New Tetra-Schiff Bases as Efficient Photostabilizers for Poly(vinyl chloride)
Abstract
:1. Introduction
2. Results and Discussion
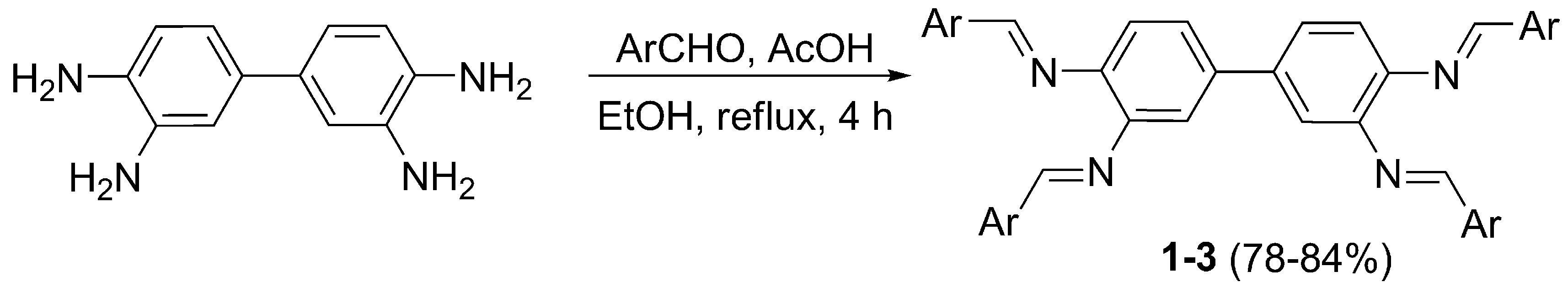
2.1. Synthesis and Characterization of Schiff Bases 1–3
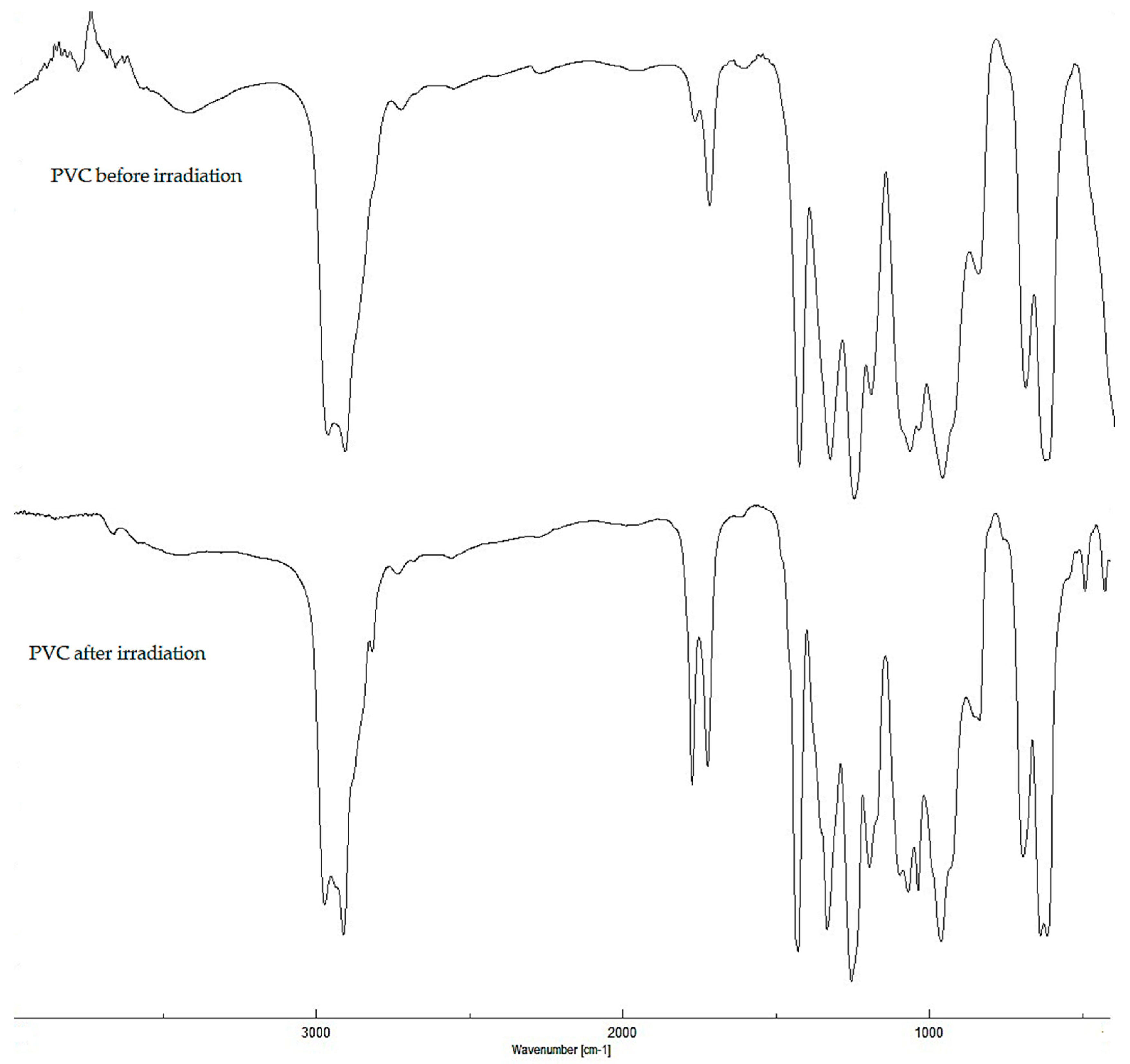
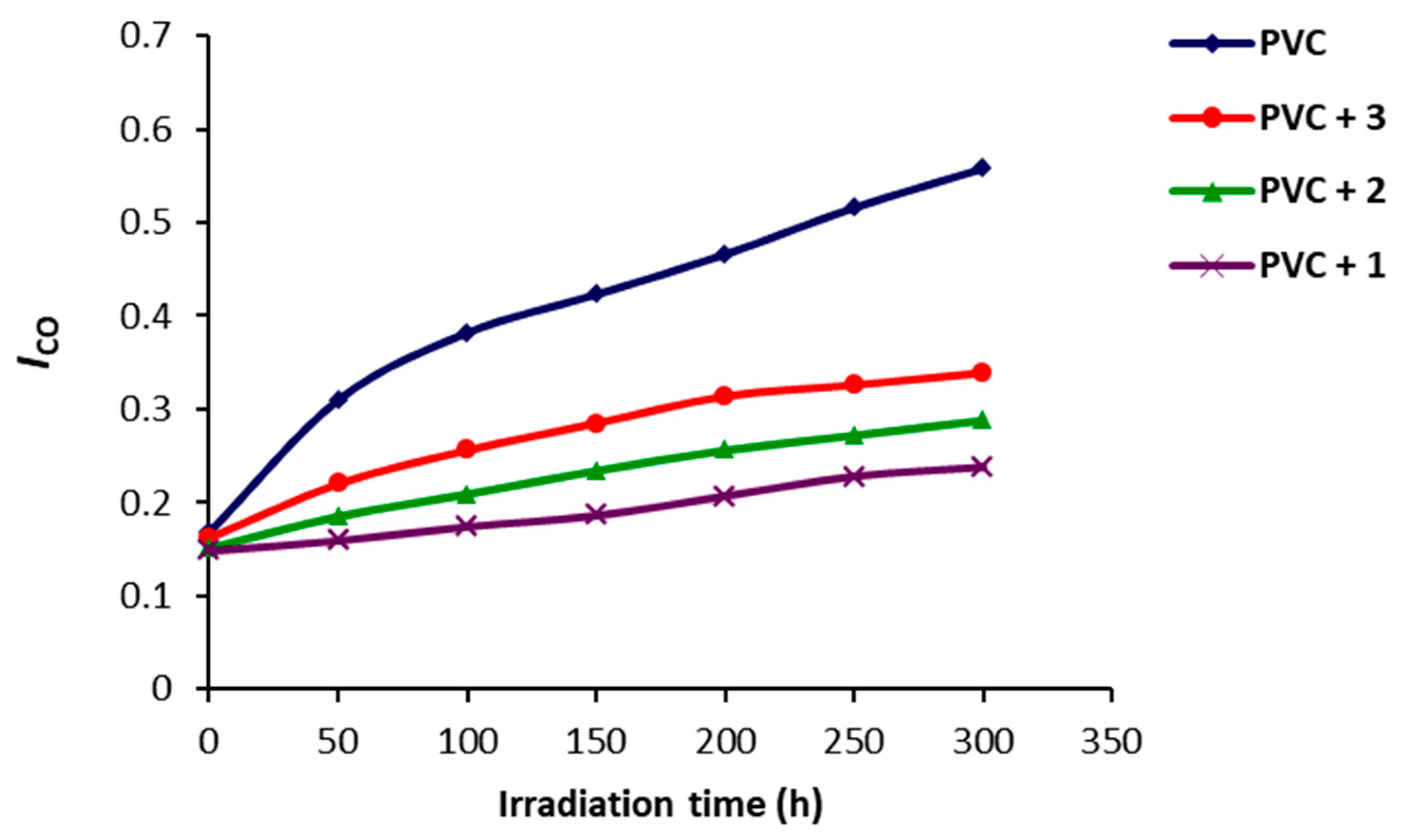
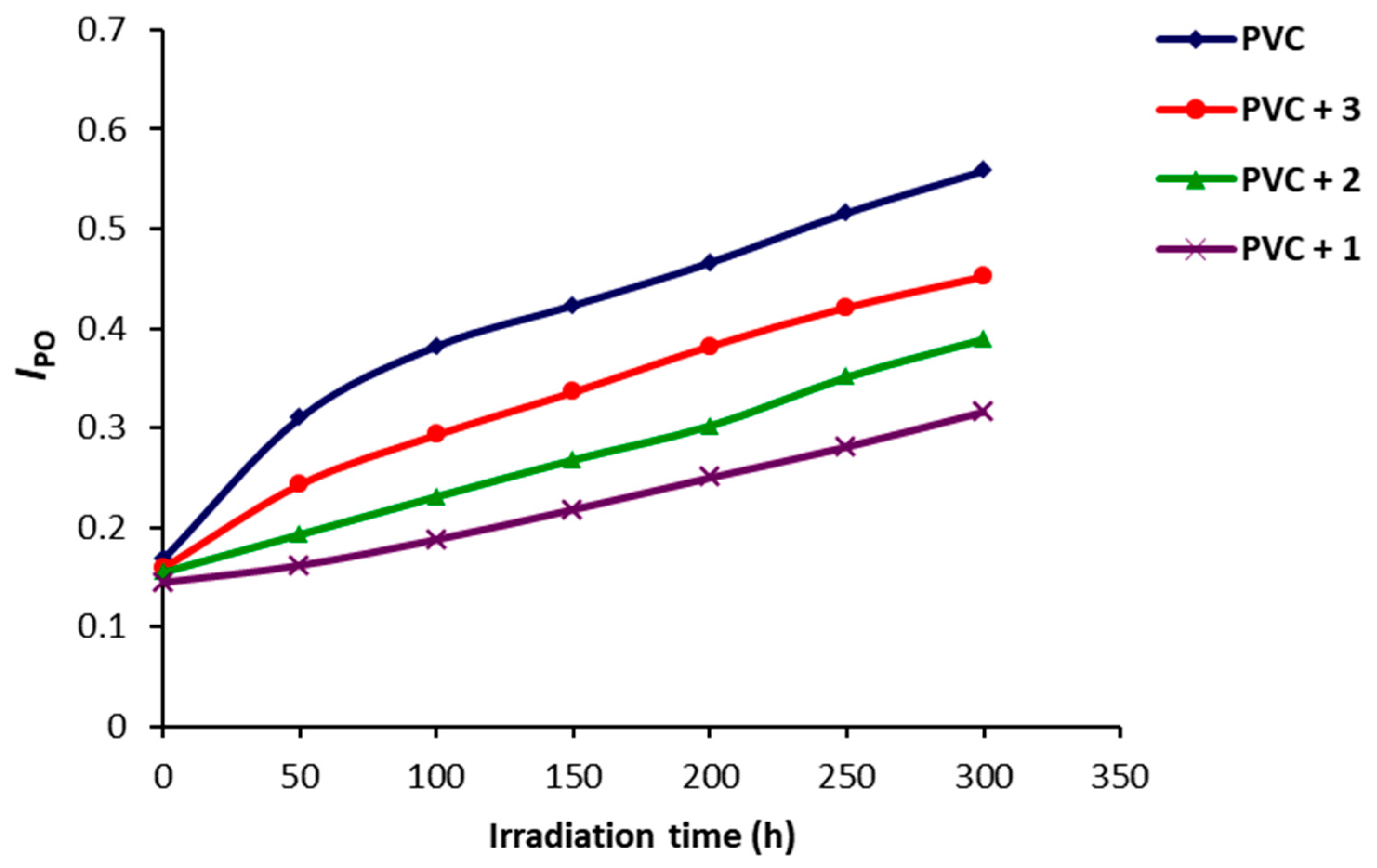
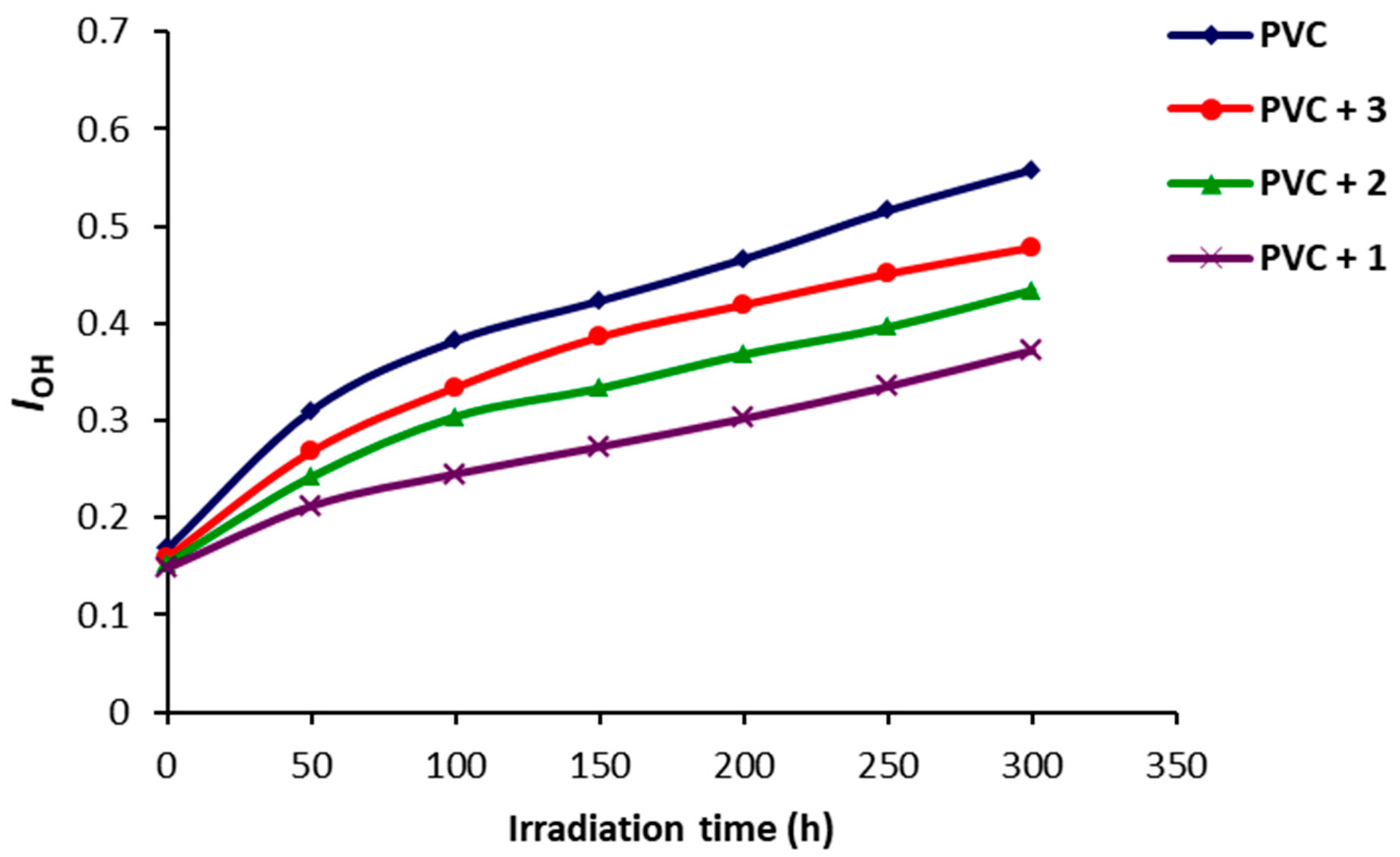
2.2. Photodegradation of PVC Films by FT-IR Spectroscopy
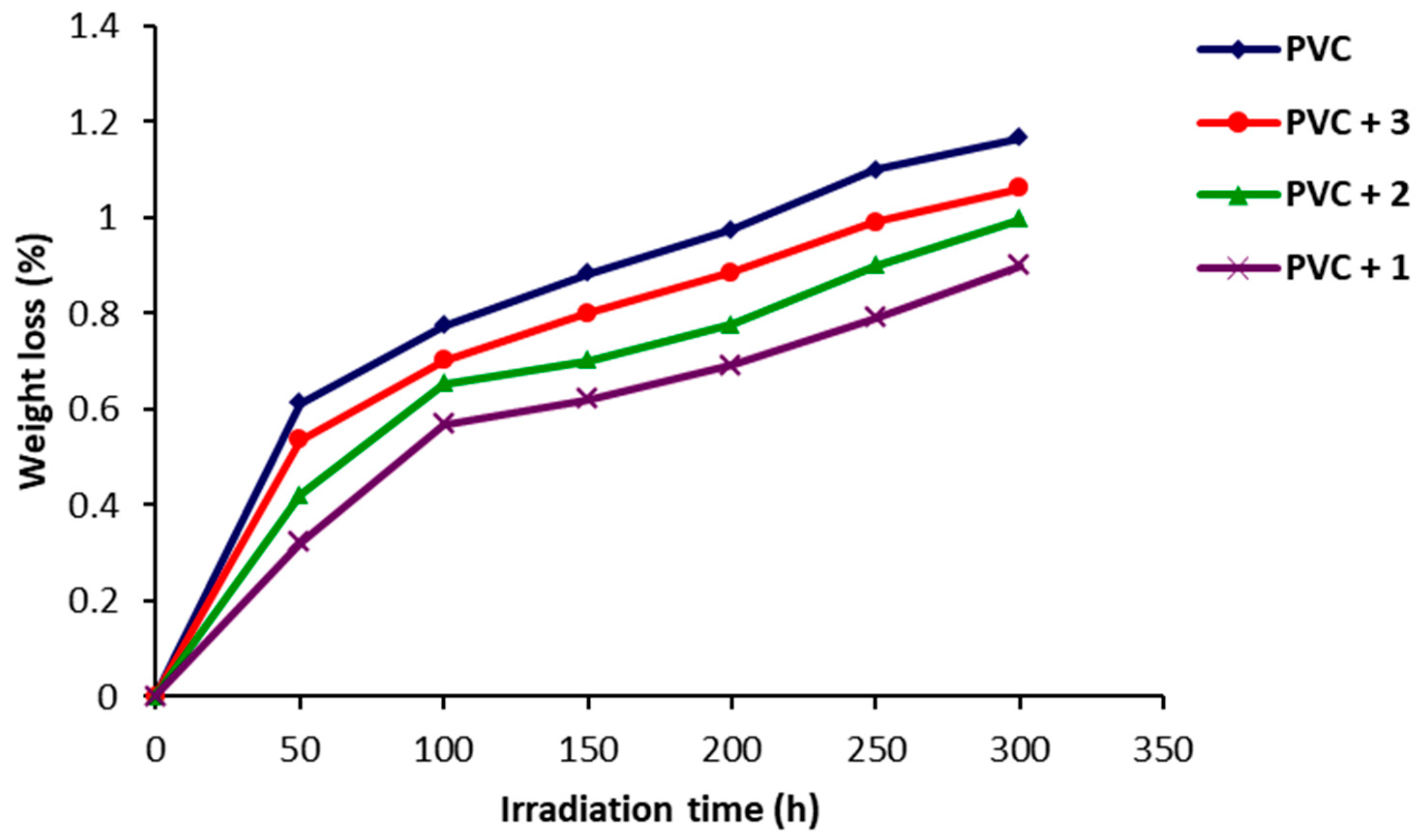
2.3. Photodegradation of PVC Films by Weight Loss
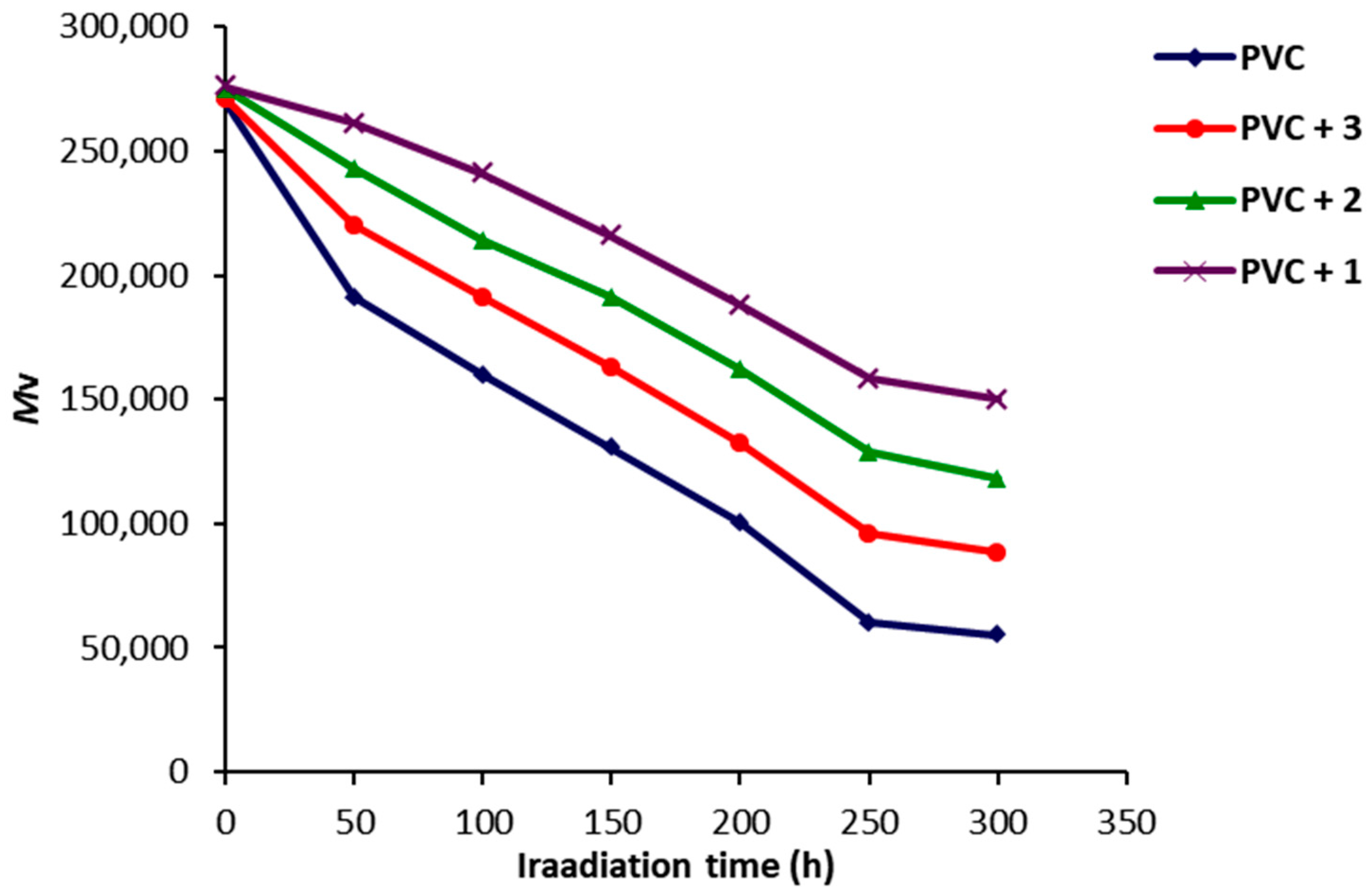
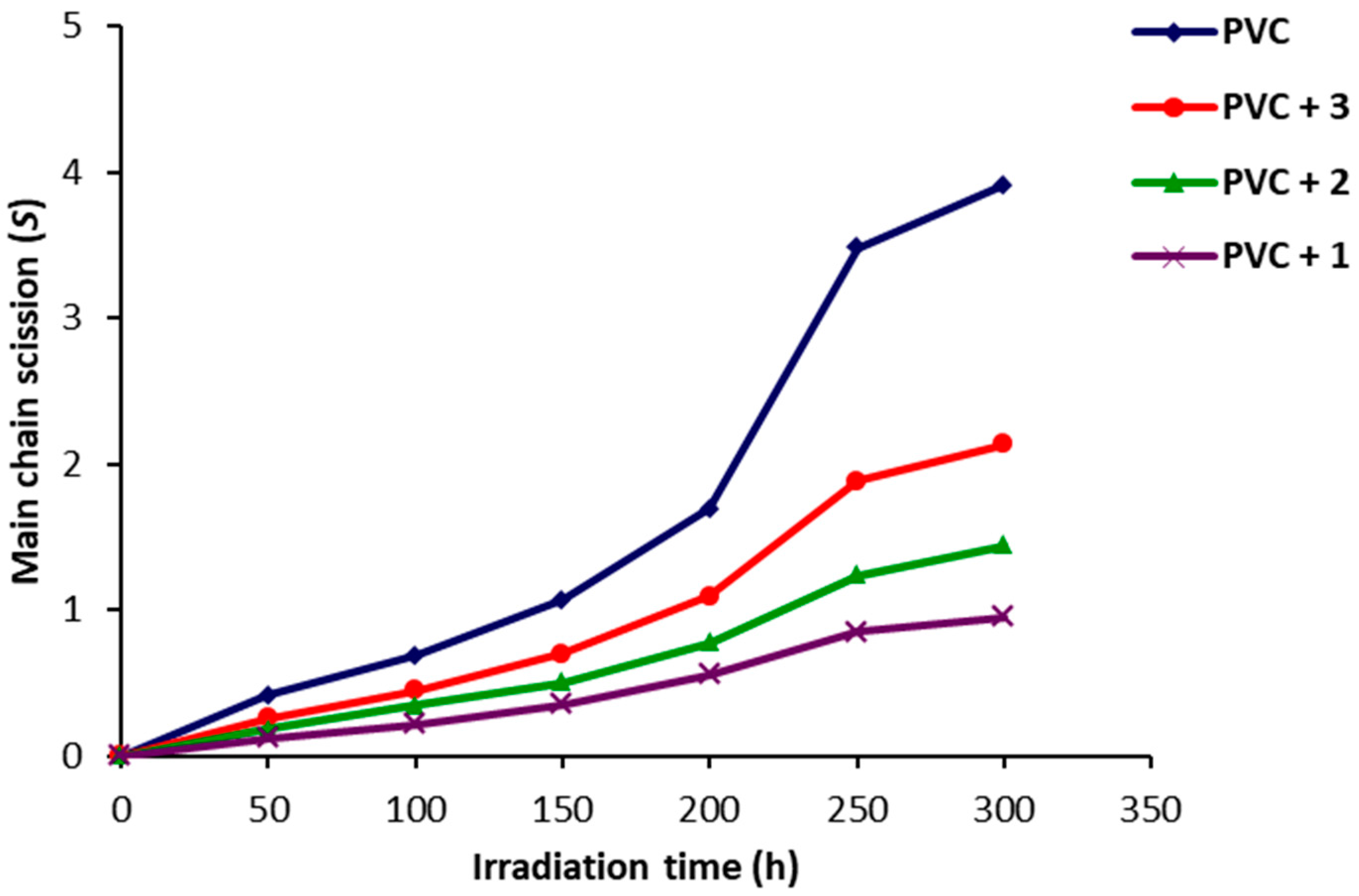
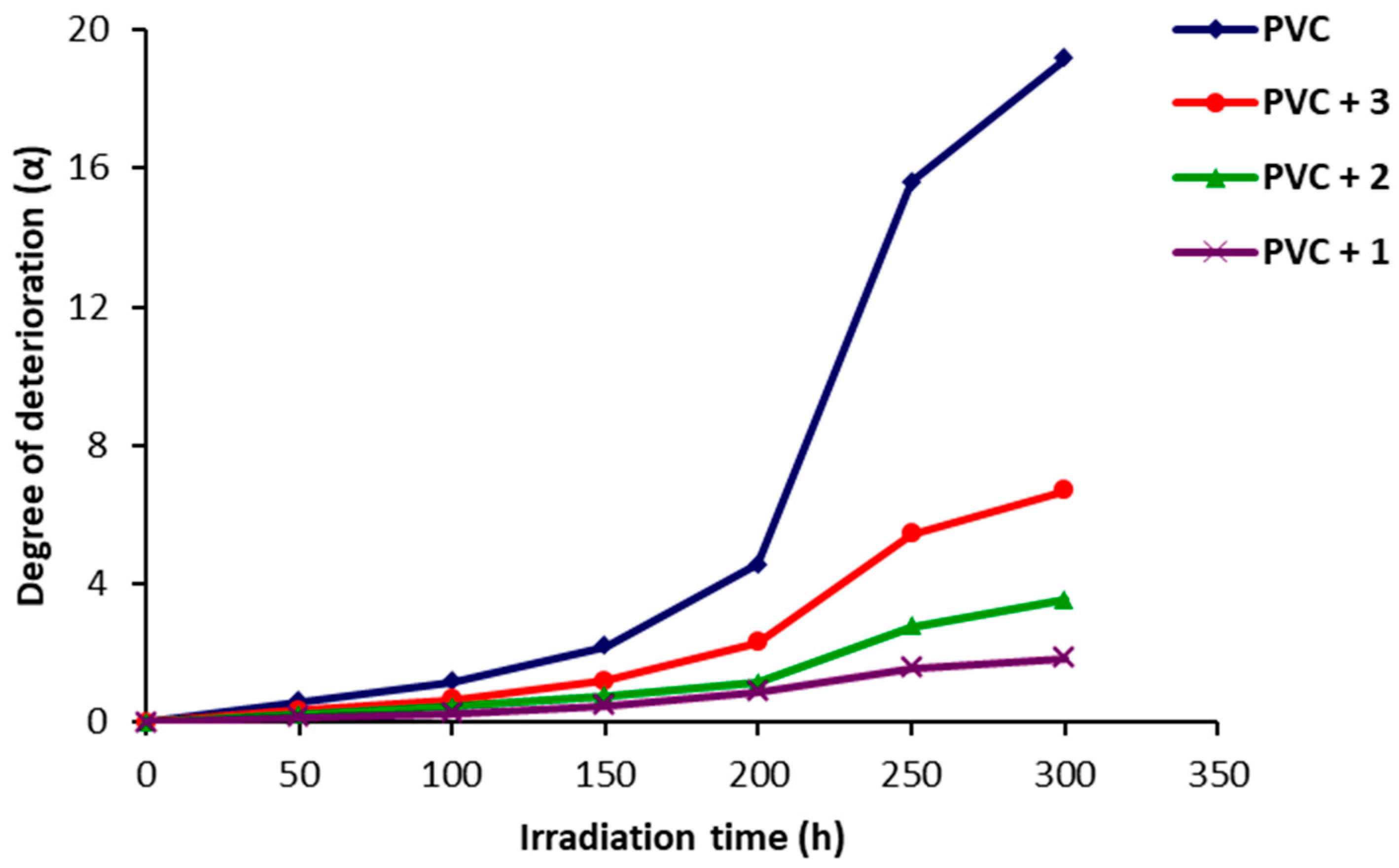
2.4. Photodegradation of PVC Films by Variation in Molecular Weight
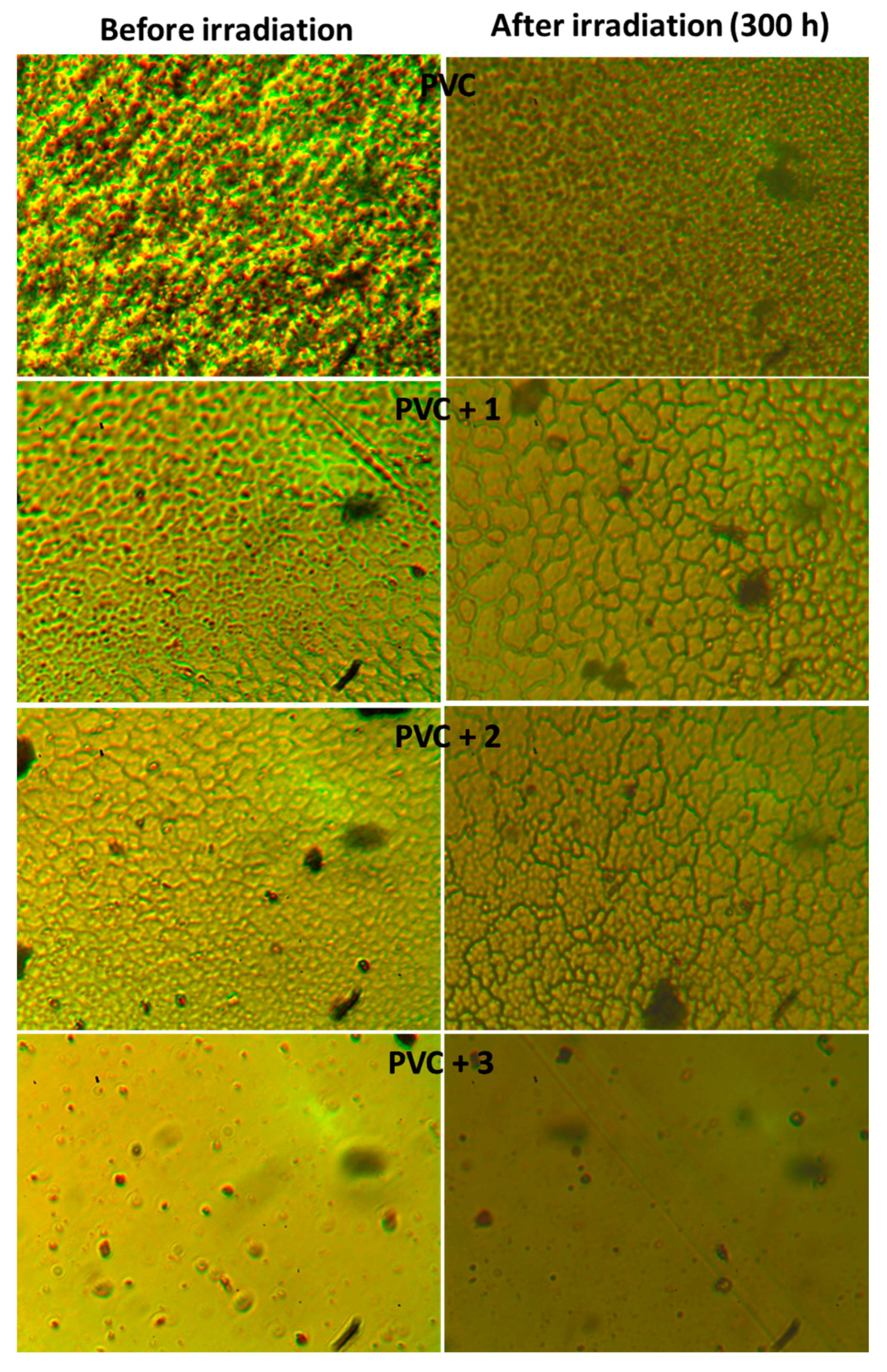
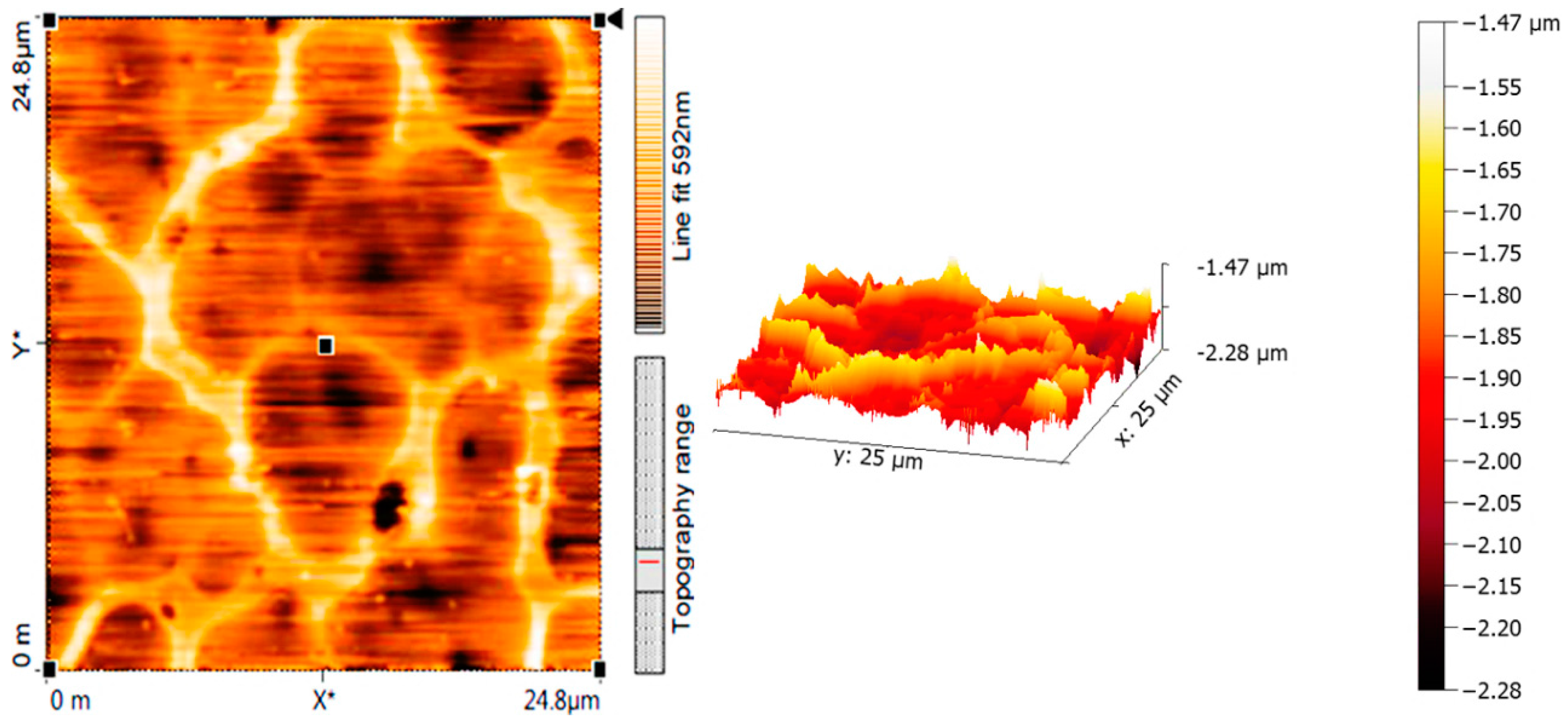
2.5. Surface Morphology of PVC Films
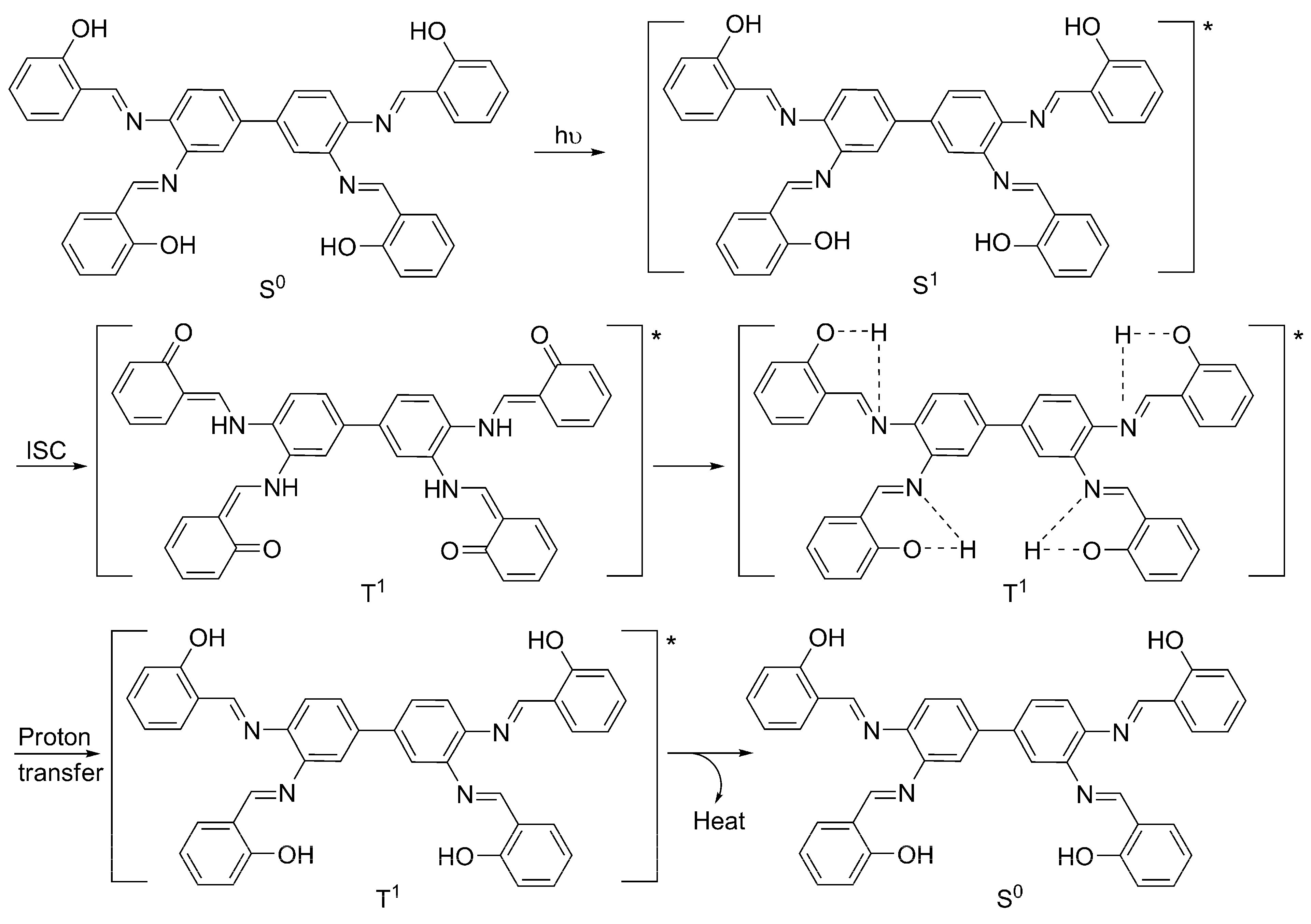
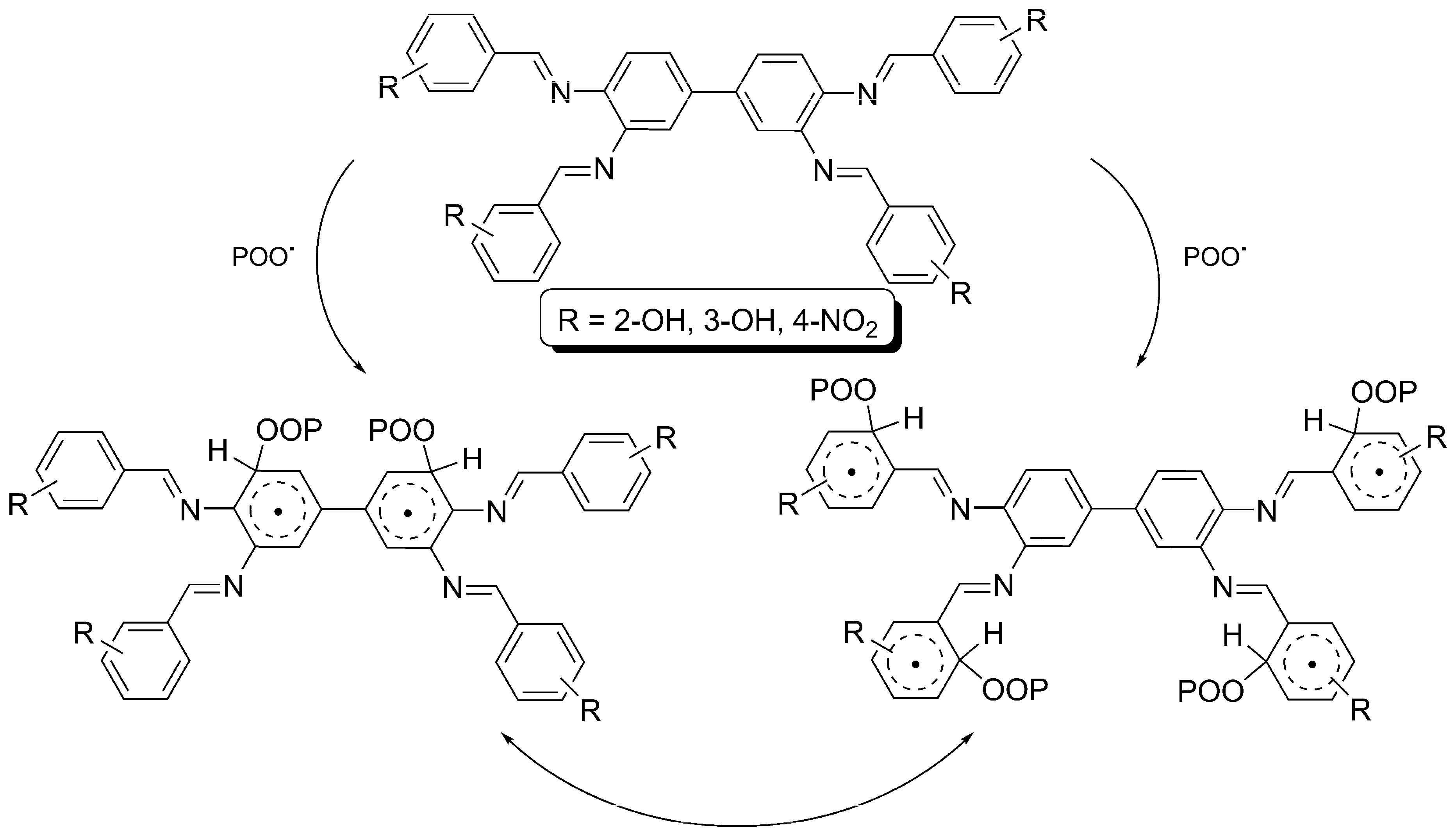
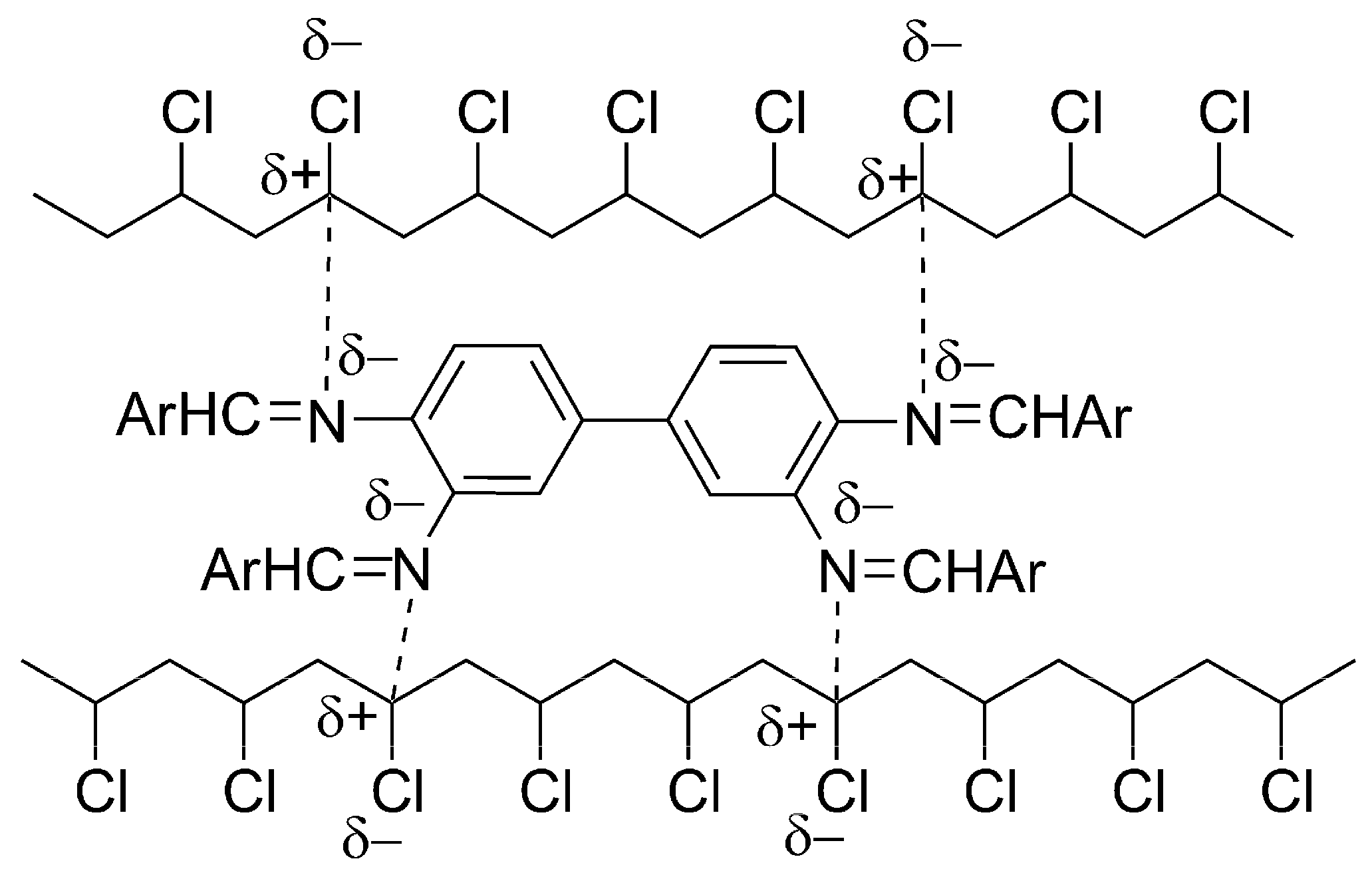
2.6. Proposed Mechanisms of PVC Film Photostabilization
3. Experimental Section
3.1. General
3.2. Synthesis of Schiff Bases 1–3
3.3. PVC Films Preparation
3.4. Light Exposure
3.5. Photodegradation of PVC Films by FT-IR Spectrophotometry
3.6. Photodegradation of PVC Films by Weight Loss
3.7. Photodegradation of PVC Films by Viscometry Method
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- National Research Council. Manufacturing: Materials and processing. In Polymer Science and Engineering: The Shifting Research Frontiers; The National Academies Press: Washington, DC, USA, 1994; Chapter 3; pp. 65–115. [Google Scholar]
- Braun, D. Thermal degradation of poly (vinyl chloride). In Developments in Polymer Degradation; Grassie, N., Ed.; Applied Science Publishers: London, UK, 1981. [Google Scholar]
- Allsopp, M.W.; Vianello, G. Poly(vinyl chloride). In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Titow, W.V. PVC Technology, 4th ed.; Elsevier Applied Science Publishers: London, UK, 1984. [Google Scholar]
