Development, Physicochemical Characterization and In Vitro Anti-Inflammatory Activity of Solid Dispersions of α,β Amyrin Isolated from Protium Oilresin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization
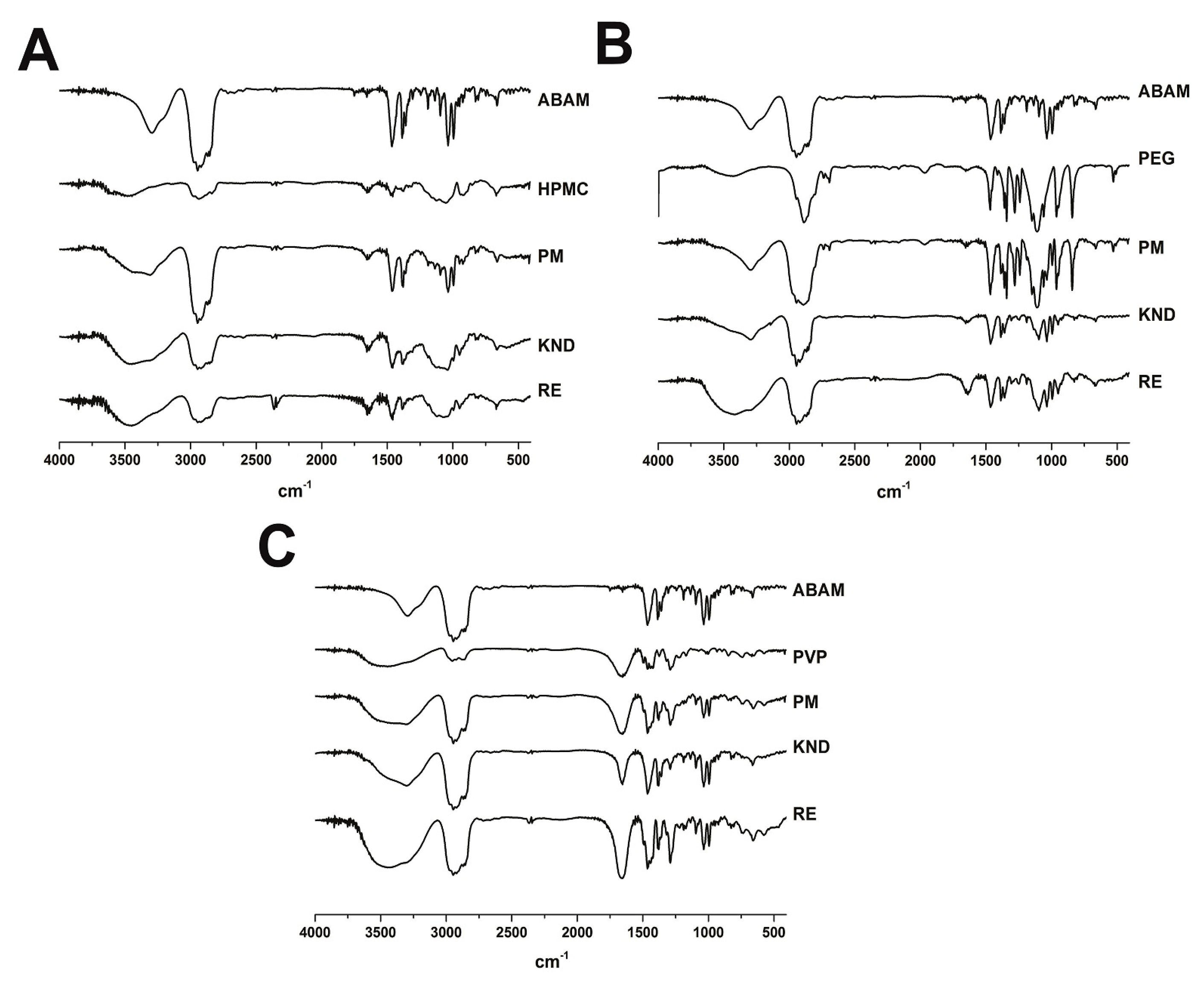
2.1.1. Fourier Transform Infrared—FTIR
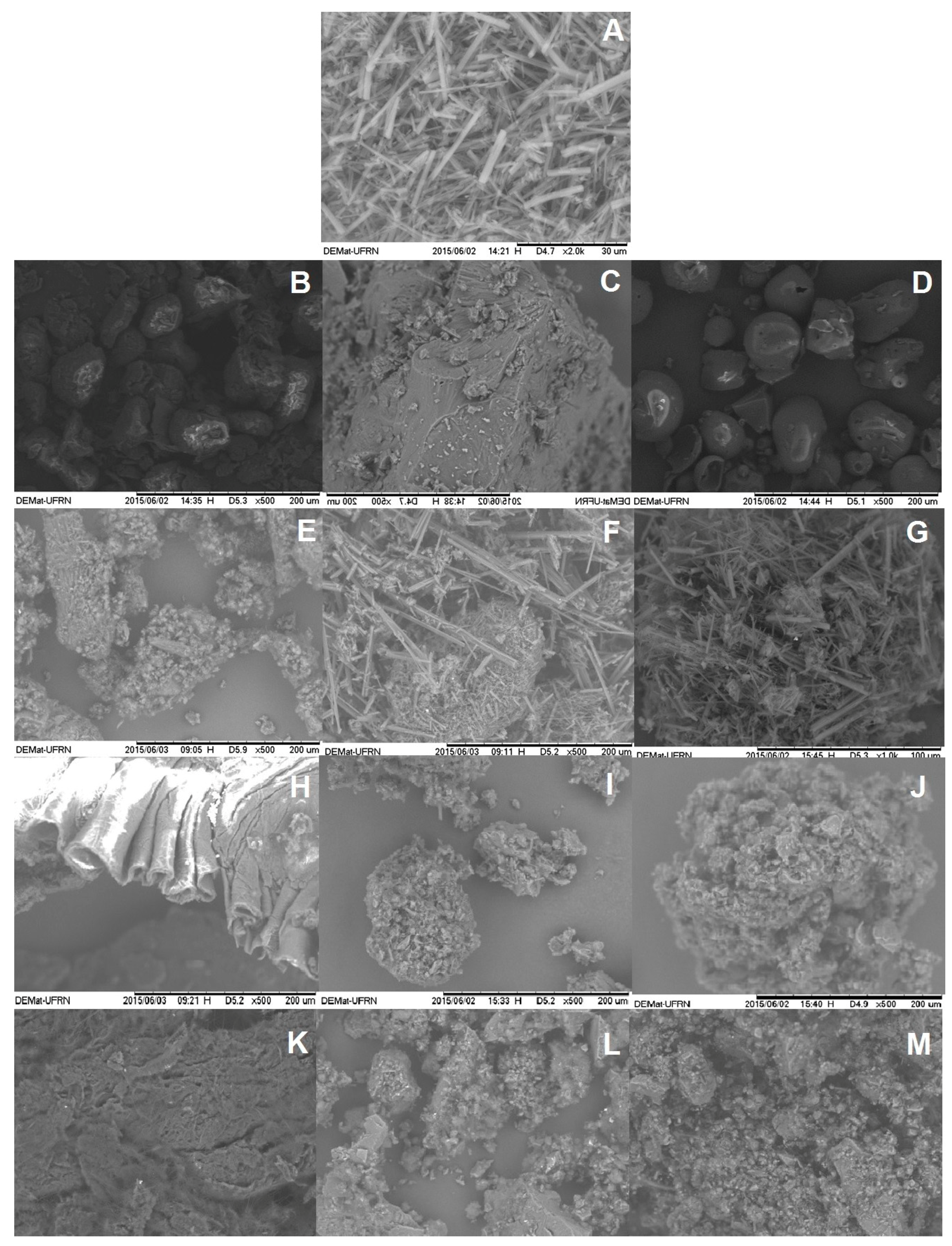
2.1.2. Scanning Electronic Microcopy
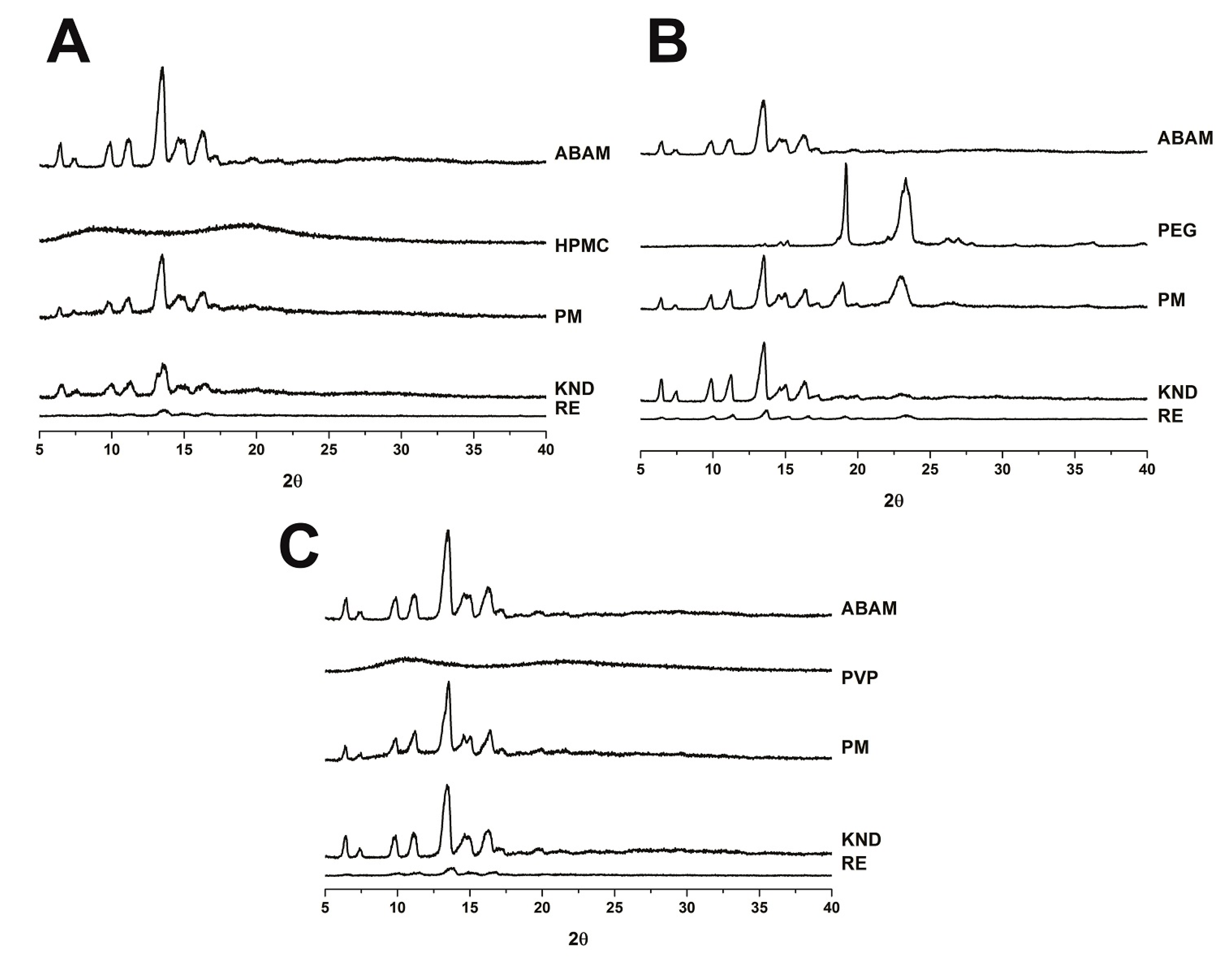
2.1.3. Powder X-ray Diffraction
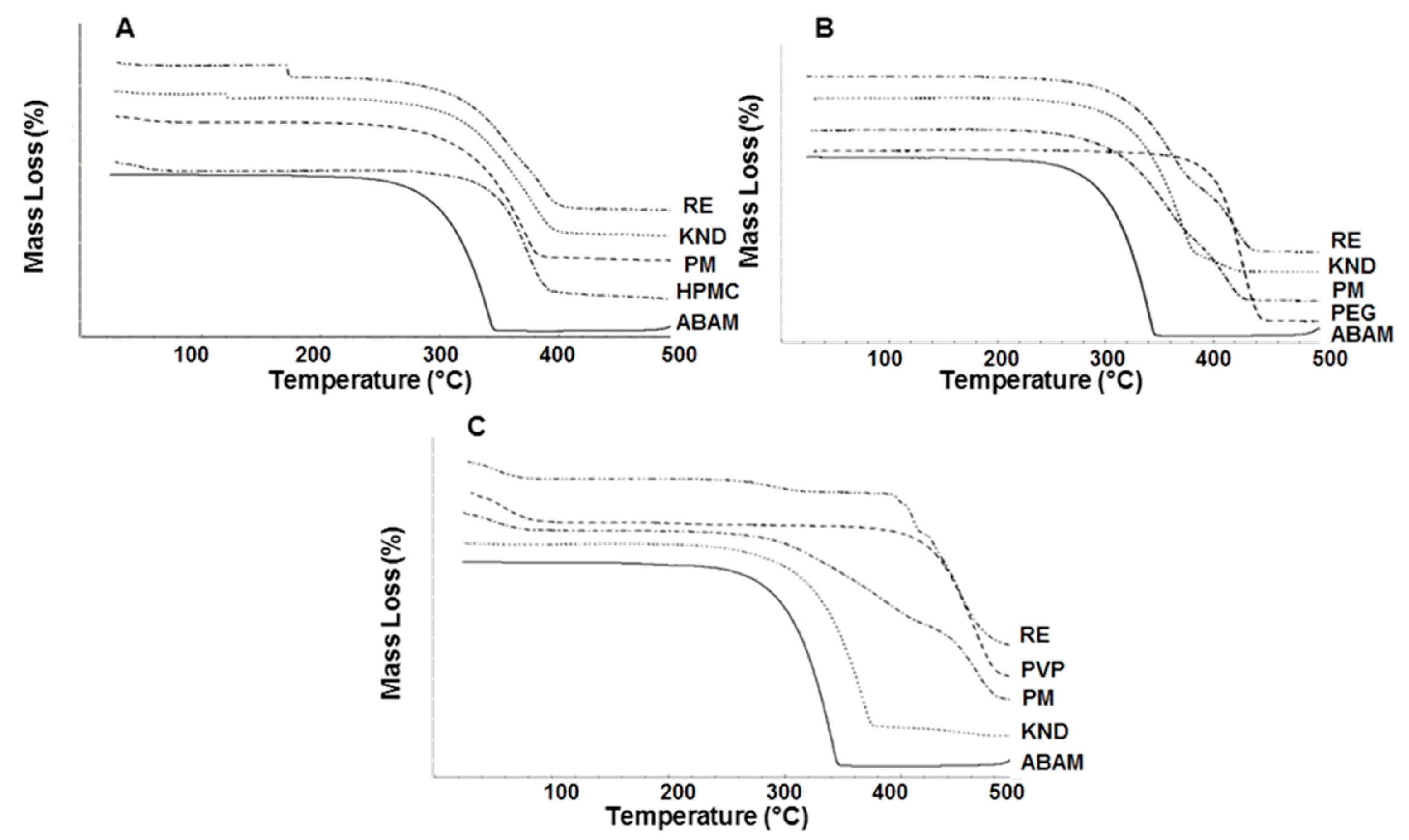
2.1.4. Thermogravimetric Analysis
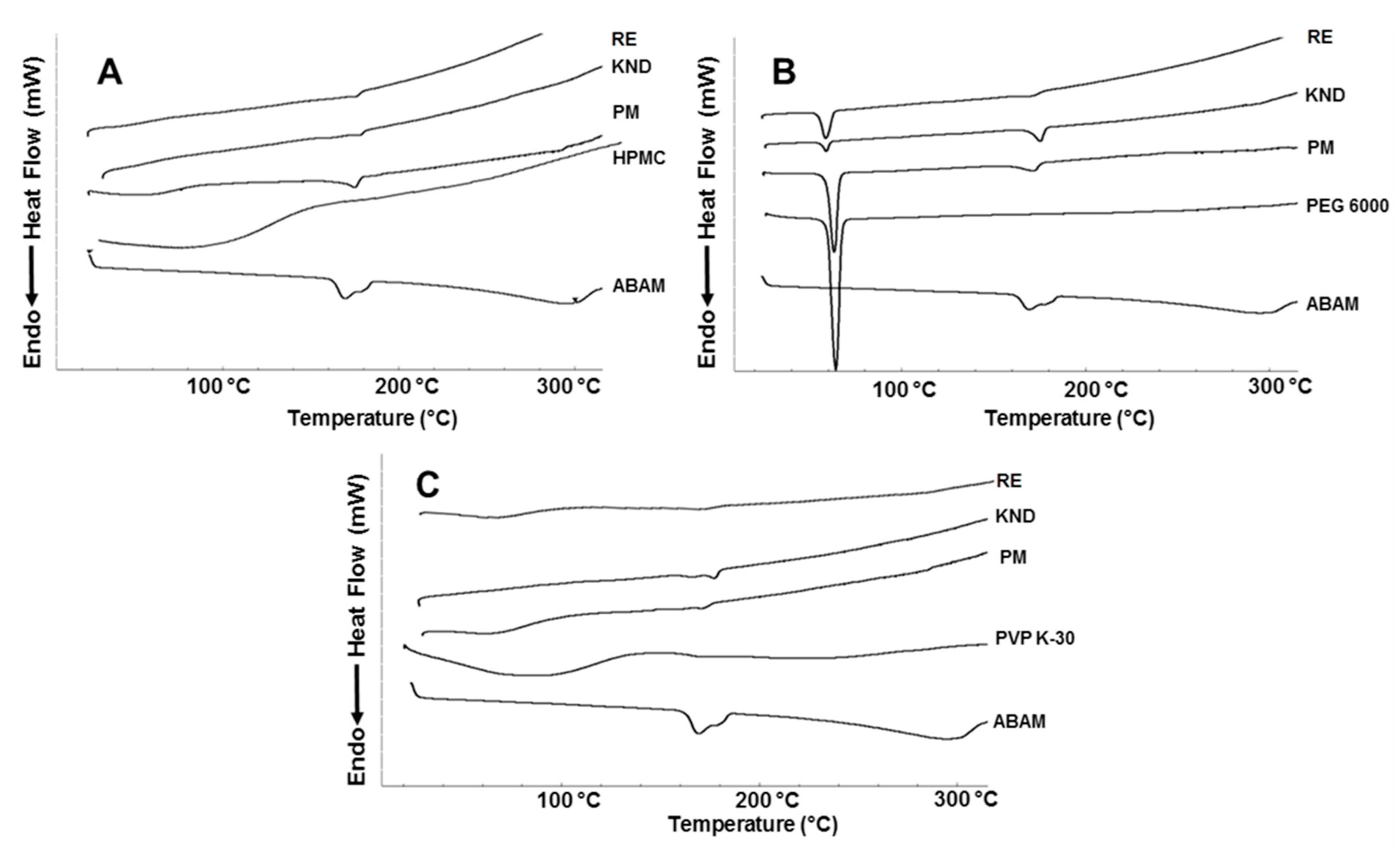
2.1.5. Differential Scanning Calorimetry
2.2. In Vitro Anti-Inflammatory Activity
2.2.1. Quantification of Nitric Oxide in LPS-Stimulated Macrophages
2.2.2. Cell Viability Assays
3. Materials and Methods
3.1. Material
3.2. Preparation of SDs
3.2.1. SD Obtained by PM
3.2.2. SD Obtained by KND
3.2.3. SD Obtained by RE
3.3. Physicochemical Characterization
3.3.1. Fourier Transform Infrared
3.3.2. Scanning Electronic Microscopy
3.3.3. Powder X-ray Diffraction
3.3.4. Thermogravimetry
3.3.5. Differential Scanning Calorimetry
3.4. In Vitro Anti-Inflammatory Study
3.4.1. Quantification of Nitric Oxide (NO)
3.4.2. Cell viability Assay
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weeks, A.; Daly, D.C.; Simpson, B.B. The phylogenetic history and historical biogeography of the frankincense and myrrh family (Burseraceae) based on nuclear and chloroplast sequence data. Mol. Phylogenet. Evol. 2005, 35, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil, 3rd ed.; Instituto Plantarum: Nova Odessa, Brazil, 2008. [Google Scholar]
