Sigma Receptor (σR) Ligands with Antiproliferative and Anticancer Activity
Abstract
:1. Introduction
2. Sigma Receptors (σRs)
3. Structure Affinity Relationship of Sigma Receptors Modulators
3.1. σ-1 Selective Ligands
3.1.1. Gilligan Model
3.1.2. Glennon/Ablordeppey Model
3.2. σ-2 Selective Ligands
3.2.1. Conformationally Restricted Amine Derivatives
3.2.2. Siramesine-Related Indole Analogs
3.2.3. Conformationally Flexible Amine Derivatives
4. σ-Receptor (σR) Ligands in Cancer Research
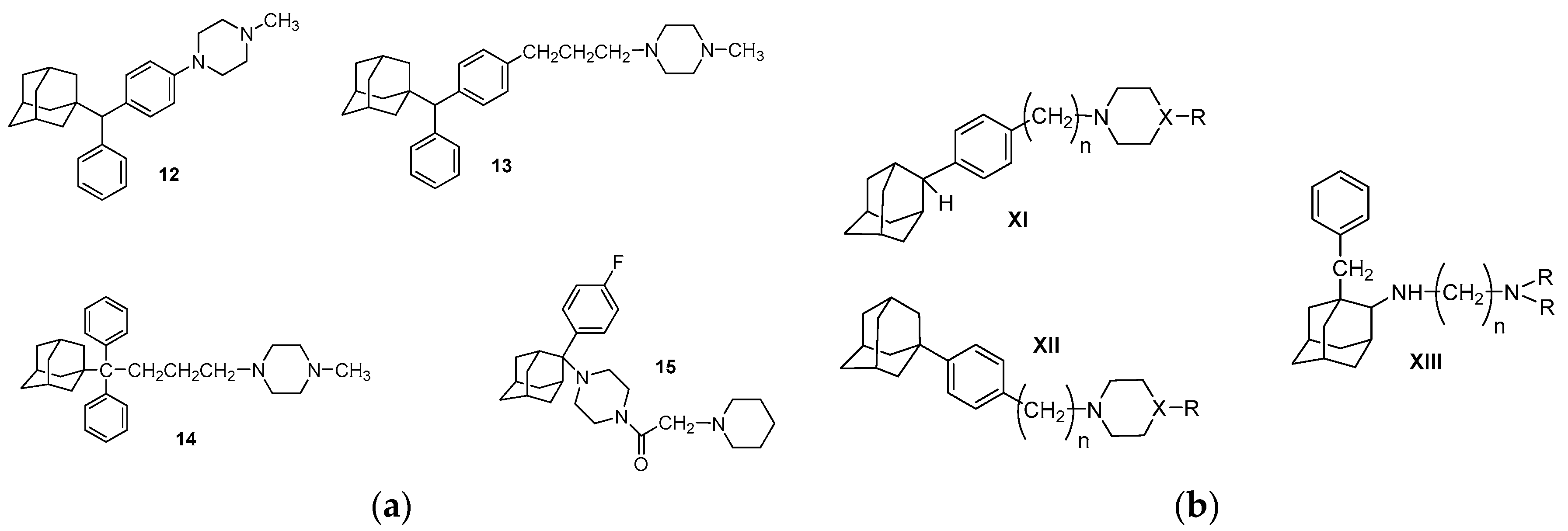
5. Adamantane Derivatives with σR Binding Affinity, Antiproliferative and Anticancer Activity
6. Conclusions
Conflicts of Interest
References
- Martin, W.R.; Eades, C.G.; Thompson, J.A.; Huppler, R.E.; Gilbert, P.E. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976, 197, 517–532. [Google Scholar] [PubMed]
- Matsumoto, R.R. Sigma Receptors: Historical perspective and background. In Sigma Receptors: Chemistry, Cell Biology and Clinical Implications; Matsumoto, R.R., Bowen, W.D., Su, T.-P., Eds.; Springer: New York, NY, USA, 2007; pp. 1–23. [Google Scholar]
- Collier, T.L.; Waterhouse, R.N.; Kassiou, M. Imaging sigma receptors: Applications in drug development. Curr. Pharm. Des. 2007, 13, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Su, T.-P.; Hayashi, T. Understanding the molecular mechanism of sigma-1 receptors: Towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr. Med. Chem. 2003, 10, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Bowen, D.W. Sigma receptors: Recent advances and new clinical potentials. Pharm. Acta Helv. 2000, 74, 211–218. [Google Scholar] [CrossRef]
- Hanner, M.; Moebius, F.; Flandorfer, A.; Knaus, H.G.; Striessnig, J.; Kempner, E.; Glossmann, H. Purification, molecular cloning, and expression of the mammalian sigma 1-binding site. Proc. Natl. Acad. Sci. USA 1996, 93, 8072–8077. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.X.; Mei, J.; Xu, J.; Wan, B.-L.; Zuckerman, A.; Pasternak, G.W. Cloning and characterization of a mouse sigma1 receptor. J. Neurochem. 1998, 70, 2279–2285. [Google Scholar] [CrossRef] [PubMed]
- Seth, P.; Fei, Y.J.; Li, H.W.; Leibach, F.H.; Ganapathy, V. Cloning and functional characterization of a sigma receptor from rat brain. J. Neurochem. 1998, 70, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Seth, P.; Leibach, F.H.; Ganapathy, V. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem. Biophys. Res. Commun. 1997, 241, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Fontanilla, D.; Johannessen, M.; Hajipour, A.; Cozzi, N.V.; Jackson, M.B.; Ruoho, A.E. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science 2009, 323, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Su, T.P.; Hayashi, T.; Vaupel, D.B. When the endogenous hallucinogenic trace amine N,N-dimethyltryptamine meets the sigma-1 receptor. Sci. Signal. 2009, 2. [Google Scholar] [CrossRef] [PubMed]
- Van Waarde, A.; Rybczynska, A.A.; Ramakrishnan, N.K.; Ishiwata, K.; Elsinga, P.H.; Dierckx, R.A. Potential applications for sigma receptor ligands in cancer diagnosis and therapy. Biochim. Biophys. Acta 2015, 1848, 2703–2714. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.; Hellewell, S.; McGarry, K. Evidence for a multi-site model of the rat brain sigma receptor. Eur. J. Pharmacol. 1989, 163, 309–318. [Google Scholar] [CrossRef]
