Dramatic Influence of Ionic Liquid and Ultrasound Irradiation on the Electrophilic Sulfinylation of Aromatic Compounds by Sulfinic Esters
Abstract
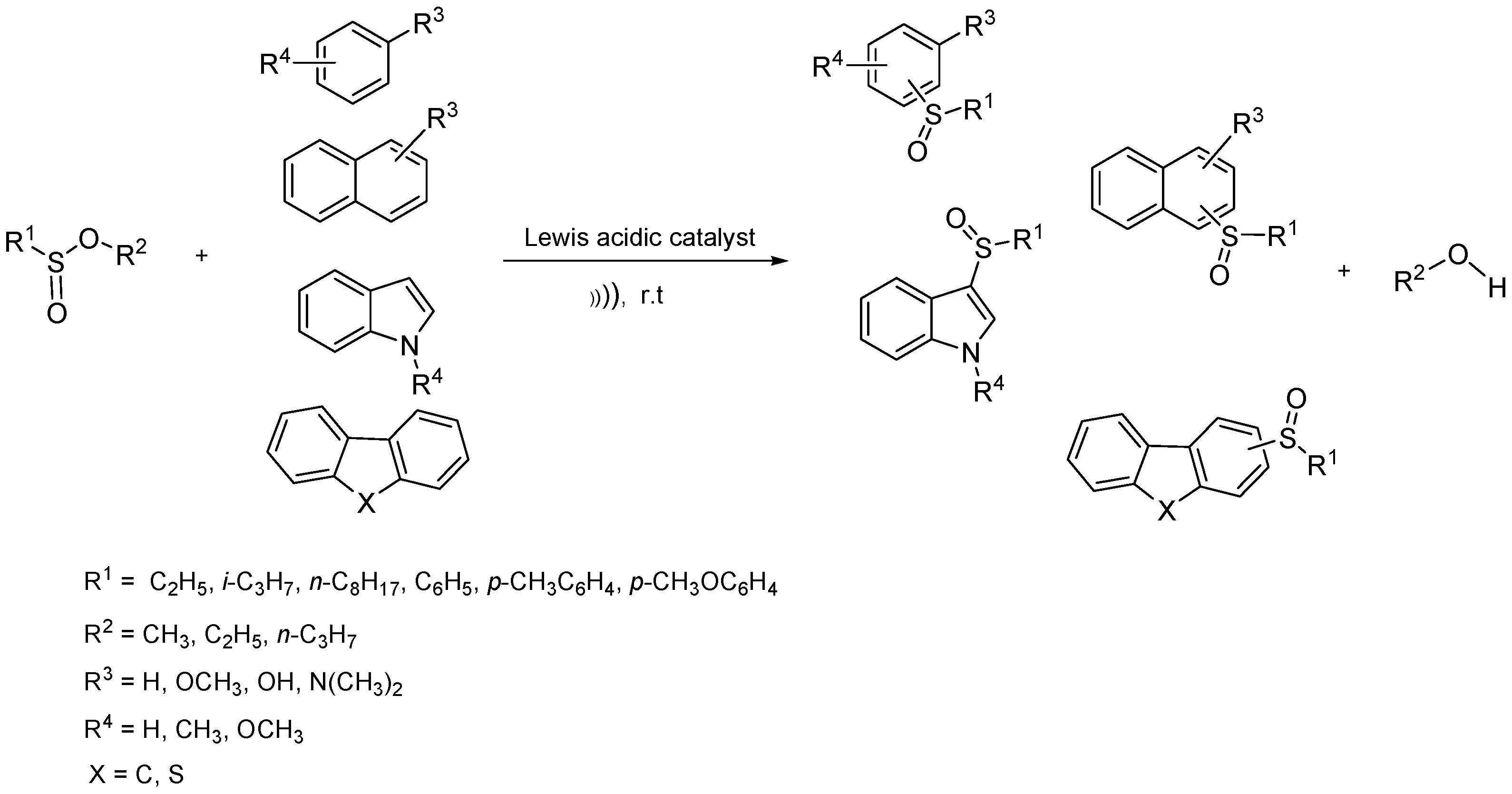
:1. Introduction
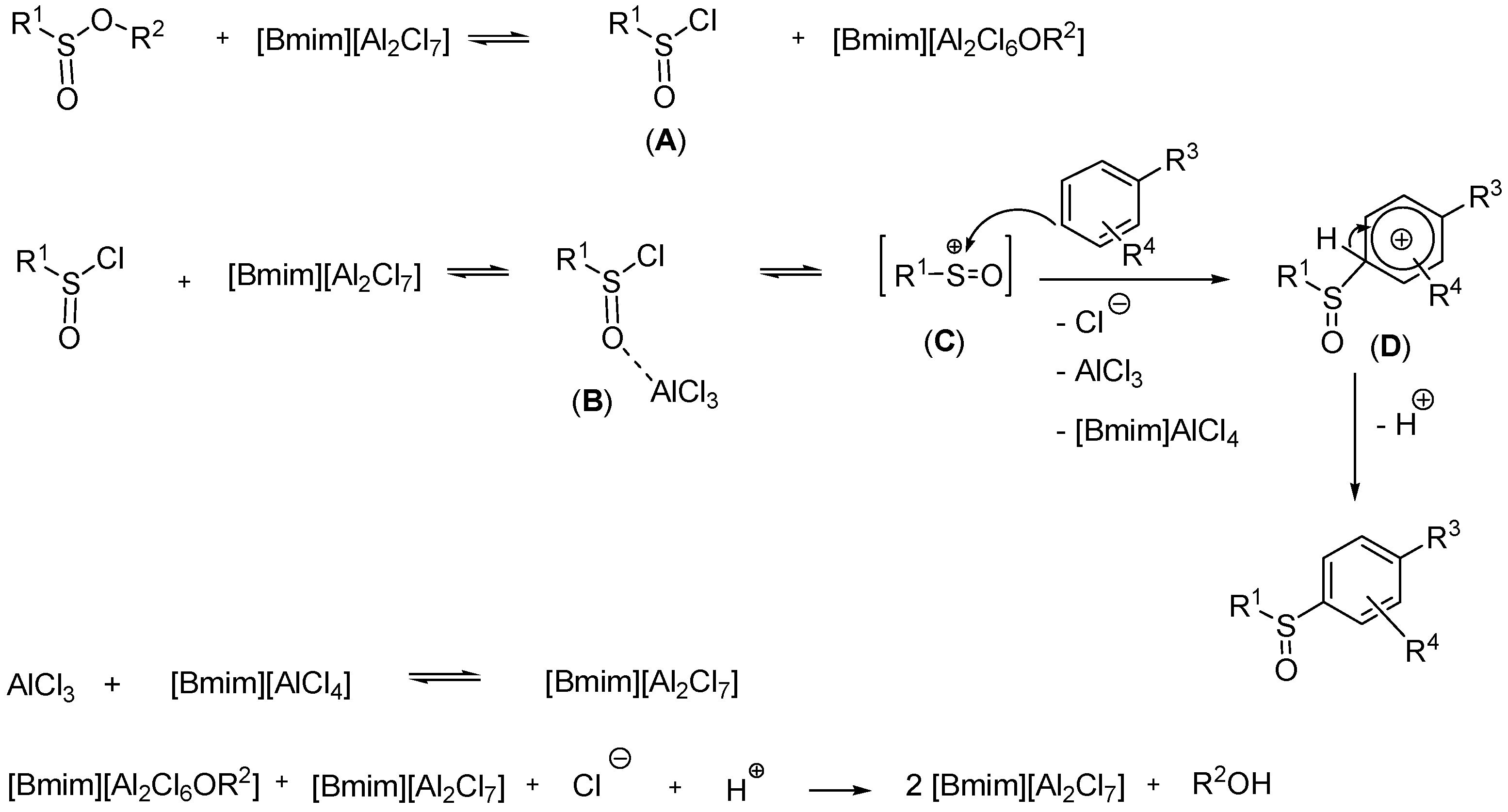
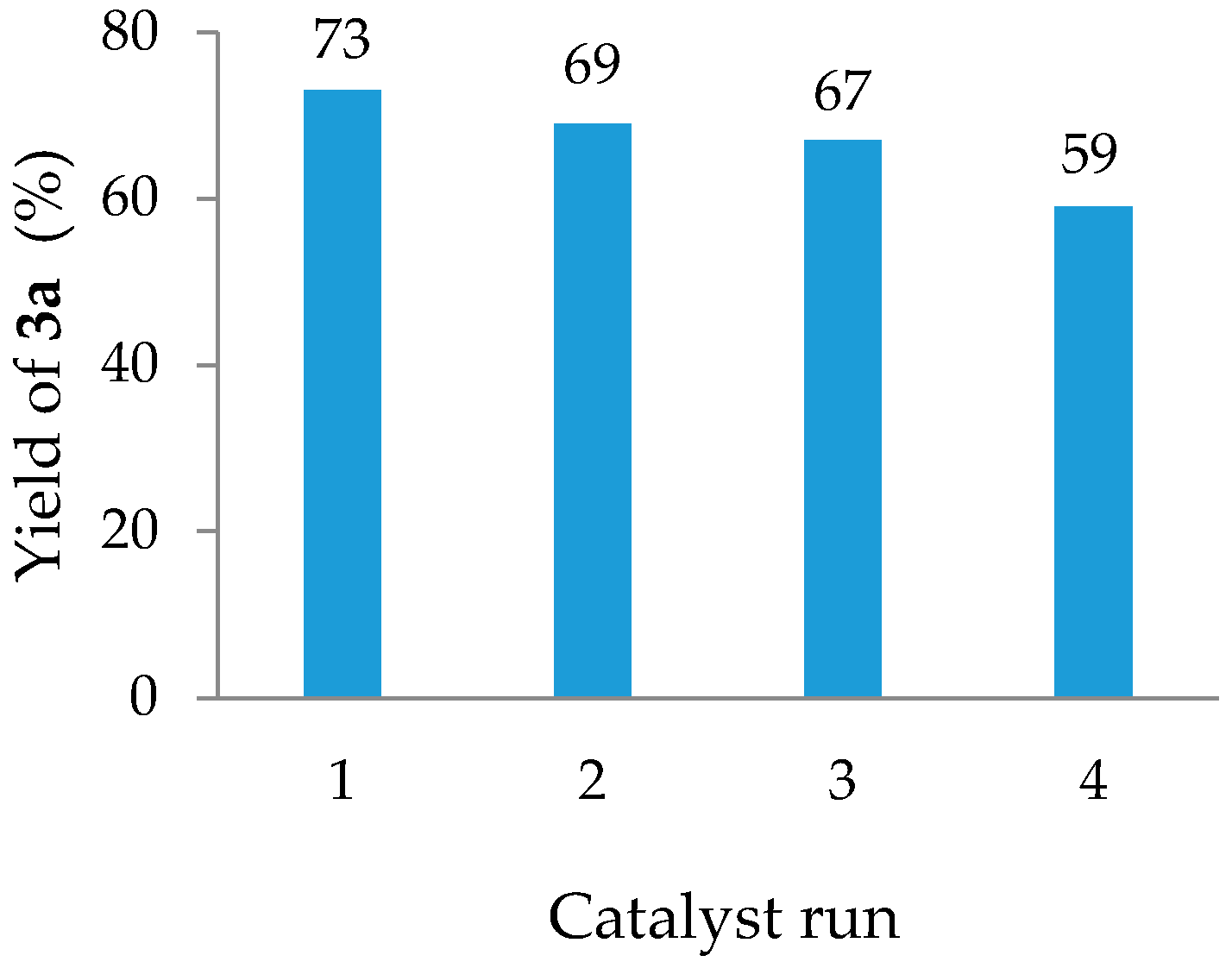
2. Results
3. Discussion
4. Materials and Methods
4.1. Instrumentation
4.2. Chemicals
4.3. General Procedure for the Sulfinylation of Aromatic Compound with Sulfinic Ester under Ultrasound Irradiation
4.4. Spectroscopic Data
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carreno, M.C. Applications of sulfoxides to asymmetric synthesis of biologically active compounds. Chem. Rev. 1995, 95, 1717–1760. [Google Scholar] [CrossRef]
- Schmied, R.; Wang, G.-X.; Korth, M. Intracellular Na+ activity and positive inotropic effect of sulmazole in guinea pig ventricular myocardium. Circ. Res. 1991, 68, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Zifko, U.A.; Rupp, M.; Schwarz, S.; Zipko, H.T.; Maida, E.M. Modafinil in treatment of fatigue in multiple sclerosis. J. Neurol. 2002, 249, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Nieves, A.V.; Lang, A.E. Treatment of excessive daytime sleepiness in patients with Parkinson’s disease with modafinil. Clin. Neuropharmacol. 2002, 25, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.; Lavin, R.C.; Durant, G.J. Asymmetric synthesis of a neuroprotective and orally active N-methyl-d-aspartate receptor ion-channel blocker, CNS 5788. Tetrahedron Asymmetry 2000, 11, 3455–3457. [Google Scholar] [CrossRef]
