Dihydrochalcones and Diterpenoids from Pteris ensiformis and Their Bioactivities
Abstract
:1. Introduction
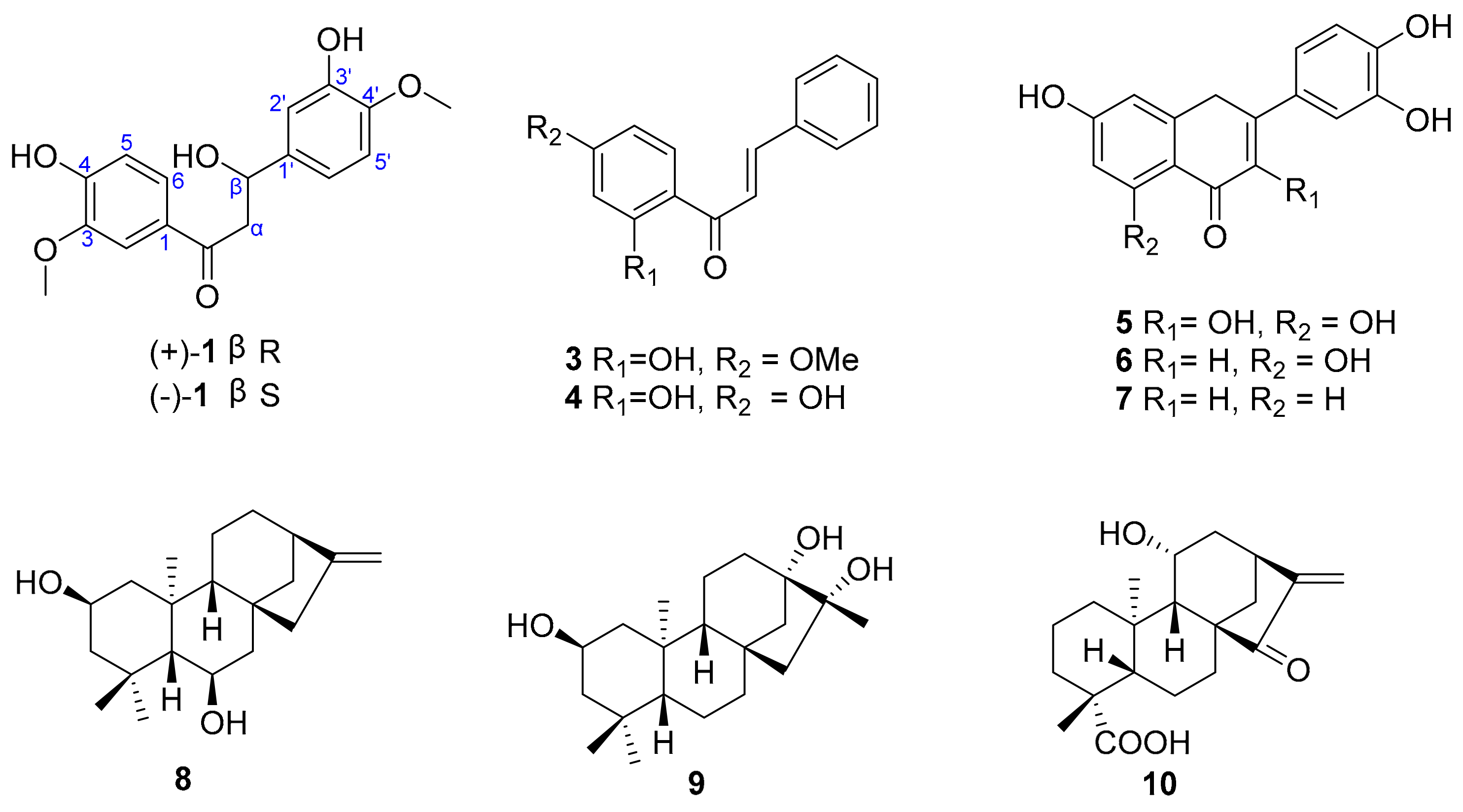
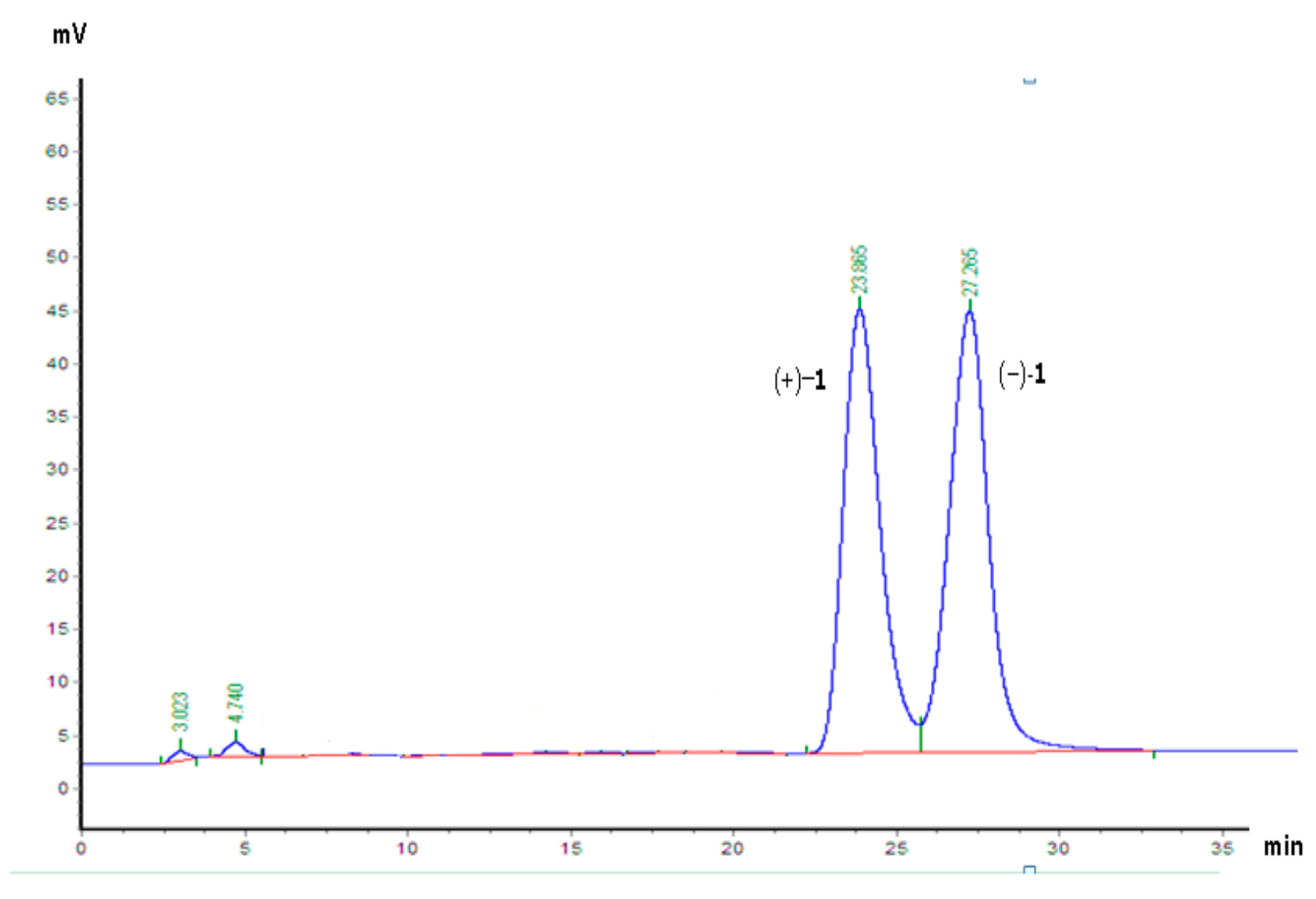
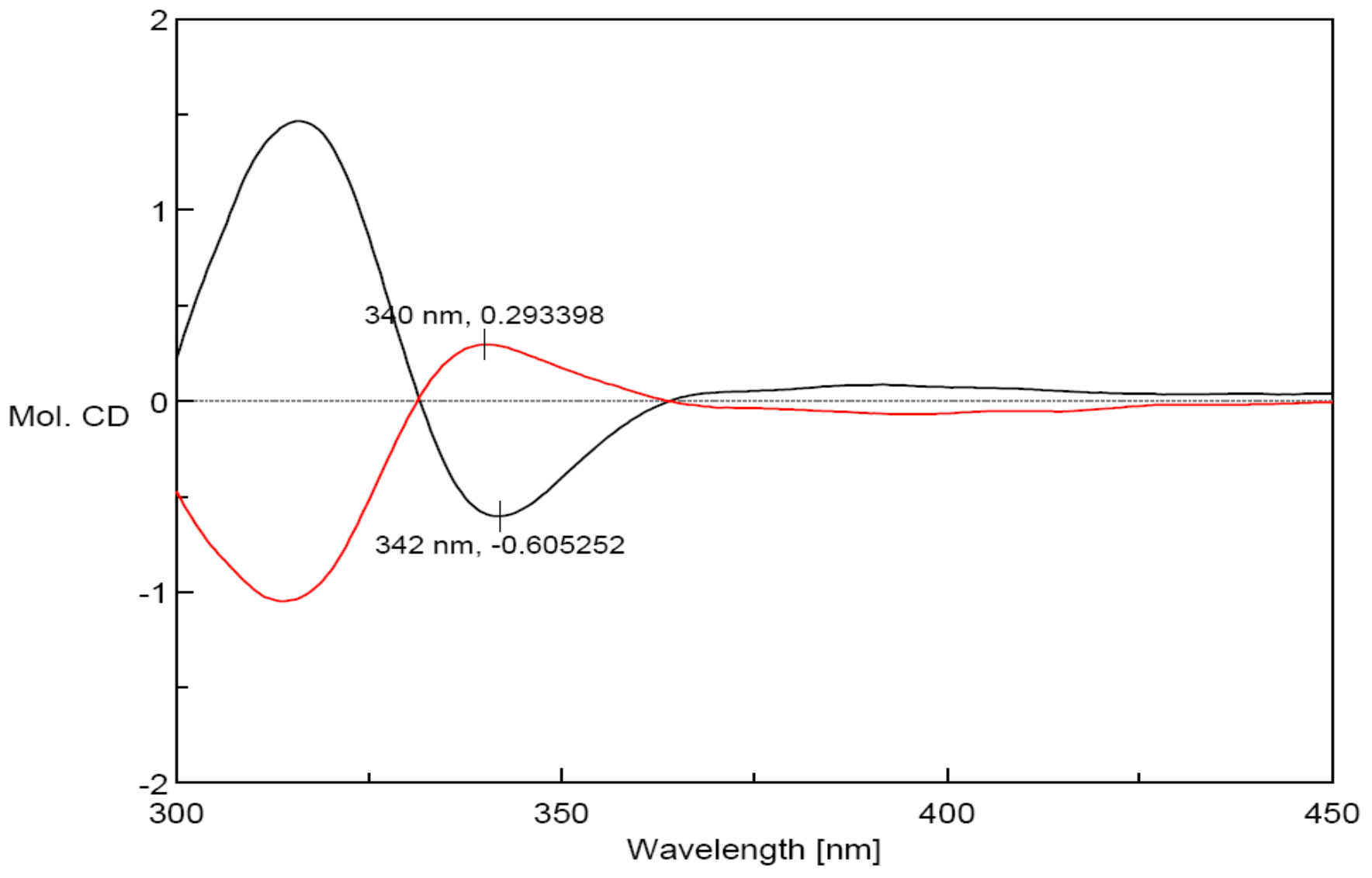
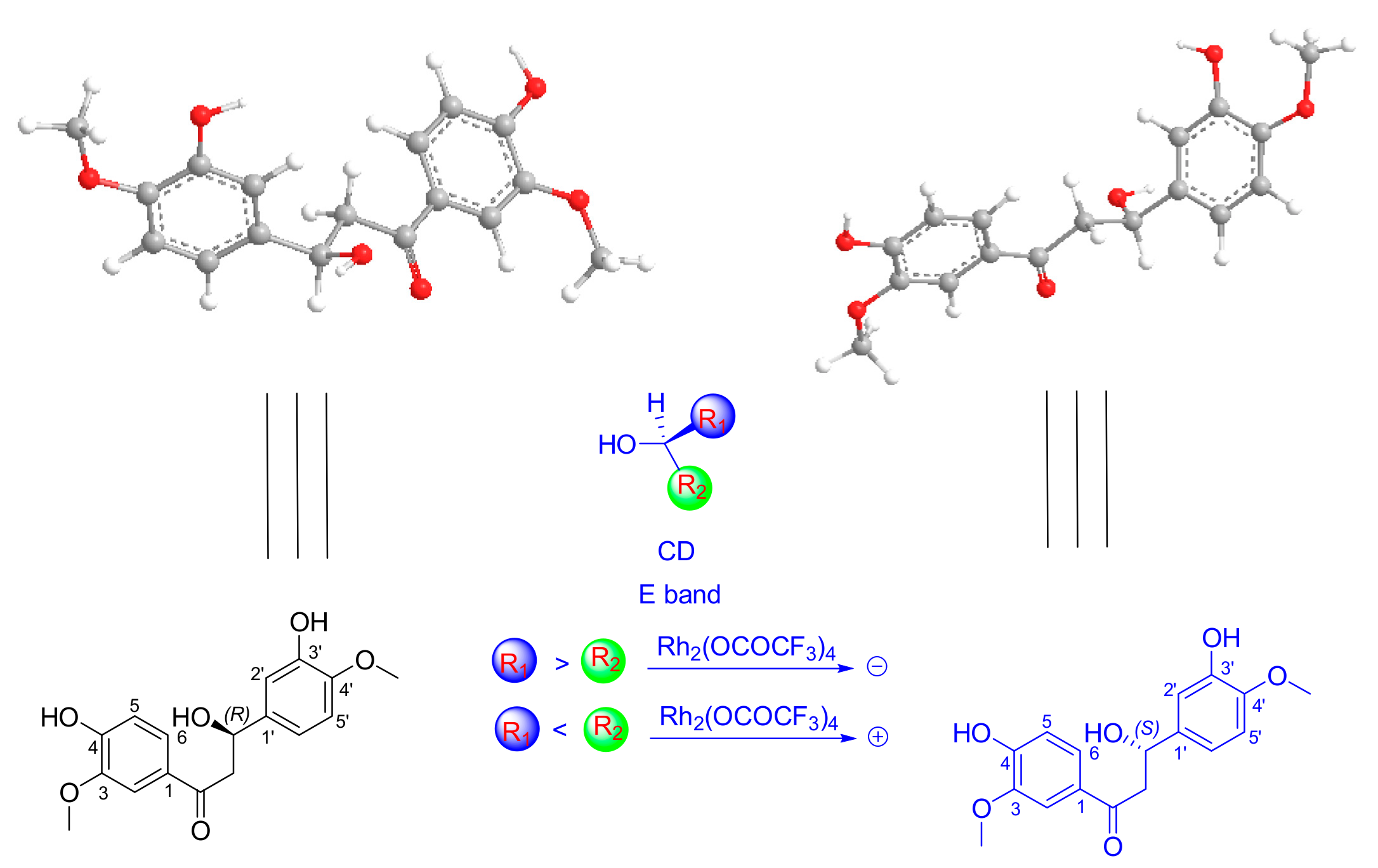
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Cytotoxic Activity Assays
3.5. Inhibitory Assay of NO Production
3.6. Cytotoxicity Assays
3.7. In Vivo Anti-Inflammatory Assays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Editorial Committee of Flora of China, CAS. Flora Republicae Popularis Sinicae; Science Press: Beijing, China, 1990. [Google Scholar]
- Gong, X.L.; Chen, Z.H.; Liang, N.C. Advances in study on chemical constituents and pharmacological activities of plants of genus pteris. Zhongguo Zhong Yao Za Zhi 2007, 32, 1382–1387. [Google Scholar] [PubMed]
- Chen, Y.H.; Chang, F.R.; Lin, Y.J.; Wang, L.; Chen, J.F.; Wu, Y.C.; Wu, M.J. Identification of phenolic antioxidants from Sword Brake fern (Pteris ensiformis Burm.). Food Chem. 2007, 105, 48–56. [Google Scholar] [CrossRef]