- Grassie, N.; Scott, G. Degradation and Stabilization of Polymers; Cambridge University Press: London, UK, 1985. [Google Scholar]
- Nicholson, J.W. The Chemistry of Polymers, 3rd ed.; RSC Publisher: Cambridge, UK, 2012. [Google Scholar]
- Decker, C. Degradation and Stabilisation of PVC; Owen, E.D., Ed.; Springer: Dordrecht, The Netherlands, 1984. [Google Scholar]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Proc. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Iván, B.; Kennedy, J.P.; Kélen, T.; Tüdòs, F.; Nagy, T.T.; Turcsanyi, B. Degradation of PVCs obtained by controlled chemical dehydrochlorination. J. Polym. Sci. Pol. Chem. 1983, 21, 2177–2188. [Google Scholar] [CrossRef]
- Caraculacu, A.; Bezdadea, E.C.; Istrate, G. Structure of branching in PVC. J. Polym. Sci. Pol. Chem. 1970, 8, 1239–1246. [Google Scholar] [CrossRef]
- Starnes, W.H. Structural and mechanistic aspects of the thermal degradation of poly(vinyl chloride). Prog. Polym. Sci. 2002, 27, 2133–2170. [Google Scholar] [CrossRef]
- Fahmy, M.M.; Mohamed, R.R.; Mohamed, N.A. Novel antimicrobial organic thermal stabilizer and co-stabilizer for rigid PVC. Molecules 2012, 17, 7927–7940. [Google Scholar] [CrossRef] [PubMed]
- Deanin, R.D.; Reynolds, H.H.; Ozcayir, Y. Thermal stabilization of polyvinyl chloride by group II metal laurates. J. Appl. Polym. Sci. 1969, 13, 1247–1252. [Google Scholar] [CrossRef]
- Birmingham, J.N. The effect of surface oxidation and titanium dioxide on exterior PVC color retention. J. Vinyl Addit. Technol. 1995, 1, 84–87. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, C.; Pavlinek, V.; Saha, P.; Wang, H. Surface-modified antibacterial TiO2/Ag+ nanoparticles: Preparation and properties. Appl. Surf. Sci. 2006, 252, 4154–4160. [Google Scholar] [CrossRef]