- Siani, A.C.; Ramos, M.F.S.; Menezes, O.; Ribeiro, R.; Fernandez, E.; Soares, R.O.; Rosas, E.C.; Susunaga, G.S.; Guimarães, A.C.; Zoghbi, M.G.; et al. Evaluation of antiinflamatory-related activity of essential oils from the leaves and resin of species of Protium. J. Ethnopharmacol. 1999, 66, 57–69. [Google Scholar] [CrossRef]
- Rüdiger, A.L.; Veiga Junior, V.F. Chemodiversity of ursane- and oleanane-type triterpenes in Amazonian Burseraceae oleoresins. Chem. Biodivers. 2013, 10, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Veiga Junior, V.F.; Silva, J.R.A.; Zoghbi, M.G.B.; Patitucci, M.L.; Pinto, A.C. Utilização de cromatografia gasosa de alta resolução na detecção de classes de terpenos em extratos brutos vegetais. Quím. Nova 1995, 18, 262–266. [Google Scholar]
- Bandeira, P.N.; Lemos, T.L.; Costa, S.M.; Santos, H.S. Obtenção de derivados da mistura triterpenoídica α e β-amirina. Rev. Bras. Farmacogn. 2007, 17, 204–212. [Google Scholar] [CrossRef]
- Matos, I.; Bento, A.F.; Marcon, R.; Claudino, R.F.; Calixto, J.B. Preventive and therapeutic oral administration of the pentacyclic triterpene α,β-amyrin ameliorates dextran sulfate sodium-induced colitis in mice: The relevance of cannabinoid system. Mol. Imunnol. 2013, 54, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Holanda, S.A.; Pinto, L.M.S.; Guedes, M.A.; Cunha, G.M.A.; Chaves, M.H.; Santos, F.A.; Rao, V.S. Antinoceptive effect of triterpenoid α,β-amyrin in rats on orofacial pain induced by