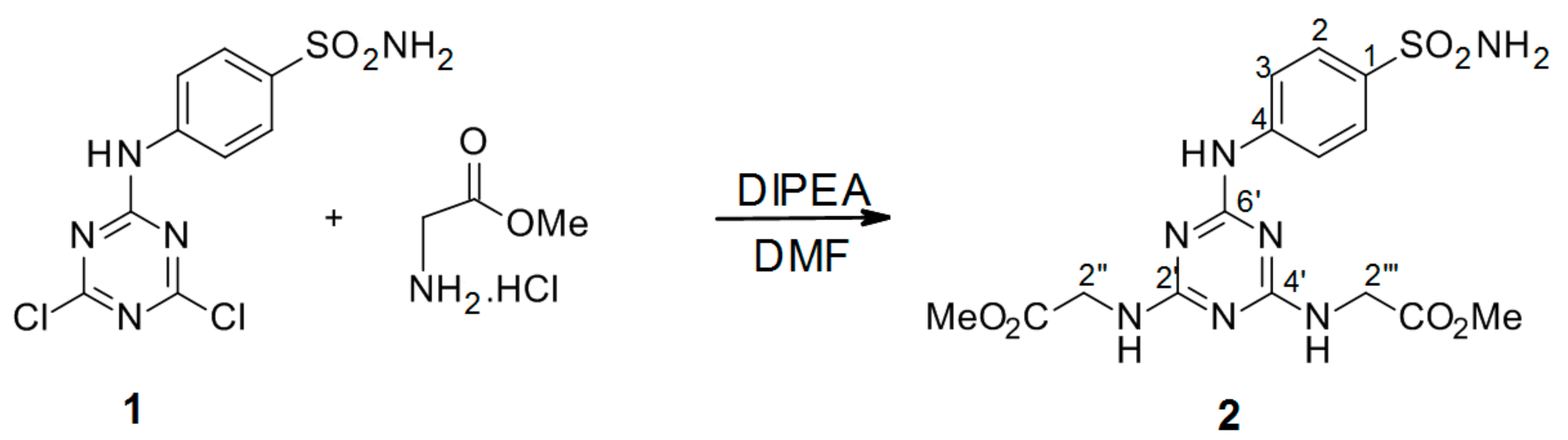
3.2.2. Synthesis of Dimethyl 2′′,2′′′-[6′-(4-Sulfamoylphenylamino)-1′,3′,5′-triazine-2′,4′-diyl]-bis(azanediyl) Diacetate Intermediate 2 (Scheme 2)
4-(4′,6′-Dichloro-1′,3′,5′-triazin-2′-ylamino)-benzene-sulfonamide 1. (0.2 g, 1.0 equiv) was dissolved in 2 mL dry DMF. The mixture of methyl 2-aminoacetate hydrochloride (4.5 equiv) in 2 mL DMF and DIPEA (9.0 equiv) was stirred for 2 h at r.t. After 2 h, a solution of 4-(4′,6′-dichloro-1′,3′,5′-triazin-2′-ylamino)-benzene-sulfonamide 1 was added dropwise and the reaction was stirred at 90 °C under an argon atmosphere. The reaction ran overnight and was monitored by TLC (Thin Layer Chromatography). The next day, the reaction was quenched with slush and the precipitate formed was collected by filtration, washed with H2O, and dried under high vacuum to give the title compound as a white solid.