- McTavish, D.; Buckley, M.M.-T.; Heel, R.C. Omeprazole: An updated review of its pharmacology and therapeutic use in acid-related disorders. Drugs 1991, 42, 138–170. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.; Hawkey, C.J.; Stack, W.A. Proton pump inhibitors. Pharmacology and rationale for use in gastrointestinal disorder. Drugs 1998, 56, 307–335. [Google Scholar] [CrossRef] [PubMed]
- Spencer, C.M.; Faulds, D. Esomeprazole. Drugs 2000, 60, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Okabe, S.; Shimosako, K.; Amagase, K. Pharmacological regulation of gastric acid secretion in the apical membrane of parietal cells; a new target for antisecretory drugs. J. Physiol. Pharmacol. 2001, 52, 639–656. [Google Scholar] [PubMed]
- Baker, D.E. New drug review: Esomeprazole magnesium (Nexium). Rev. Gastrolenterol. Disord. 2001, 1, 32–41. [Google Scholar]
- Salas, M.; Ward, A.; Caro, J. Are proton pump inhibitors the first choice for acute treatment of gastric ulcers? A meta analysis of randomized clinical trials. BMC Gastroenterol. 2002, 2, 17. [Google Scholar] [CrossRef]
- Lai, K.C.; Lam, S.K.; Chu, K.M.; Wong, B.C.Y.; Hui, W.M.; Hu, W.H.C.; Lau, G.K.K.; Wong, W.M.; Yuen, M.F.; Chan, A.O.O.; et al. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N. Engl. J. Med. 2002, 346, 2033–2038. [Google Scholar] [CrossRef] [PubMed]