formalin and capsaicin. Phytomedcine 2008, 15, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.A.; Vieira-Júnior, G.M.; Chaves, M.H.; Almeida, F.R.C.; Florêncio, M.G.; Lima-Junior, R.C.P.; Silva, R.M.; Santos, F.A.; Rao, V.S.N. Gastroprotective and anti-inflammatory effects of resin from Protium heptaphyllum in mice and rats. Pharmacol. Res. 2004, 49, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.A.; Chaves, M.H.; Almeida, F.R.; Lima-Júnior, R.C.; Silva, R.M.; Maia, J.L.; Brito, G.A.; Santos, F.A.; Rao, V.S. Protective effect of α- and β-amyrin, a triterpene mixture from Protium heptaphyllum (Aubl.) March. trunk wood resin, against acetaminophen-induced liver injury in mice. J. Ethnopharmacol. 2005, 98, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Burton, L.; Ying, W.; Gandhi, R.; West, R.; Huang, C.; Zhou, S.; Shak, K.; Chen, J.; Shen, X. Development of a precipitation-resistant solution formulation to increase in vivo exposure of a poorly water-soluble compound. Int. J. Pharm. 2012, 433, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Sahoo, J.; Dixit, P.K. Formulation and process optimization of naproxen nanosuspensions stabilized by hydroxy propryl methyl cellulose. Carbohydr. Polym. 2015, 127, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Gulsun, T.; Gursoy, R.N.; Oner, L. Design and characterization of nanocrystal formulations containing ezetimibe. Chem. Pharm. Bull. 2011, 59, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Thadkala, K.; Nanam, P.K.; Rambabu, B.; Sailu, C.; Aukunuru, J. Preparation and characterization of amorphous ezetimibe nanosuspensions intended for enhancement of oral bioavailability. Int. J. Pharm. Investig. 2014, 4, 131–137. [Google Scholar] [PubMed]