2′′,2′′′-[6′-(4-Sulfamoylphenylamino)-1′,3′,5′-triazine-2′,4′-diyl]-bis(azanediyl)diacetate (2): The product was obtained as a white solid in 37% yield. IR (KBr): 3427 (m), 3367 (m), 3263 (m), 3005 (w), 2361 (w), 1734 (s), 1569 (s), 1500 (s), 1411 (s), 1317 (s), 1153 (s), 987 (m), 903 (w), 807 (s), 590 (m), 541 (m); 1H-NMR (DMSO-d6, 80°C) δ: 9.16 (s, 1H, Ar-NH), 7.86 (d, 2H, J = 8.8 Hz, H-2), 7.66 (d, 2H, J = 8.8 Hz, H-3), 7.07 (s, 2H, CH2-NH-), 6.95 (s, 2H, SO2NH2), 4.01 (d, 4H, J = 5.3 Hz, H-2′′, H-2′′′), 3.64 (s, 6H, 2 × CH3); 13C-NMR (DMSO-d6, 80 °C) δ: 171.27, 166.33, 164.50, 143.99, 137.00, 126.54, 119.35, 51.80, 42.65. HRMS (ESI/QTOF) m/z: [M + H]+ Calcd. for [C15H19N7SO6H]+ 426.1190; Found: 426.1190.
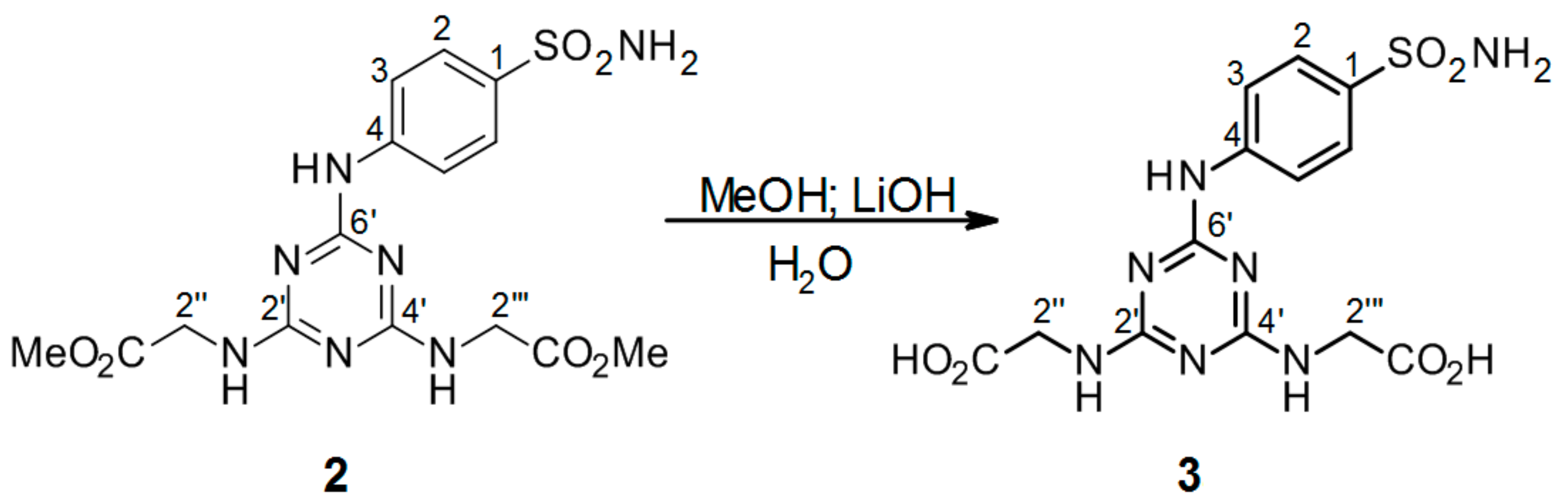
3.2.3. Hydrolysis of Intermediate 2 (Scheme 2) to Product 2′′,2′′′-[6′-(4-Sulfamoylphenylamino)-1′,3′,5′-triazine-2′,4′-diyl]-bis(azanediyl)diacetic Acid 3 (Scheme 3)
2′′,2′′′-[6′-(4-Sulfamoylphenylamino)-1′,3′,5′-triazine-2′,4′-diyl]-bis(azanediyl) diacetate 2. (0.10048 g) was dissolved in 4 mL MeOH. LiOH (4.0 eguiv) was dissolved in 4 mL H2O and added to the methanol solution of compound 2. The mixture was stirred at r.t. and monitored by TLC. After 3 h, the hydrolysis was terminated (before the addition of 1M HCl, methanol was evaporated) by the addition of a few drops of 1M HCl until a white precipitate was no longer produced. The precipitated mixture was filtered and the precipitate was dried under high vacuum.