- Bai, R.; Zhou, Y.; Deng, S.; Dong, P.P.; Zhang, B.; Huang, S.; Wang, C.; Zhang, H.L.; Zhao, Y.Y.; Wang, L.; et al. Two new ent-kaurane diterpenoids from Pteris semipinnata. J. Asian Nat. Prod. Res. 2013, 15, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Yang, B.; Cao, D.; Zhu, J.; Jin, J.; Chen, Y.; Zhou, L.; Luo, X.; Zhao, Z. Two new hydroxylated ent-kauranoic acids from Pteris semipinnata. Phytochem. Lett. 2016, 16, 156–162. [Google Scholar] [CrossRef]
- Wang, L.; Li, D.; Wang, C.; Zhang, Y.; Xu, J. Recent progress in the development of natural ent-kaurane diterpenoids with anti-tumor activity. Mini Rev. Med. Chem. 2016, 11, 910–919. [Google Scholar] [CrossRef]
- Harinantenaina, L.; Matsunami, K.; Otsuka, H. Chemical and biologically active constituents of Pteris multifida. J. Nat. Med. 2008, 62, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.C.; Ti, M.C.; Lo, S.C.; Yang, C.C. Free radical-scavenging activity of aqueous extract of Pteris multifida Poiret. Fitoterapia 2007, 78, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.S.; Zhang, Y.; Hu, W.Z.; Zhang, L.H.; Chen, X.; Zhang, N.; Li, G.; Tan, L.Y. Cytotoxic diterpenoids from Pteris ensiformis. J. Asian Nat. Prod. Res. 2017, 19, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Frelek, J.; Szczepek, W.J. [Rh2(OCOCF3)4] as an auxiliary chromophore in chiroptical studies on steroidal alcohols. Tetrahedron Asymmetry 1999, 10, 1507–1520. [Google Scholar] [CrossRef]
- Teshima, T.; Takeishi, M.; Arai, T. Red fluorescence from tautomers of 2′-hydroxychalcones induced by intra-molecular hydrogen atom transfer. New J. Chem. 2009, 33, 1393–1401. [Google Scholar] [CrossRef]
- Wollenweber, E.; Seigler, D.S. Flavonoids from the exudate of Acacia neovernicosa. Phytochemistry 1982, 21, 1063–1066. [Google Scholar] [CrossRef]
- Luca, C.; Giulio, R.; Pratima, B.; Anna, I.; Mariagrazia, S.; Luciano, A.; Umberto, M.A. 1-Benzopyran-4-one Antioxidants as Aldose Reductase Inhibitors. J. Med. Chem. 1999, 42, 1881–1893. [Google Scholar]