- Duarte, I.; Corvo, M.L.; Serôdio, P.; Vicente, J.; Pinto, J.F.; Temtem, M. Production of nano-solid dispersions using a novel solvente-controlled precipitation process—Benchmarking their in vivo performance with an amorphous micro-sized solid dispersion produced by spray drying. Eur. J. Pharm. Sci. 2016, 93, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.; Buttchevitzs, A.; Kruger, J.H.; Kratz, J.M.; Simões, C.M.O.; Benedet, P.O.; Oliveira, P.R.; Silva, M.A.S. Inclusion complexes of hydrochlorothiazide and β-cyclidextrin: Physicochemical characteristics, in vitro and in vivo studies. Eur. J. Pharm. Sci. 2016, 83, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.D.S.; de La Roca, M.F.S.; Albuquerque, C.T.; Silva, É.R.; Vieira, A.C.C.; Fontes, D.A.F.; Figueirêdo, C.B.M.; Soares-Sobrinho, J.L.; Rolim-Neto, P.J. Solid dispersion of efavirenz in PVP K-30 by conventional solvent and kneading methods. Carbohydr. Polym. 2014, 104, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, D.; Zhao, S.; Huang, X.; Jianbin, Z.; Yan, L.V.; Liu, X.; Guojun, L.V.; Xiaojun, M. Evaluate the ability of PVP to inhibit crystallization of amorphous solid dispersions by density functional theory and experimental verify. Eur. J Pharm. Sci. 2017, 96, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Marsac, P.; Li, T.; Taylor, L. Estimation