- Folarin, O.M.; Sadiku, E.R. Thermal stabilizers for poly(vinyl chloride): A review. Int. J. Phys. Sci. 2011, 6, 4323–4330. [Google Scholar]
- Bojinov, V.B.; Grabchev, I.K. Novel functionalized 2-(2-hydroxyphenyl)-benzotriazole-benzo[de]isoquinoline-1,3-dione fluorescent UV absorbers: Synthesis and photostabilizing efficiency. J. Photochem. Photobiol. A 2005, 172, 308–315. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Oraby, E.H.; Naby, A.S.A.; Mohamed, R.R. N-Phenyl-3-substituted-5-pyrazolone derivatives as organic stabilizer for rigid PVC against photodegradation. J. Appl. Polym. Sci. 2005, 101, 1543–1555. [Google Scholar] [CrossRef]
- Yousif, E.; Salih, N.; Salimon, J. Improvement of the photostabilization of PVC films in the presence of 2N-salicylidene-5-(substituted)-1,3,4-thiadiazole. J. Appl. Polym. Sci. 2011, 120, 2207–2214. [Google Scholar] [CrossRef]
- Yousif, E.; Hameed, A.; Salih, N.; Salimon, J.; Abdullah, B. New photostabilizers for polystyrene based on 2,3-dihydro-(5-mercapto-1,3,4-oxadiazol-2-yl)-phenyl-2-(substituted)-1,3,4-oxazepine-4,7-dione compounds. SpringerPlus 2013, 2, 104. [Google Scholar] [CrossRef] [PubMed]
- Balakit, A.A.; Ahmed, A.; El-Hiti, G.A.; Smith, K.; Yousif, E. Synthesis of new thiophene derivatives and their use as photostabilizers for rigid poly(vinyl chloride). Int. J. Polym. Sci. 2015, 2015, 510390. [Google Scholar] [CrossRef]
- Ali, M.A.; El-Hiti, G.A.; Yousif, E. Photostabilizing efficiency of poly(vinyl chloride) in the presence of organotin(IV) complexes as photostabilizers. Molecules 2016, 21, 1151. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.; Hameed, A.; Rasheed, R.; Mansoor, H.; Farina, Y.; Graisa, A.; Salih, N.; Salimon, J. Synthesis and photostability study of some modified poly(vinyl chloride) containing pendant benzothiazole and benzimidozole ring. Int. J. Chem. 2010, 2, 65–80. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Mikhael, M.G.; Mohamed, N.A.; Yassin, A.A. N-Substituted maleimides as thermal stabilizers for rigid poly (vinyl chloride). Angew. Makromol. Chem. 1989, 168, 23–35. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Oraby, E.H.; Abdel Naby, A.S.; Mohammed, R.R. Anthraquinone derivatives as organic stabilizers for rigid poly(vinyl chloride) against photo-degradation. Eur. Polym. J. 2005, 41, 2530–2543. [Google Scholar] [CrossRef]
- Zhao, Y.; Dan, Y. Preparation and characterization of a high molecular weight UV-stabilizer based on a derivative of 2,4-dihydroxybenzophenone and its application in polymer materials. J. Appl. Polym. Sci. 2006, 102, 2203–2211. [Google Scholar] [CrossRef]
- Tomohito, K.; Masahiko, O.; Guido, G.; Tadaaki, M.; Toshiaki, Y. Antibacterial effect of thiocyanate substituted poly (vinyl chloride). J. Polym. Res. 2011, 18, 945–947. [Google Scholar]
- Tomi, I.H.R.; Ali, G.Q.; Jawad, A.H.; Yousef, E. Synthesis and characterization of gallic acid derivatives and their utilized as organic photo-stabilizers for poly (vinyl chloride). J. Polym. Res. 2017, 24, 119. [Google Scholar] [CrossRef]
- Yousif, E.; Hasan, A.; El-Hiti, G.A. Spectroscopic, physical and topography of photochemical process of PVC films in the presence of Schiff base metal complexes. Polymers 2016, 8, 204. [Google Scholar] [CrossRef]
- Yousif, E.; El-Hiti, G.A.; Hussain, Z.; Altaie, A. Viscoelastic, spectroscopic and microscopic study of the photo irradiation effect on the stability of PVC in the presence of sulfamethoxazole Schiff’s bases. Polymers 2015, 7, 2190–2204. [Google Scholar] [CrossRef]
- Yousif, E.; Al-Amiery, A.A.; Kadihum, A.; Kadhum, A.H.; Mohamad, A. Photostabilizing efficiency of PVC in the presence of Schiff bases as photostabilizers. Molecules 2015, 20, 19886–19899. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; El-Hiti, G.A.; Ahmed, A.; Altaee, N.; Yousif, E. Photocatalytic degradation of polyhydroxybutyrate films using titanium dioxide nanoparticles as a photocatalyst. Russ. J. Appl. Chem. 2016, 89, 1536–1543. [Google Scholar] [CrossRef]