- Adetumbi, M.A.; Lau, B.H.S. Allium sativum (garlic)-A natural antibiotic. Med. Hypotheses 1983, 12, 227–237. [Google Scholar] [CrossRef]
- Yoshida, S.; Kasuga, S.; Hayashi, N.; Ushiroguchi, T.; Matsuura, H.; Nakagawa, S. Antifungal activity of ajoene derived from garlic. Appl. Environ. Microbiol. 1987, 53, 615–617. [Google Scholar] [PubMed]
- Focke, M.; Feld, A.; Lichtenthaler, H.K. Allicin-A naturally occurring antibiotic from garlic, specifically inhibits acetyl-CoA synthetase. FEBS Lett. 1990, 261, 106–108. [Google Scholar] [CrossRef]
- Agarwal, K.C. Therapeutic actions of garlic constituents. Med. Res. Rev. 1996, 16, 111–124. [Google Scholar] [CrossRef]
- Pérez-Giraldo, C.; Cruz-Villalón, G.; Sánchez-Silos, R.; Martínez-Rubio, R.; Blanco, M.T.; Gómez-García, A.C. In vitro activity of allicin against Staphylococcus epidermidis and influence of subinhibitory concentrations on biofilm formation. J. Appl. Microbiol. 2003, 95, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Kotelanski, B.; Grozmann, R.J.; Cohn, J.N. Positive inotropic effect of oral esproquin in normal subjects. Clin. Pharmacol. Ther. 1973, 14, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.; Mitka, K.; Ossowska, K.; Kolarska, Z. Oxidation of sulfides to sulfoxides. Part 1: Oxidation using halogen derivatives. Tetrahedron 2005, 61, 1933–1953. [Google Scholar] [CrossRef]
- Kaczorowska, K.; Kolarska, Z.; Mitka, K.; Kowalski, P. Oxidation of sulfides to sulfoxides. Part 2: Oxidation by hydrogen peroxide. Tetrahedron 2005, 61, 8315–8327. [Google Scholar] [CrossRef]