of drug-polymer miscibility and solubility in amorphous solid dispersions using experimentally determined interaction parameters. Pharm. Res. 2009, 26, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Meaurio, E.; Zuza, E.; Sarasua, J.R. Miscibility and specific interactions in blends of poly(l-lactide) with poly(vinylphenol). Macromolecules 2005, 38, 1207–1215. [Google Scholar] [CrossRef]
- Park, Y.; Veytsman, B.; Coleman, M.; Painter, P. The miscibility of hydrogenbonded polymer blends: Two self-associating polymers. Macromolecules 2005, 38, 3703–3707. [Google Scholar] [CrossRef]
- Li, B.; Wen, M.; Li, W.; He, M.; Yang, X.; Li, S. Preparation and characterization of baicalin-poly-vinylpyrrolidone coprecipitate. Int. J. Pharm. 2011, 408, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Arya, P.V.; Sharma, A.; Dobhal, M.P.; Gupta, R.S. Modulatory potential of α-amyrin against hepatic oxidative stress through antioxidant status in wistar albino rats. J. Ethnopharmcol. 2015, 161, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Al-Homaidan, A.A.; Ibraheem, I.B.M.; Al-Othman, M.R.; Hatamleh, A.A. Antibacterial β-amyrin isolated from Laurencia microcladia. Arab. J. Chem. 2015, 8, 32–37. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D. Spectrometric Identification of Organic Compounds, 7th ed.; Wiley: New York, NY, USA, 2005. [Google Scholar]
- Sharma, V.K.; Mazumdar, B. Feasibility and characterization of gummy exudates of Cochlospermum religiosum as pharmaceutical excipiente. Ind. Crop. Prod. 2013, 50, 776–786. [Google Scholar] [CrossRef]