2′′,2′′′-[6′-(4-Sulfamoylphenylamino)-1′,3′,5′-triazine-2′,4′-diyl]-bis(azanediyl)diacetic acid (3): The product was obtained as a white solid in 64% yield. IR (KBr): 3264 (w), 2361 (w), 1734 (w), 1591 (s), 1504 (s), 1406 (s), 1312 (m), 1229 (m), 1155 (s), 905 (w), 779 (m), 587 (m), 544 (m); 1H-NMR (DMSO-d6, 80 °C) δ: 9.29 (s, 1H, Ar-NH), 7.88 (d, 2H, J = 8.9 Hz, H-2), 7.68 (d, 2H, J = 8.9 Hz, H-3), 7.01 (br s, 4H, SO2NH2, 2 × CH2-NH), 3.96 (br s, 4H, 2 × CH2-NH); 13C-NMR (DMSO-d6, 80 °C) δ: 171.87, 165.72, 163.98, 143.83, 137.12, 126.65, 119.33, 42.70. HRMS (ESI/QTOF) m/z: [M + H]+ Calcd. for [C13H15N7SO6H]+ 398.0877; Found: 398.0877.
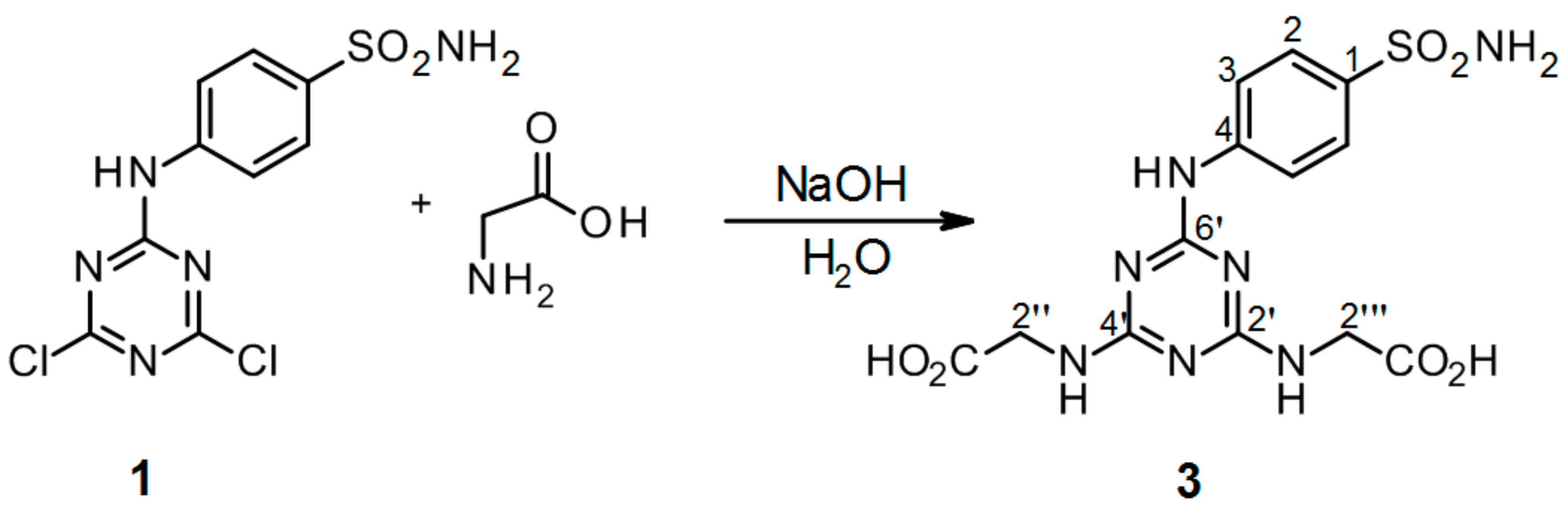
3.2.4. Synthesis of 2′′,2′′′-[6′-(4-Sulfamoylphenylamino)-1′,3′,5′-triazine-2′,4′-diyl]-bis(azanediyl)diacetic Acid 3 (Scheme 4)
4-(4′,6′-Dichloro-1′,3′,5′-triazin-2′-ylamino)-benzene-sulfonamide 1. (0.1 g, 1.0 equiv) was stirred in H2O (2 ml) at r.t. for 10 min. Then alkaline solution of glycine (2.4 equiv of Gly and 5.0 equiv of NaOH) was added dropwise into precursor 1. The mixture was refluxed for 24 h and monitored by TLC. The synthesis was terminated by the addition of a few drops of 1M HCl until a white precipitate was no longer produced. The product was filtered off and dried under high vacuum.