- Kitaichi, K.; Chabot, J.G.; Moebius, F.F.; Flandorfer, A.; Glossmann, H.; Quirion, R. Expression of the purported sigma1 (σ1) receptor in the mammalian brain and its possible relevance in deficits induced by antagonism of the NMDA receptor complex as revealed using an antisense strategy. J. Chem. Neuroanat. 2000, 20, 375–387. [Google Scholar] [CrossRef]
- Skuza, G.; Rogoz, Z. The synergistic effect of selective sigma receptor agonists and uncompetitive NMDA receptor antagonists in the forced swim test in rats. J. Physiol. Pharmacol. 2006, 57, 217–229. [Google Scholar] [PubMed]
- Yang, S.; Bhardwaj, A.; Cheng, J.; Alkayed, N.J.; Hurn, P.D.; Kirsch, J.R. Sigma receptor agonists provide neuroprotection in vitro by preserving bcl-2. Anesth. Analg. 2007, 104, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Alon, A.; Schmidt, H.R.; Wood, M.D.; Sahn, J.J.; Martin, S.F.; Kruse, A.C. Identification of the gene that codes for the σ2 receptor. Proc. Natl. Acad. Sci. USA 2017, 114, 7160–7165. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.M.; Charifson, P.S.; Owens, C.E.; Kula, N.S.; McPhail, A.T.; Baldessarini, R.J.; Booth, R.G.; Wyrick, S.D. Conformational analysis, pharmacophore identification, and comparative molecular field analysis of ligands for the neuromodulatory sigma 3 receptor. J. Med. Chem. 1994, 37, 4109–4117. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Su, P.T. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef] [PubMed]
- Mégalizzi, V.; Mathieu, V.; Mijatovic, T.; Gailly, P.; Debeir, O.; De Neve, N.; Van Damme, M.; Bontempi, G.; Haibe-Kains, B.; Decaestecker, C.; et al. Tissue microen- vironment modulates CXCR4 expression and tumor metastasis in Neuroblastoma. Neoplasia 2007, 9, 36–46. [Google Scholar]
- Aliprantis, A.O.; Yang, R.-B.; Mark, M.R.; Suggett, S.; Devaux, B.; Radolf, J.D.; Klimpel, G.R.; Godowski, P.; Zychlinsky, A. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 1999, 285, 736–739. [Google Scholar] [CrossRef]
- Kashiwagi, H.; McDunn, E.J.; Simon, O.P., Jr.; Goedegebuure, S.P.; Vangveravong, S.; Chang, K.; Hotchkiss, S.R.; Mach, H.R.; Hawkins, G.W. Sigma-2 receptor ligands potentiate conventional chemotherapies and improve survival in models of pancreatic adeno- carcinoma. J. Transl. Med. 2009, 26. [Google Scholar] [CrossRef]
- Ostenfeld, M.S.; Fehrenbacher, N.; Hoyer-Hansen, M.; Thomsen, C.; Farkas, T.; Jäättelä, M. Effective tumor cell death by σ-2 receptor ligand Siramesine involves lysosomal leakage and Oxidative Stress. Cancer Res. 2005, 65, 8975–8983. [Google Scholar] [CrossRef] [PubMed]
- Vilner, B.; John, C.S.; Bowen, W.D. Sigma-1 and Sigma-2 receptors Are Expressed in a wide variety of human and rodent tumor cell lines. Cancer Res. 1995, 55, 408–413. [Google Scholar] [PubMed]
- Zamora, P.O.; Moody, T.W.; John, C.S. Increased binding to sigma sites of N-[1-(2-piperidinyl)ethyl)-4-[I-125]-iodobenzamide (I-125-PAB) with onset of tumor cell proliferation. Life Sci. 1998, 63, 1611–1618. [Google Scholar] [CrossRef]