- Papadimitriou, S.A.; Barmpalexis, P.; Karavas, E.; Bikiaris, D.N. Optimizing the ability of PVP/PEG mixtures to be used as ppropriate carriers for the preparation of drug solid dispersions by melt mixing technique usingartificial neural networks: I. Eur. J. Pharm. Biopharm. 2012, 82, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Riekes, M.K.; Kuminek, G.; Rauber, G.S.; Campos, C.E.M.; Bortoluzzi, A.J.; Stulzer, H.K. HPMC as a potential enhancer of nimodipine biopharmaceutical properties via ball-milled solid dispersions. Carbohydr. Polym. 2015, 99, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Tong, Q.; Fang, J.; Wang, C.; Wu, J.; Wang, W. Preparation, chacarterization and in vivo studies of amorphous solid dispersion of berberine with hydrogenated phosphatidylcoline. Eur. J. Pharm. Sci. 2015, 74, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.A.; Cavalheiro, T.G. Thermal behavior of loratadine. J. Therm. Anal. Calorim. 2007, 87, 831–834. [Google Scholar] [CrossRef]
- Kumar, S.G.V.; Mishra, D.N. Preparation, characterization and in vitro dissolution studies of solid dispersion of meloxicam with PEG 6000. Yakugaku Zasshi 2006, 126, 657–664. [Google Scholar] [CrossRef]
- Sethia, S.; Squillante, E. Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int. J. Pharm. 