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Influence of titanium dioxide particle size on the photostability of the chemical UV-filters butyl methoxy dibenzoylmethane and octocrylene in a microemulsion. Cosmetics 2014, 1, 128–139. [Google Scholar] [CrossRef]
- Farrar, R.R., Jr.; Shapiro, M.; Javaid, I. Photostabilized titanium dioxide and a fluorescent brightener as adjuvants for a nucleopolyhedrovirus. BioControl 2003, 48, 543–560. [Google Scholar] [CrossRef]
- Smith, K.; Balakit, A.A.; Pardasani, R.T.; El-Hiti, G.A. New polymeric sulfide-borane complexes: Convenient hydroborating and reducing reagents. J. Sulfur Chem. 2011, 32, 287–295. [Google Scholar] [CrossRef]
- Smith, K.; Balakit, A.A.; El-Hiti, G.A. Poly(propylene sulfide)-borane: Convenient and versatile reagent for organic synthesis. Tetrahedron 2012, 68, 7834–7839. [Google Scholar] [CrossRef]
- Yousif, E.; El-Hiti, G.A.; Haddad, R.; Balakit, A.A. Photochemical stability and photostabilizing efficiency of poly(methyl methacrylate) based on 2-(6-methoxynaphthalen-2-yl) propanoate metal ion complexes. Polymers 2015, 7, 1005–1019. [Google Scholar] [CrossRef]
- Smith, K.; Al-Zuhairi, A.J.; El-Hiti, G.A.; Alshammari, M.B. Comparison of cyclic and polymeric disulfides as catalysts for the regioselective chlorination of phenols. J. Sulfur Chem. 2015, 36, 74–85. [Google Scholar] [CrossRef]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Biodegradation of different formulations of polyhydroxybutyrate films in soil. SpringerPlus 2016, 5, 762. [Google Scholar] [CrossRef] [PubMed]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Screening and evaluation of poly(3-hydroxybutyrate) with Rhodococcus. equi using different carbon sources. Arab. J. Sci. Eng. 2017, 42, 2371–2379. [Google Scholar] [CrossRef]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Hameed, A.S.; Abdalla, M. New eco-friendly phosphorus organic polymers as gas storage media. Polymers 2017, 9, 336. [Google Scholar] [CrossRef]