2′′,2′′′-[6′-(4-Sulfamoylphenylamino)-1′,3′,5′-triazine-2′,4′-diyl]-bis(azanediyl)diacetic acid (3). The product was obtained as a white solid in 81% yield. IR (KBr): 2968 (s), 2361 (s), 2343 (m), 1560 (m), 1498 (s), 1405 (s), 1308 (m), 1157 (s), 837 (w), 779 (m), 587 (m), 544 (s); 1H-NMR (DMSO-d6, 80 °C) δ: 9.14 (s, 1H, Ar-NH), 7.89 (d, 2H, J = 8.9 Hz, H-2), 7.67 (d, 2H, J = 8.9 Hz, H-3), 6.94 (s, 2H, SO2NH2), 6.88 (br s, 2H, 2 × CH2-NH), 3.94 (d, 4H, J = 6.2 Hz 2 × CH2-NH); 13C-NMR (DMSO-d6, 80 °C) δ: 172.06, 166.40, 164.50, 144.09, 136.83, 126.60, 119.28, 42.67. HRMS (ESI/QTOF) m/z: [M + H]+ Calcd. for [C13H15N7SO6H]+ 398.0877; Found: 398.0877 [C13H15N7SO6H]+; 341.0663 [C11H12N6SO5H]+; 284.0448 [C9H9N5SO4H]+.
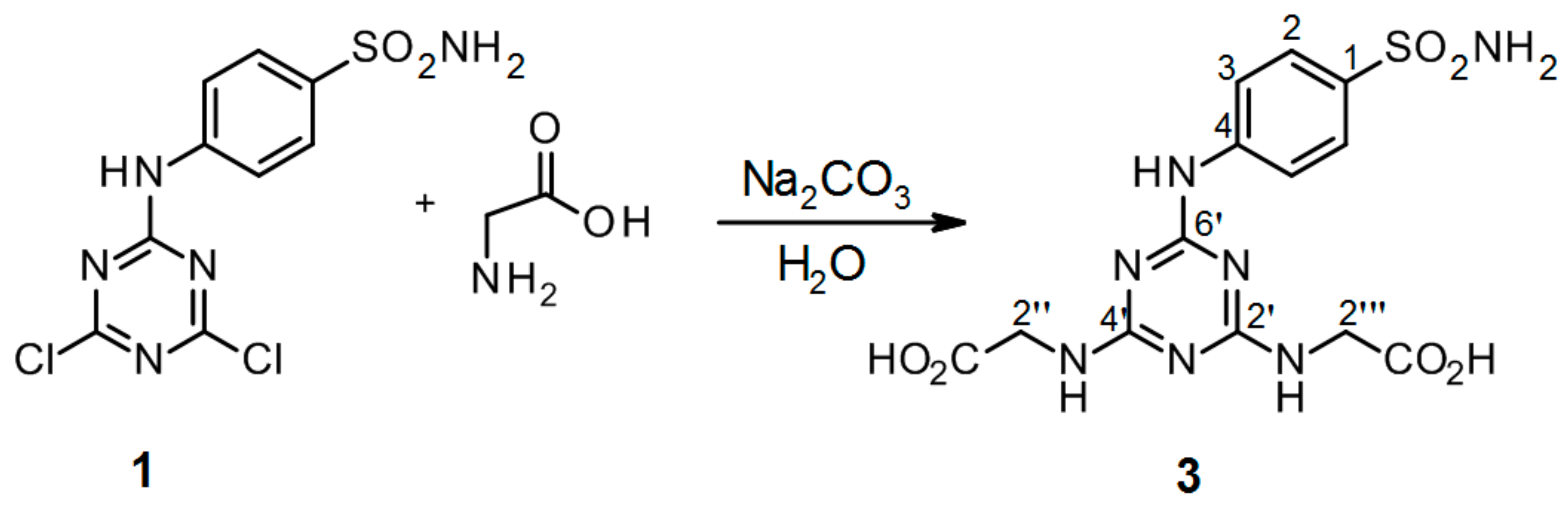
3.2.5. Synthesis of 2′′,2′′′-[6′-(4-Sulfamoylphenylamino)-1′,3′,5′-triazine-2′,4′-diyl]-bis(azanediyl)diacetic Acid 3 (Scheme 5)