- Ni, G.; Fu, N.J.; Zhang, D.; Yang, H.Z.; Chen, X.G.; Yu, D.Q. An unusual dihydrobenzofuroisocoumarin and ent-kaurane diterpenoids from Pteris multifida. J. Asian Nat. Prod. Res. 2015, 17, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Li, W.F.; Wang, J.; Zhang, J.J.; Song, X.; Ku, C.F.; Zou, J.; Li, J.X.; Rong, L.J.; Pan, L.T.; Zhang, H.J.; et al. Henrin A: A new anti-HIV ent-kaurane diterpene from Pteris henryi. Int. J. Mol. Sci. 2015, 16, 27978–27987. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Shen, Y.; Yang, J.; He, J.; Zhao, Y.; Peng, L.Y.; Leng, Y.; Zhao, Q.S. Discovery and structure-activity relation-ships of ent-Kaurene diterpenoids as potent and selective 11β-HSD1 inhibitors: Potential impact in diabetes. Eur. J. Med. Chem. 2013, 65, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, C.; Dong, B.; Ma, X.; Hou, L.; Cao, X.; Wang, C. Anti-inflammatory activity and mechanism of surfactin in lipopolysaccharide-activated macrophages. Inflammation 2015, 38, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Garcia, A.T.; Parra, D.H.; Velazquez, G.; Martinez, V.M. Anti-inflammatory activity of coumarins from Decatropis bicolor on TPA ear mice model (letter). Planta Med. 2000, 66, 279–281. [Google Scholar] [CrossRef]
- Sacco, R.E.; Waters, W.R.; Rudolph, K.M.; Drew, M.L. Comparative nitric oxide production by LPS-stimulated monocyte-derived macrophages from Ovis canadensis and Ovis aries. Comp. Immunol. Microb. 2006, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.S.; Zhang, Y.; Hu, W.Z.; Chen, X.; Fu, X.; Lv, X.; Zhang, L.H.; Zhang, N.; Li, G. Anti-inflammatory Triterpene Glycosides from the Radix of Ilex dunniana Levl. Molecules 2017, 22, 1206. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–10 are available from the authors. |
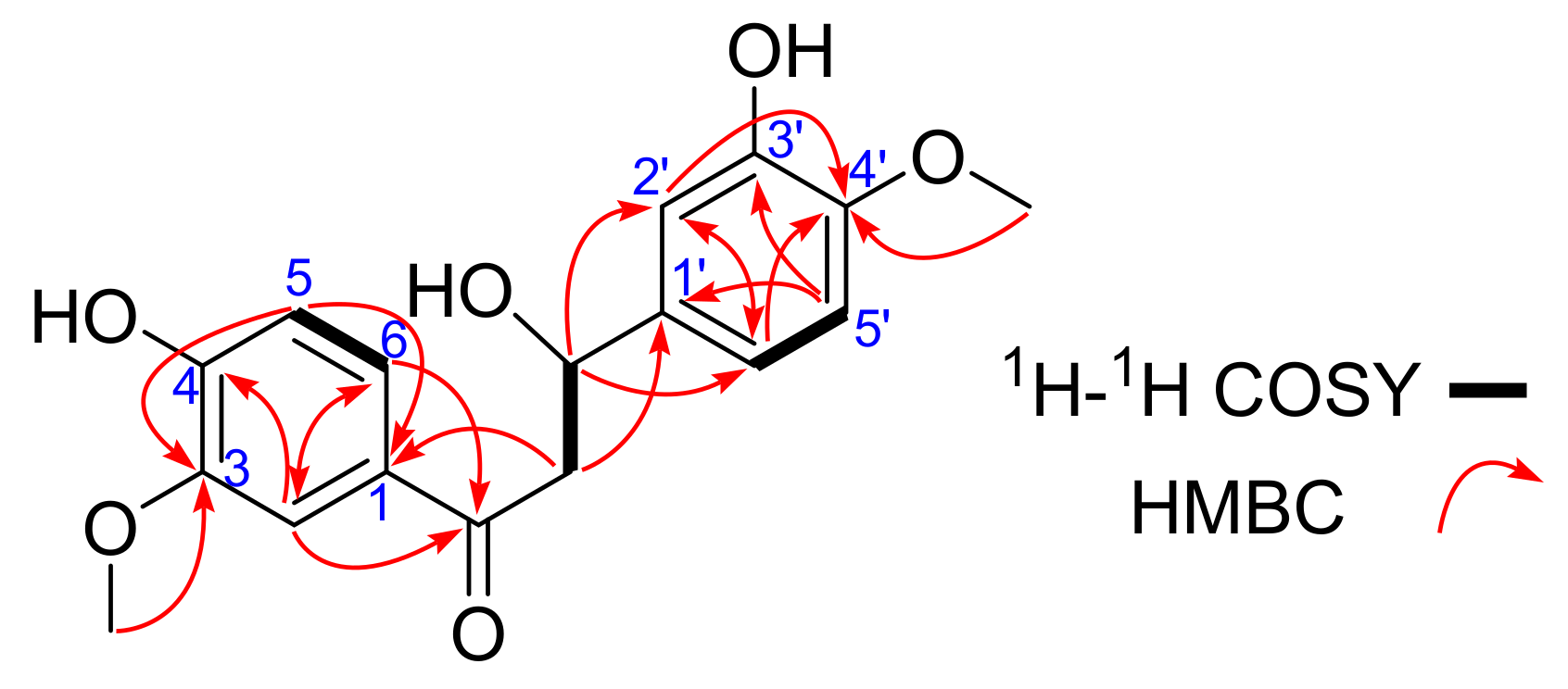
| Position | δC | δH (J in Hz) | HMBC |
|---|---|---|---|
| 1 | 130.3 | ||