- Curreli, S.; Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. A modular approach towards nonsymmetrical bis(metallosalen) building blocks. Eur. J. Inorg. Chem. 2008, 2863–2873. [Google Scholar] [CrossRef]
- Karabocek, N.; Eski, P.; Baskan, O.; Karabocek, S. Synthesis, characterization and biochemical activity of dinuclear Cu(II)/Ni(II)/Co(II) and tetranuclear Cu(II) complexes of Schiff base. Asian J. Chem. 2012, 24, 387–390. [Google Scholar]
- Rabek, J.; Ranby, B. Photodegradation., Photooxidation. and Photostabilization of Polymer; John Wiley: New York, NY, USA, 1975. [Google Scholar]
- Jafari, A.J.; Donaldson, J.D. Determination of HCl and VOC emission from thermal degradation of PVC in the absence and presence of copper, copper(II) oxide and copper(II) chloride. E-J. Chem. 2009, 6, 685–692. [Google Scholar] [CrossRef]
- Martinsson, E.; Hjertberg, T.; Sorvik, E. Catalytic effect of hydrochloric acid dehydrochlorination of PVC. Macromolecules 1988, 21, 136–141. [Google Scholar] [CrossRef]
- Pepperl, G. Molecular weight distribution of commercial PVC. J. Vinyl Addit. Technol. 2000, 6, 88–92. [Google Scholar] [CrossRef]
- Skillicorn, D.E.; Perkins, G.G.A.; Slark, A.; Dawkins, J.V. Molecular weight and solution viscosity characterization of PVC. J. Vinyl Addit. Technol. 1993, 15, 105–108. [Google Scholar] [CrossRef]
- Goldberg, A.I.; Hohenstein, W.P.; Mark, H. Intrinsic viscosity-molecular weight relationship for polystyrene. J. Polym. Sci. Pol. Chem. 1947, 2, 503–510. [Google Scholar] [CrossRef]
- Mark, J.E. Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2007. [Google Scholar]
- Jellinek, H.H.G. Aspects of Degradation and Stabilization of Polymers; Elsevier: New York, NY, USA, 1978. [Google Scholar]