- Still, I.W.J.; Ablenas, F.J. Conversion of sulfones to sulfoxides via hydride reduction of (aryloxy)sulfoxonium salt intermediates. J. Org. Chem. 1983, 48, 1617–1620. [Google Scholar] [CrossRef]
- Andersen, K.K. Synthesis of (+)-ethyl p-tolyl sulfoxide from (−)-menthyl (−)-p-toluenesulfinate. Tetrahedron Lett. 1962, 3, 93–95. [Google Scholar] [CrossRef]
- Andersen, K.K.; Gaffield, W.; Papanikolaou, N.E.; Foley, J.W.; Perkins, R.I. Optically active sulfoxides. The synthesis and rotatory dispersion of some diaryl sulfoxides. J. Am. Chem. Soc. 1964, 86, 5637–5646. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Cardellicchio, C.; Naso, F. Enantioselective routes to sulfoxides based upon the use of carbanionic leaving groups. Eur. J. Org. Chem. 2004, 1855–1865. [Google Scholar] [CrossRef]
- Mohile, S.S.; Potdar, M.K.; Salunkhe, M.M. An ionic liquid-mediated expeditious route to the syntheses of diaryl sulfoxides. Tetrahedron Lett. 2003, 44, 1255–1258. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Rao, R.S.; Kumar, S.P.; Nagaiah, K. Scandium triflate catalyzed one-pot synthesis of diaryl sulfoxides. Synlett 2002, 5, 784–786. [Google Scholar] [CrossRef]
- Schöberl, A.; Wagner, A. Herstellung und Umwandlungvon Sulfoxydenund Sulfiniminen. In Houben-Weyl, 4th ed.; Müller, E., Ed.; Thieme: Stuggart, Germany, 1955; p. 217. [Google Scholar]