| 2 | 112.5 | 7.56, d, 2.0 | C-1, C-3, C-4, C-6, C=O |
| 3 | 149.0 | C-1, C-2, C-4, C-5 | |
| 4 | 153.3 | ||
| 5 | 115.7 | 6.80, d, 8.5 | C-1, C-3, C-4, C-6 |
| 6 | 125.2 | 7.62, dd, 8.5, 2.0 | C-1, C-2, C-4, C-5 |
| C=O | 199.6 | ||
| α | 65.5 | 4.26, dd, 11.0, 8.5, 3.71, dd, 11.0, 5.5 | C-1, C-1′, C=O, C-β |
| β | 56.3 | 4.76, dd, 8.5, 5.0 | C-1′,C-2′, C-6′, C=O, C-α |
| 1′ | 129.9 | C-α, C-β, C-2′, C-3′, C-5′, C-6′ | |
| 2′ | 112.7 | 6.89, d, 2.0 | C-β, C-1′,C-3′, C-4′, C-6′ |
| 3′ | 149.3 | ||
| 4′ | 147.0 | ||
| 5′ | 116.6 | 6.73, d, 8.5 | C-1′, C-3′, C-4′, C-6′ |
| 6′ | 122.2 | 6.76, dd, 8.5, 2.0 | C-β, C-1′,C-2′, C-4′, C-5′ |
| 3-OCH3 | 56.3 | 3.86, s | C-3 |
| 4′-OCH3 | 56.4 | 3.82, s | C-4′ |
| Extracts | Dose (mg/kg) | Edema Degree (mg) | Inhibitation Rate (%) |
|---|---|---|---|
| 95% Ethanol extract | 200.0 | 13.89 ± 3.25 * | 38.6 |
| polyamide resin at 30% ethanol extract | 200.0 | 9.89 ± 1.33 ** | 56.3 |
| Dexamethasone a | 1.0 | 6.61 ± 0.89 ** | 70.8 |