- Spruce, B.A.; Campbell, L.A.; McTavish, N.; Cooper, M.A.; Appleyard, M.V.L.; O’Neil, M.; Howie, J.; Samson, J.; Watt, S.; Murray, K.; et al. Small molecule antagonists of the 1 receptor cause selective release of the death program in tumor and self-reliant cells and inhibit tumor growth in vitro and in vivo. Cancer Res. 2004, 64, 4875–4886. [Google Scholar] [CrossRef] [PubMed]
- Colabufo, N.A.; Berardi, F.; Contino, M.; Niso, M.; Abate, C.; Perrone, R.; Tortorella, V. Synthesis, Biological and spectroscopic evaluation of some ligands with intrisinc fluorescent properties. Naunyn Schmiedeberg’s Arch. Pharmacol. 2004, 370, 106–113. [Google Scholar]
- Kim, F.J.; Maher, C.M. Sigma 1 Pharmacology in the Context of Cancer. Handb. Exp. Pharmacol. 2017. [Google Scholar] [CrossRef]
- Villard, V.; Espallergues, J.; Keller, E.; Alkam, T.; Nitta, A.; Ya-mada, K.; Nabeshima, T.; Vamvakides, A.; Maurice, T. Antiamnesic and neuroprotective effects of the aminotetrahydrofuran derivative ANAVEX1–41 against amyloid beta(25–35)-induced toxicity in mice. Neuropsychopharmacology 2009, 34, 1552–1566. [Google Scholar] [CrossRef] [PubMed]
- Meunier, J.; Hayashi, T. Sigma-1 Receptors Regulate Bcl-2 Expression by reactive oxygen species-dependent transcriptional regulation of nuclear factor {kappa}B. J. Pharmacol. Exp. Ther. 2010, 332, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Groth-Pedersen, L.; Ostenfeld, M.S.; Høyer-Hansen, M.; Nylandsted, J.; Jäätelä, M. Vincristine induces dramatic lysosomal changes and sensitizes cancer cells to lysosome-destabilizing siramesine. Cancer Res. 2007, 67, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Rui, M.; Rossi, D.; Marra, A.; Paolillo, M.; Schinelli, S.; Curti, D.; Tesei, A.; Cortesi, M.; Zamagni, A.; Laurini, E.; et al. Synthesis and biological evaluation of new aryl-alkyl(alkenyl)-4-benzylpiperidines, novel sigma receptor (SR) modulators, as potential anticancer-agents. Eur. J. Med. Chem. 2016, 124, 649–665. [Google Scholar] [CrossRef] [PubMed]
- John, C.S.; Bowen, W.D.; Varma, V.M.; McAfee, J.G.; Moody, T.W. Sigma receptors are expressed in human non-small cell lung carcinoma. Life Sci. 1995, 56, 2385–2392. [Google Scholar] [CrossRef]
- Bem, W.T.; Thomas, G.; Mamone, J.; Homan, S.M.; Levy, B.K.; Johnson, F.K.; Coscia, C.J. Overexpression of sigma receptors in nonneural human tumors. Cancer Res. 1991, 51, 6558–6562. [Google Scholar] [PubMed]
- Thomas, G.E.; Szucs, M.; Mamone, J.Y.; Bem, W.T.; Rush, M.D.; Johnson, F.E.; Coscia, C.J. Sigma and opioid receptors in human brain tumors. Life Sci. 1990, 46, 1279–1286. [Google Scholar] [CrossRef]
- Zeng, C.; Rothfuss, J.; Zhang, J.; Chu, W.; Vangveravong, S.; Tu, Z.; Pan, F.; Chang, K.C.; Hotchkiss, R.; Mach, R.H. Sigma-2 ligands induce tumour cell death by multiple signalling pathways. Br. J. Cancer 2012, 106, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Mach, R.; Zeng, C.; Hawkins, G. The σ2 receptor: A novel protein for the imaging and treatment of cancer. J. Med. Chem. 2013, 56, 7137–7160. [Google Scholar] [CrossRef] [PubMed]