- Mori, F.; Koyama, M.; Oki, Y. Studies on photodegradation of poly(vinyl chloride) (part 1). Macromol. Mater. Eng. 1977, 64, 89–99. [Google Scholar]
- Júnior, G.C.; Silva, A.P.S.; Guinesi, L.S. Synthesis, characterization and electropolymerization of a new polypyrrole iron(II) Schiff-base complex. Polyhedron 2004, 23, 1953–1960. [Google Scholar] [CrossRef]
- Valko, L.; Klein, E.; Kovařík, P.; Bleha, T.; Šimon, P. Kinetic study of thermal dehydrochlorination of poly(vinyl chloride) in the presence of oxygen: III. Statistical thermodynamic interpretation of the oxygen catalytic activity. Eur. Polym. J. 2001, 37, 1123–1133. [Google Scholar] [CrossRef]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic-force microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Kara, F.; Aksoy, E.; Yuksekdagd, Z.; Hasirci, N.; Aksoy, S. Synthesis and surface modification of polyurethanes with chitosan forantibacterial properties. Carbohydr. Polym. 2014, 112, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-G.; Tang, L.-H.; Zhang, N.; Gao, Q.-H.; Zhang, C.-F.; Zhu, Z.-B. Dehydrochlorination of PVC materials at high temperature. Energy Fuel 2003, 17, 896–900. [Google Scholar] [CrossRef]
- Kasha, M. Characterization of electronic transitions in complex molecules. Discuss. Faraday Soc. 1950, 9, 14–19. [Google Scholar] [CrossRef]
- Pospíšil, J.; Klemchuk, P.P. Oxidation Inhibition in Organic Materials; CRC Press: Boca Raton, FL, USA, 1989; Volume 1, pp. 48–49. [Google Scholar]
- Yousif, E.; Bakir, E.; Salimon, J.; Salih, N. Evaluation of Schiff bases of 2,5-dimercapto-1,3,4-thiadiazole as photostabilizer for poly(methyl methacrylate). J. Saudi Chem. Soc. 2012, 16, 279–285. [Google Scholar] [CrossRef]
Sample Availability: Samples of the tetra-Schiff bases are available from the authors. |



| Schiff Base | Ar | Color | Yield (%) | Mp (°C) | Calcd. (Found; %) | ||
|---|---|---|---|---|---|---|---|
| C | H | N | |||||