2004, 272, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.H.; Abdel-Wahab, O.; Patel, J.P.; Levine, R.L. The role of mutations in epigenetic regulators in myeloid malignancies. Nat. Rev. Cancer 2012, 12, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Biskup, C.S.; Sánchez, C.L.; Arrant, A.; Van Swearingen, A.E.; Kuhn, C.; Zepf, F.D. Effects of acute tryptophan depletion on brain serotonin function and concentrations of dopamine and norepinephrine in C57BL/6J and BALB/cJ mice. PLoS ONE. 2012, 7, e35916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, N.; Tripathi, S.K.; Sahu, D.; Das, H.R.; Das, R.H. Evaluation of inhibitory activities of plant extracts on production of LPS-stimulated pro-inflammatory mediators in J774 murine macrophages. Mol. Cell. Biochem. 2010, 336, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Julião, L.S.; Piccinelli, A.L.; Marzocco, S.; Leitão, S.G.; Lotti, C.; Autore, G.; Rastrelli, L. Phenylethanoid glycosides from Lantana fucata with in vitro anti-inflammatory activity. J. Nat. Prod. 2009, 72, 1424–1428. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.S.; Amano, Y.; Narukawa, Y.; Takeda, T. Nitric oxide production inhibitory activity of flavonoids contained in trunk exudates of Dalbergia sissoo. J. Nat. Prod. 2008, 71, 98–101. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds α,β amyrin. are available from the authors. |


| Sample | Concentration (μg/mL) | NO− Concentration | % Inhibition |
|---|---|---|---|
| LPS | 1 | 45.75 ± 0.855 | 0 |
| ABAM | 20 | 25.37 ± 1.237 | 44.55 |
| PM-HPMC | 20 | 17.99 ± 2.037 | 60.68 |
| PM-PEG | 20 | 16.70 ± 0.200 | 63.50 |
| PM-PVP | 20 | 18.81 ± 1.114 | 58.88 |
| KND-HPMC | 20 | 20.06 ± 1.195 | 56.15 |