| Control group | - | 22.63 ± 3.16 | - |
| Compounds | IC50 (μM) | Compounds | IC50 (μM) |
|---|---|---|---|
| (+)-1 | 2.0 ± 0.21 | 7 | >50 |
| (−)-1 | 2.5 ± 0.82 | 8 | 8.0 ± 0.98 |
| 3 | >50 | 9 | 9.5 ± 0.75 |
| 4 | >50 | 10 | 5.6 ± 0.69 |
| 5 | >50 | dexamethasone a | 0.025 ± 0.37 |
| 6 | >50 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.-S.; Zhang, Y.; Hu, W.-Z.; Zhang, X.-F.; Fu, X.; Lv, X. Dihydrochalcones and Diterpenoids from Pteris ensiformis and Their Bioactivities. Molecules 2017, 22, 1413. https://doi.org/10.3390/molecules22091413
Shi Y-S, Zhang Y, Hu W-Z, Zhang X-F, Fu X, Lv X. Dihydrochalcones and Diterpenoids from Pteris ensiformis and Their Bioactivities. Molecules. 2017; 22(9):1413. https://doi.org/10.3390/molecules22091413
Chicago/Turabian StyleShi, Yu-Sheng, Yan Zhang, Wen-Zhong Hu, Xiu-Fu Zhang, Xin Fu, and Xia Lv. 2017. "Dihydrochalcones and Diterpenoids from Pteris ensiformis and Their Bioactivities" Molecules 22, no. 9: 1413. https://doi.org/10.3390/molecules22091413