- Replogle, L.L.; Maynard, J.R. Organosulfur derivatives of azulene. II. 1-Azulyl sulfoxides and sulfones. J. Org. Chem. 1967, 32, 1909–1915. [Google Scholar] [CrossRef]
- Douglass, I.B.; Farah, B.S. Some reactions of methanesulfinyl chloride. J. Org. Chem. 1958, 23, 805–807. [Google Scholar] [CrossRef]
- Fujisawa, T.; Kakutani, M.; Kobayashi, N. On the reaction of p-toluenesulfinyl chloride with anisole. Bull. Chem. Soc. Jpn. 1973, 46, 3615–3617. [Google Scholar] [CrossRef]
- Olah, G.A.; Nishimura, J. Aromatic substitution. XXXII. Aluminum chloride catalyzed arenesulfinylation of benzene and toluene with benzenesulfinyl and substituted benzenesulfinyl chlorides in nitromethane solution. J. Org. Chem. 1974, 39, 1203–1205. [Google Scholar] [CrossRef]
- Yuste, F.; Linares, A.H.; Mastranzo, V.M.; Ortiz, B.; Sánchez-Obregón, R.; Fraile, A.; Ruano, J.L.G. Methyl sulfinates as electrophiles in Friedel-Crafts reactions. Synthesis of aryl sulfoxides. J. Org. Chem. 2011, 76, 4635–4644. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.E.; Lazarova, T.I. Preparation of (phenylsulfinyl)phenols from aryl phenylsulfinates: “Thia-Fries rearrangement”. Tetrahedron Lett. 1996, 37, 7–8. [Google Scholar] [CrossRef]
- Sugden, S.; Wilkins, H. CLXVII.-The parachor and chemical constitution. Part XII. Fused metals and salts. J. Chem. Soc. 1929, 1291–1298. [Google Scholar] [CrossRef]