- Su, T.P.; Su, T.C.; Nakamura, Y.; Tsai, S.Y. The sigma-1 receptor as a pluripotent modulator in living systems. Trends Pharmacol. Sci. 2016, 37, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Mavlyutov, T.A.; Guo, L.W.; Epstein, M.L.; Ruoho, A.E. Role of the sigma-1 receptor in Amyotrophic Lateral Sclerosis (ALS). J. Pharmacol. Sci. 2015, 127, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Alon, A.; Schmidt, H.; Zheng, S.; Kruse, A.C. Structural perspectives on sigma-1 receptor function. In Sigma Receptors: Their Role in Disease and as Therapeutic Targets; Smith, S.B., Su, T.P., Eds.; Spinger: Berlin, Germany, 2017; Volume 964, pp. 5–13. [Google Scholar]
- Kekuda, R.; Prasad, P.D.; Fei, Y.J.; Leibach, F.H.; Ganapathy, V. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1). Biochem. Biophys. Res. Commun. 1996, 229, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Seth, P.; Ganapathy, M.E.; Conway, S.J.; Bridges, C.D.; Smith, S.B.; Casellas, P.; Ganapathy, V. Expression pattern of the type 1 sigma receptor in the brain and identity of critical anionic amino acid residues in the ligand-binding domain of the receptor. Biochim. Biophys. Acta 2001, 1540, 59–67. [Google Scholar] [CrossRef]
- Hayashi, T.; Justinova, Z.; Hayashi, E.; Cormaci, G.; Mori, T.; Tsai, S.-Y.; Barnes, C.; Goldberg, S.R.; Su, T.-P. Regulation of sigma-1 receptors and endoplasmic reticulum chaperones in the brain of methamphetamine self-administering rats. J. Pharm. Exp. Therap. 2010, 332, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-Y.; Rothman, R.K.; Su, T.-P. Insights into the sigma-1 receptor chaperone’s cellular functions: A microarray report. Synapse 2012, 66, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-Y.; Hayashi, T.; Mori, T.; Su, T.-P. Sigma-1 receptor chaperones and diseases. Cent. Nerv. Syst. Agents Med. Chem. 2009, 9, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Soriani, O.; Vaudry, H.; Mei, Y.A.; Roman, F.; Cazin, L. Sigma ligands stimulate the electrical activity of frog pituitary melanotrope cells through a G-protein-dependent inhibition of potassium conductances. J. Pharmacol. Exp. Ther. 1998, 286, 163–171. [Google Scholar] [PubMed]
- Lupardus, P.J.; Wilke, R.A.; Aydar, E.; Palmer, C.P.; Chen, Y.; Ruoho, A.E.; Jackson, M.B. Membrane-delimited coupling between sigma receptors and K+ channels in ratneurohypophysial terminals requires neither G-protein nor ATP. J. Physiol. 2000, 3, 527–539. [Google Scholar] [CrossRef]
- Maeno, E.; Ishizaki, Y.; Kanaseki, T.; Hazama, A.; Okada, Y. Normotonic cell shrinkage because of disordered volume regulation is an early rerequisite to apoptosis. Proc. Natl. Acad. Sci. USA 2000, 97, 9487–9492. [Google Scholar] [CrossRef] [PubMed]
- Aydar, E.; Palmer, C.P.; Klyachko, V.A.; Jackson, M.B. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron 2002, 34, 399–410. [Google Scholar] [CrossRef]
- Fernandez, P.C.; Frank, S.R.; Wang, L.; Schroeder, M.; Liu, S.; Greene, J.; Cocito, A.; Amati, B. Genomic targets of the human c-Myc protein. Genes Dev. 2003, 17, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Persaud, L.; Dejoie, J.; Happy, M.; Brannigan, O.; De Jesus, D.; Sauane, M. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) activates caspases in human prostate cancer cells through sigma 1 receptor. Biochem. Biophys. Res. Commun. 2016, 470, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Maurice, T.; Su, T.