| 1 | 2-HOC6H4 | Deep orange | 84 | 234–236 | 8.88 (8.92) | 4.79 (4.85) | 76.17 (76.22) |
| 2 | 3-HOC6H4 | Light brown | 80 | 207–209 | 8.88 (8.91) | 4.79 (4.83) | 76.17 (76.19) |
| 3 | 4-NO2C6H4 | Dark red | 78 | 274–276 | 15.01 (15.23) | 3.51 (3.56) | 64.34 (64.40) |
| Schiff Base | FR-IR (υ, cm−1) | |||
|---|---|---|---|---|
| OH | CH=N | C=C (Ar) | C-N | |
| 1 | 3414 | 1616 | 1558 | 1273 |
| 2 | 3417 | 1600 | 1527 | 1273 |
| 3 | - | 1624 | 1516 | 1342 |
| Schiff Base | 1H-NMR (400 MHz: DMSO-d6, δ, ppm, J in Hz) |
|---|---|
| 1 | 9.17 (s, exch., 4 H, OH), 9.08 (s, 4 H, CH), 7.93 (s, 2 H, Ar), 7.71 (d, J = 8.2 Hz, 4 H, Ar), 7.64 (d, J = 8.5 Hz, 2 H, Ar), 7.44 (t, J = 8.2 Hz, 4 H, Ar), 7.25 (d, J = 8.5 Hz, 2 H, Ar), 7.00 (t, J = 8.2 Hz, 4 H, Ar), 6.99 (d, J = 8.2 Hz, 4 H, Ar) |
| 2 | 8.08 (s, exch., 4 H, OH), 8.01 (s, 4 H, CH), 7.63 (s, 2 H, Ar), 7.53 (d, J = 8.1 Hz, 4 H, Ar), 7.48 (d, J = 8.5 Hz, 2 H, Ar), 7.48 (s, 4 H, Ar), 7.35 (d, J = 8.5 Hz, 2 H, Ar), 7.18 (t, J = 8.1 Hz, 4 H, Ar), 6.95 (d, J = 8.1 Hz, 4 H, Ar) |
| 3 | 8.61 (s, 4 H, CH), 8.32 (d, J = 8.3 Hz, 8 H, Ar), 8.05 (d, J = 8.3 Hz, 8 H, Ar), 7.70 (s, 2 H, Ar), 7.50 (d, J = 8.4 Hz, 2 H, Ar), 7.35 (d, J = 8.4 Hz, 2 H, Ar) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, D.S.; El-Hiti, G.A.; Hameed, A.S.; Yousif, E.; Ahmed, A. New Tetra-Schiff Bases as Efficient Photostabilizers for Poly(vinyl chloride). Molecules 2017, 22, 1506. https://doi.org/10.3390/molecules22091506
Ahmed DS, El-Hiti GA, Hameed AS, Yousif E, Ahmed A. New Tetra-Schiff Bases as Efficient Photostabilizers for Poly(vinyl chloride). Molecules. 2017; 22(9):1506. https://doi.org/10.3390/molecules22091506
Chicago/Turabian StyleAhmed, Dina S., Gamal A. El-Hiti, Ayad S. Hameed, Emad Yousif, and Ahmed Ahmed. 2017. "New Tetra-Schiff Bases as Efficient Photostabilizers for Poly(vinyl chloride)" Molecules 22, no. 9: 1506. https://doi.org/10.3390/molecules22091506