- Wassercheid, P.; Keim, W. Ionic liquid-New “solutions” for transition metal catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Welton, I. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Boon, J.A.; Levinsky, J.A.; Pflug, J.I.; Wilkes, J.S. Friedel-Crafts reactions in ambient-temperature molten salts. J. Org. Chem. 1986, 51, 480–483. [Google Scholar] [CrossRef]
- Mason, T.J.; Lorimer, J.P. Sonochemistry: Theory, Applications and Uses of Ultrasound in Chemistry; Ellis Horwood: West Sussex, UK, 1988; pp. 74–98. [Google Scholar]
- Luche, J.-L. Synthetic Organic Sonochemistry; Plemum Press: New York, NY, USA, 1998; pp. 246–263. [Google Scholar]
- Seddon, K.R.J. Ionic liquids for clean technology. J. Chem. Technol. Biotechnol. 1997, 68, 351–356. [Google Scholar] [CrossRef]
- Tillett, J.G. Nucleophilic substitution at tricoordinate sulfur. Chem. Rev. 1976, 76, 747–772. [Google Scholar] [CrossRef]
- Nara, S.J.; Harjani, J.R.; Salunkhe, M.M. Friedel-Crafts sulfonylation in 1-butyl-3-methylimidazolium chloroaluminate ionic liquids. J. Org. Chem. 2001, 66, 8616–8620. [Google Scholar] [CrossRef] [PubMed]
- Miao, T.; Li, P.; Zhang, Y.; Wang, L. A sulfenylation reaction: Direct synthesis of 3-arylsulfinylindoles from arylsulfinic acids and indoles in water. Org. Lett. 2015, 17, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-A.T.; Le, N.T.; Chau, T.-P.; Duus, F.; Luu, T.X.T. “One pot” synthesis of sulfinic esters from ultrasound irradiation accelerated tanderm reaction of thiols and alcohols with N-Bromosuccinimide. Manuscript in Preparation.