-P. The pharmacology of sigma-1 receptors. Pharmacol. Ther. 2009, 124, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Happy, M.; Dejoie, J.; Zajac, C.K.; Cortez, B.; Chakraborty, K.; Aderemi, J.; Sauane, M. Sigma 1 Receptor antagonist potentiates the anti-cancer effect of p53 by regulating ER stress, ROS production, Bax levels, and caspase-3 activation. Biochem. Biophys. Res. Commun. 2015, 456, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zeng, W.; Chu, F.; Pan, J.; Rothfuss, M.; Zhang, F.; Tu, Z.; Zhou, D.; Zeng, D.; Vangveravong, S.; et al. Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nat. Commun. 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Lu, H.L.; Zhang, L.J.; Wu, Z. Sigma-2 receptor ligands and their perspectives in cancer diagnosis and therapy. Med. Res. Rev. 2014, 34, 532–566. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.D.; Crawford, K.W.; Huang, S.; Walker, J.W. Activation of sigma-2 receptors causes changes in ceramide levels in neuronal and non-neuronal cell lines. Soc. Neurosci. Abstr. 2000, 26, 601–611. [Google Scholar]
- Wheeler, K.T.; Wang, L.M.; Wallen, C.A.; Childers, S.R.; Cline, J.M.; Keng, P.C.; Mach, R.H. Sigma-2 receptors as a biomarker of proliferation in solid tumors. Br. J. Cancer 2000, 82, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Toyohara, J.; Sakata, M.; Ishiwata, K. Imaging of sigma1 receptors in the human brain using PET and [11C]SA4503. Cent. Nerv. Syst. Agents Med. Chem. 2009, 9, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-R.; Yang, B.; Plossl, K.; Chumpradit, S.; Wey, S.-P.; Acton, P.D.; Wheeler, K.T.; Mach, R.H.; Kung, H.F. Development of a Tc-99m labeled sigma-2 receptor-specific ligand as a potential breast tumor imaging agent. Nucl. Med. Biol. 2001, 28, 657–666. [Google Scholar] [CrossRef]
- John, C.S.; Gulden, M.E.; Li, J.; Bowen, W.D.; McAfee, J.G.; Thakur, M.L. Synthesis, in vitro binding, and tissue distribution of radioiodinated 2-[125I]N-(N-benzylpiperidin-4-yl)-2-iodo benzamide, 2-[125I]BP: A potential sigma receptor marker for human prostate tumors. Nucl. Med. Biol. 1998, 25, 189–194. [Google Scholar] [CrossRef]
- Abate, C.; Perrone, R.; Berardi, F. Classes of sigma 2 receptor ligands: Structure affinity relationship (SAfiR) studies and antiproliferative activity. Curr. Pharm. Des. 2012, 18, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Crawford, K.W.; Bowen, W.D. Sigma-2 receptor agonists activate a novel apoptotic pathway and potentiate antineoplastic drugs in breast tumor cell lines. Cancer Res. 2002, 62, 313–322. [Google Scholar] [PubMed]
- Mir, S.U.; Schwarze, S.R.; Jin, L.; Zhang, J.; Friend, W.; Miriyala, D.; St Clair, D.; Craven, R.J. Progesterone receptor membrane component 1/Sigma-2 receptor associates with MAP1LC3B and promotes autophagy. Autophagy 2013, 9, 1566–1578. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.D.; Jin, B.; Blann, E.; Vilner, B.J.; Lyn-Cook, B.D. σ Receptor ligands modulate expression of the multi-drug resistance gene in human and rodent brain tumor cell lines. Proc. Am. Assoc. Cancer Res. 1997, 38, 3206. [Google Scholar]