4-(4′,6′-Dichloro-1′,3′,5′-triazin-2′-ylamino)-benzene-sulfonamide 1. (0.2 g, 1.0 equiv) and glycine (3.0 equiv) were stirred in H2O (2 mL) at r.t. for 10 min. Then aqueous solution of Na2CO3 (4.0 equiv) was added dropwise into the mixture of precursor 1 and glycine. The mixture was refluxed for 24 h and monitored by TLC. The synthesis was terminated by the addition of a few drops of 1M HCl (up to pH = 2.0) until a white precipitate was no longer produced. The product was filtered off and dried under high vacuum.
2′′,2′′′-[6′-(4-Sulfamoylphenylamino)-1′,3′,5′-triazine-2′,4′-diyl]-bis(azanediyl)diacetic acid (3). The product was obtained as a white solid in 96% yield. IR (KBr): 2968 (w), 2360 (w), 2343 (w), 1683 (m), 1598 (m), 1507 (s), 1407 (s), 1159 (s), 1096 (w), 847 (w), 779 (s), 588 (m), 544 (s); 1H-NMR (DMSO-d6, 80 °C) δ: 9.17 (s, 1H, Ar-NH), 7.89 (d, 2H, J = 8.9 Hz, H-2), 7.67 (d, 2H, J = 8.9 Hz, H-3), 6.94 (br s, 4H, SO2NH2, 2 × CH2-NH), 3.95 (d, 4H, J = 5.2 Hz, 2 × CH2-NH); 13C-NMR (DMSO-d6, 80 °C) δ: 172.03, 166.23, 164.40, 144.04, 136.92, 126.66, 119.32, 42.70. HRMS (ESI/QTOF) m/z: [M + H]+ Calcd. for [C13H15N7SO6H]+ 398.0877; Found: 398.0878.