- Furukawa, N.; Ogawa, S.; Matsumara, K.; Fujihara, H. Extremely facile ligand-exchange and disproportionation reactions of diaryl sulfoxides, selenoxides, and triarylphosphine oxides with organolithium and Grignard reagents. J. Org. Chem. 1991, 56, 6341–6348. [Google Scholar] [CrossRef]
- Oae, S.; Yamada, O.; Maeda, T. The reaction of sulfinic acids with N,N-dimethylaniline. Bull. Chem. Soc. Jpn. 1974, 47, 166–169. [Google Scholar] [CrossRef]
- Bell, K.H.; McCaffery, L.F. Use of menthyl 2-methoxynaphthalene1–1-sulfinates in the Andersen synthesis of optically active sulfoxides. Facile cleavage by Grignard reagents of some aromatic methyl ethers. Aust. J. Chem. 1994, 47, 1925. [Google Scholar] [CrossRef]
- Garcia, J.; Ortiz, C.; Greenhouse, R. The regiospecific Pummerer-like introduction of chlorine atoms into pyrrol-3-yl and indol-3-yl sulfoxides. J. Org. Chem. 1988, 53, 2634–2637. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

| Entry | Acidic Catalyst (mmol) | Yield b (%) |
|---|---|---|
| 1 | Bi(OTf)3(0.05), solvent-free | 3 |
| 2 | La(OTf)3(0.05), solvent-free | - |
| 3 | Cu(OTf)2(0.05), solvent-free | - |
| 4 | AlCl3(2.0), solvent-free | 16 |
| 5 | AlCl3(2.0) in 1,2-dichloroethane (1 mL) | 7 |
| 6 | [(HSO3)4C4C1Im]HSO4(1.0) | 6 |
| 7 | [Choline]Cl·3ZnCl2 (1.0) | - |
| 8 | [Bmim]Cl·2ZnCl2 (1.0) | 3 |
| 9 | [Bmim]Cl·2ZnCl2 (1.2) | 4 |
| 10 | [Bmim]Cl·2NiCl2(1.0) | - |
| 11 | [Bmim]Cl·p-TSA (2.0) | - |
| 12 | [Bmim]Cl·AlCl3(1.0) | 32 |
| 13 | [Bmim]Cl·2AlCl3(1.0) | 55 |
| 14 | [Bmim]Cl·3AlCl3(1.0) | 25 |
| 15 | [Bmim]Cl·2AlCl3(1.1) | 69 |
| 16 | [Bmim]Cl·2AlCl3(1.2) | 72 |
| 17 | [Bmim]Cl·2AlCl3(1.5) | 68 |

| Entry | Sulfinic Ester | Product | Time (h) | Yield b (%) | |
|---|---|---|---|---|---|
| 1 |  |  | 3a | 1.0 1.5 | 72 73 |
| 2 |  | 1.0 | 72 | ||
| 3 |  | 1.0 | 77 | ||
| 4 |  |  | 3b | 1.0 4.0 | 15 70 |
| 5 |  |  | 3c | 1.0 4.5 | 10 68 |
| 6 |  |  | 3d | 1.0 4.0 | 17 72 |
| 7 |  |  | 3e | 1.0 | 73 |
| 8 |  |  | 3f | 1.0 | 75 |
| Entry | Arene | Product | Time (h) | Yield b (%) | |
|---|---|---|---|---|---|
| 1 |  |  | 3g | 1.0 | 61 |
| 2 |  |  | 3h | 1.0 | 68 |
| 3 |  |  |  | 1.0 | 75 |
| 4 |  |  | 3j | 1.0 | 81 |
| 5 |  |  | 3k | 2.0 | 58 |
| 6 |  |  | 3l | 3.0 | 53 |
| 7 |  |  | 3m | 0.5 | 73 |
| 8 |  |  | 3n | 0.5 | 40 |
| 9 |  |  | 3p | 8 | 13 |
| 10 |  |  | 3q | 8 | 6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.-L.T.; Vo, H.-T.; Duus, F.; Luu, T.X.T. Dramatic Influence of Ionic Liquid and Ultrasound Irradiation on the Electrophilic Sulfinylation of Aromatic Compounds by Sulfinic Esters. Molecules 2017, 22, 1458. https://doi.org/10.3390/molecules22091458
Nguyen N-LT, Vo H-T, Duus F, Luu TXT. Dramatic Influence of Ionic Liquid and Ultrasound Irradiation on the Electrophilic Sulfinylation of Aromatic Compounds by Sulfinic Esters. Molecules. 2017; 22(9):1458. https://doi.org/10.3390/molecules22091458
Chicago/Turabian StyleNguyen, Ngoc-Lan Thi, Hong-Thom Vo, Fritz Duus, and Thi Xuan Thi Luu. 2017. "Dramatic Influence of Ionic Liquid and Ultrasound Irradiation on the Electrophilic Sulfinylation of Aromatic Compounds by Sulfinic Esters" Molecules 22, no. 9: 1458. https://doi.org/10.3390/molecules22091458