- Abate, C.; Ferorelli, S.; Contino, M.; Marottoli, R.; Colabufo, N.A.; Perrone, R.; Berardi, F. Arylamides hybrids of two high-affinity σ2 receptor ligands as tools for the development of PET radiotracers. Eur. J. Med. Chem. 2011, 46, 4733–4741. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Bowen, W.D.; Walker, F.O.; Matsumoto, R.R.; De Costa, B.; Rice, K.C. Sigma receptors: Biology and function. Pharmacol. Rev. 1990, 42, 355–402. [Google Scholar] [PubMed]
- De Costa, B.R.; He, X. Structure-activity relationships and evolution of σ receptor ligands. In Sigma Receptors; Itzhak, Y., Ed.; Academic Press: London, UK, 1994; pp. 45–111. [Google Scholar]
- Glennon, R.A.; Ablordeppey, S.Y.; Ismaiel, A.M.; El-Ashmawy, M.B.; Fischer, J.B.; Burke Howie, K. Structural features important for sigma-1 receptor binding. J. Med. Chem. 1994, 37, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Glennon, R.A.; Smith, J.D.; Ismaiel, A.M.; El-Ashmawy, M.B.; Battaglia, G.; Fischer, J.B. Identification and exploitation of the sigma-opioid pharmacophore. J. Med. Chem. 1991, 34, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Manallack, D.T.; Wong, M.G.; Costa, M.; Andrews, P.R.; Beart, P.M. Receptor site topographies for phencyclidine-like and sigma drugs: Predictions from quantitative, conformational, electrostatic potential, and radioreceptor analyses. Mol. Pharmacol. 1989, 34, 863–879. [Google Scholar]
- Glennon, R.A.; Ismaiel, A.M.; Yousif, M.; El-Ashmawy, M.; Herndon, J.L.; Fischer, J.B.; Burke Howie, K.; Server, A.C. Binding of substituted and conformationally restricted derivatives of N-(3-phenyl-n-propyl)-l-phenyl-2-aminopropanes at sigma receptors. J. Med. Chem. 1991, 34, 1855–1859. [Google Scholar] [CrossRef] [PubMed]
- Glennon, R.A.; El-Ashmawy, M.B.; Fischer, J.B.; Burke Howie, K.; Ismaiel, A.M. N-Substituted 5-phenylpentylamines: A new class of sigma ligands. Med. Chem. Res. 1991, 1, 207–212. [Google Scholar]
- El-Ashmawy, M.B.; Ablordeppey, S.Y.; Hassan, I.; Gad, L.; Fischer, J.B.; Burke Howie, K.; Glennon, R.A. Further investigation of 5-phenylethylamines derivatives as novel sigma receptor ligands. Med. Chem. Res. 1992, 2, 119–126. [Google Scholar]
- Glennon, R.A.; Yousif, M.Y.; Ismaiel, A.M.; El-Ashmawy, M.B.; Herndon, J.L.; Fischer, J.B.; Server, A.C.; Burke Howie, K. Novel 1-phenylpiperazine and 4-phenylpiperidine derivatives as high affinity sigma ligands. J. Med. Chem. 1991, 34, 3360–3365. [Google Scholar] [CrossRef] [PubMed]
- Ablordeppey, S.Y.; Issa, H.; Fischer, J.B.; Howie, B.; Glennon, R.A. Synthesis and structure-affinity relationship studies of sigma ligands related to haloperidol. Med. Chem. Res. 1993, 3, 131–138. [Google Scholar]
- Ablordeppey, S.Y.; Fischer, J.B.; Law, H.; Glennon, R.A. Probing the proposed phenyl-A region of the sigma-1 receptor. Bioorg. Med. Chem. 2002, 10, 2759–2765. [Google Scholar] [CrossRef]
- Ablordeppey, S.Y.; El-Ashmawy, M.B.; Fischer, J.B.; Glennon, R.A. A CoMFA investigation of sigma receptor binding affinity: Reexamination of a spurious sigma ligand. Eur. J. Med. Chem. 1998, 33, 625–633. [Google Scholar] [CrossRef]
- Itzhak, Y. (Ed.) Sigma Receptors; Academic Press: London, UK, 1994. [Google Scholar]
- Ablordeppey, S.Y.; Fischer, J.B.; Glennon, R.A. Is a nitrogen atom an important pharmacophoric element in sigma ligand binding? Bioorg. Med. Chem. 2000, 8, 2105–2111. [Google Scholar] [CrossRef]