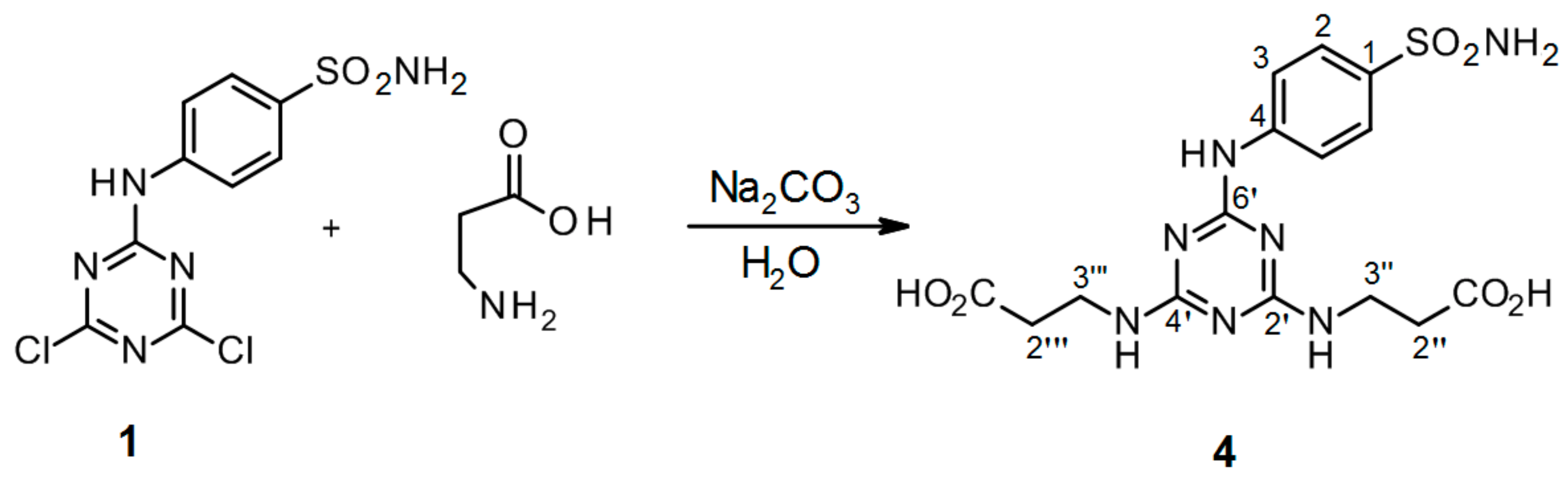
3.2.6. Synthesis of 3′′,3′′′-[6′-(4-Sulfamoylphenylamino)-1′,3′,5′-triazine-2′,4′-diyl]bis(azanediyl) Dipropanoic acid 4 (Scheme 6)
4-(4′,6′-Dichloro-1′,3′,5′-triazin-2′-ylamino)-benzene-sulfonamide 1. (0.2 g, 1.0 equiv) and β-alanine (3.0 equiv) were stirred in H2O (2 mL) at r.t. for 10 min. Then aqueous solution of Na2CO3 (4.0 equiv) was added dropwise into the mixture of precursor 1 and β-alanine. The mixture was refluxed for 24 h and monitored by TLC. The synthesis was terminated by the addition of a few drops of 1 M HCl (up to pH = 3.0) until a white precipitate was no longer produced. The product was filtered off and dried under high vacuum.
3′′,3′′′-[6′-(4-Sulfamoylphenylamino)-1′,3′,5′-triazine-2′,4′-diyl]bis(azanediyl) dipropanoic acid (4). The product was obtained as a white solid in 98% yield. IR (KBr): 2967 (m), 2361 (s), 2343 (m), 1630 (m), 1592 (s), 1509 (s), 1399 (m), 1163 (s), 889 (w), 678 (w), 581 (m), 552 (m); 1H-NMR (DMSO-d6, 80 °C) δ: 9.32 (br s, 1H, Ar-NH), 7.92 (d, 2H, J = 8.8 Hz, H-2), 7.69 (d, 2H, J = 8.8 Hz, H-3), 6.96 (br s, 4H, SO2NH2, 2 × CH2-NH), 3.53 (t, J = 7.1 Hz, 4H, H-2′′, H-2′′′), 2.54 (t, 4H, J = 7.1 Hz, H-3′′, H-3′′′); 13C-NMR (DMSO-d6, 80 °C) δ: 173.19, 164.70, 163.70, 143.83, 137.17, 126.68, 119.42, 36.98, 34.54. HRMS (ESI/QTOF) m/z: [M + H]+ Calcd. for [C15H19N7SO6H]+ 426.1190; Found 426.1190.