| KND-PEG | 20 | 21.41 ± 1.437 | 53.20 |
| KND-PVP | 20 | 17.16 ± 0.830 | 62.49 |
| RE-HPMC | 20 | 26.53 ± 1.425 | 42.09 |
| RE-PEG | 20 | 28.20 ± 0.762 | 38.35 |
| RE-PVP | 20 | 22.26 ± 2.306 | 51.13 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Silva Júnior, W.F.; Pinheiro, J.G.d.O.; De Menezes, D.L.B.; E Silva, N.E.d.S.; De Almeida, P.D.O.; Lima, E.S.; Da Veiga Júnior, V.F.; De Azevedo, E.P.; De Lima, Á.A.N. Development, Physicochemical Characterization and In Vitro Anti-Inflammatory Activity of Solid Dispersions of α,β Amyrin Isolated from Protium Oilresin. Molecules 2017, 22, 1512. https://doi.org/10.3390/molecules22091512
Da Silva Júnior WF, Pinheiro JGdO, De Menezes DLB, E Silva NEdS, De Almeida PDO, Lima ES, Da Veiga Júnior VF, De Azevedo EP, De Lima ÁAN. Development, Physicochemical Characterization and In Vitro Anti-Inflammatory Activity of Solid Dispersions of α,β Amyrin Isolated from Protium Oilresin. Molecules. 2017; 22(9):1512. https://doi.org/10.3390/molecules22091512
Chicago/Turabian StyleDa Silva Júnior, Walter Ferreira, Jonas Gabriel de Oliveira Pinheiro, Danielle Lima Bezerra De Menezes, Natan Emanuell de Sobral E Silva, Patrícia Danielle Oliveira De Almeida, Emerson Silva Lima, Valdir Florêncio Da Veiga Júnior, Eduardo Pereira De Azevedo, and Ádley Antonini Neves De Lima. 2017. "Development, Physicochemical Characterization and In Vitro Anti-Inflammatory Activity of Solid Dispersions of α,β Amyrin Isolated from Protium Oilresin" Molecules 22, no. 9: 1512. https://doi.org/10.3390/molecules22091512