- Ramachandran, S.; Chu, U.B.; Mavlyutov, T.A.; Pal, A.; Pyne, S.; Ruoho, A.E. The sigma1 receptor interacts with N-alkyl amines and endogenous sphingolipids. Eur. J. Pharmacol. 2009, 609, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Nastasi, G.; Miceli, C.; Pittalà, V.; Modica, M.N.; Prezzavento, O.; Romeo, G.; Rescifina, A.; Marrazzo, A.; Amata, E. S2RSLDB: A comprehensive manually curated, internet-accessible database of the sigma-2 receptor selective ligands. J. Cheminform. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.D.; Bertha, C.M.; Vilner, B.J.; Rice, K.C. CB-64D and CB-184: Ligands with high sigma-2 receptor affinity and subtype selectivity. Eur. J. Pharmacol. 1995, 278, 257–260. [Google Scholar] [CrossRef]
- Mach, R.H.; Vangveravong, S.; Huang, Y.S.; Yang, B.; Blair, J.B.; Wu, L. Synthesis of N-substituted 9-azabicyclo[3.3.1]nonan-3α-yl phenylcarbamate analogs as sigma-2 receptor ligands. Med. Chem. Res. 2003, 11, 380–398. [Google Scholar]
- Mach, R.H.; Wheeler, K.T. Development of molecular probes for imaging sigma-2 receptors in vitro and in vivo. Cent. Nerv. Syst. Agents Med. Chem. 2009, 9, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Xu, J.; Zhou, D.; Zhang, F.; Jones, L.A.; Wheeler, K.T.; Mach, R.H. New N-substituted 9-azabicyclo[3.3.1]nonan-3alpha-yl phenylcarbamate analogs as sigma 2 receptor ligands: Synthesis, in vitro characterization, and evaluation as PET imaging and chemosensitization agents. Bioorg. Med. Chem. 2009, 17, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Perregaard, J.; Moltzen, E.K.; Meier, E.; Sanchez, C. Sigma ligands with subnanomolar affinity and preference for the sigma 2 binding site. 1. 3-(omega-Aminoalkyl)-1H-indoles. J. Med. Chem. 1995, 38, 1998–2008. [Google Scholar] [CrossRef] [PubMed]
- Moltzen, E.K.; Perregaard, J.; Meier, E. Sigma ligands with subnanomolar affinity and preference for the sigma 2 binding site. 2. Spiro-joined benzofuran, isobenzofuran, and benzopyran piperidines. J. Med. Chem. 1995, 38, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Vangveravong, S.; Xu, J.; Chang, K.C.; Hotchkiss, R.S.; Wheeler, K.T.; Shen, D.; Zhuang, Z.P.; Kung, H.F.; Mach, R.H. Subcellular localization of sigma-2 receptors in breast cancer cells using two-photon and confocal microscopy. Cancer Res. 2007, 67, 6708–6716. [Google Scholar] [CrossRef] [PubMed]
- Mach, R.H.; Huang, Y.; Freeman, R.A.; Wu, L.; Vangveravong, S.; Luedtke, R.R. Conformationally-flexible benzamide analogues as dopamine D3 and sigma-2 receptor ligands. Bioorg. Med. Chem. Lett. 2004, 14, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Lever, J.R.; Lever, S.Z. Synthesis and in vitro evaluation of tetrahydroisoquinolinylbenzamidesas ligands for sigma receptors. Bioorg. Med. Chem. Lett. 2007, 17, 2594–2597. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.H.; Lever, J.R.; Lever, S.Z. Effect of structureal modification in the amine portion of substituted aminobutyl-benzamides as ligands for binding sigma-1 and sigma-2 receptors. Bioorg. Med. Chem. 2011, 19, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, A.R.; Guo, L.W.; Pal, A.; Mavlyutov, T.; Ruoho, A.E. Electron-donating para-methoxy converts a benzamide-isoquinoline derivative into a highly sigma-2 receptor selective ligand. Bioorg. Med. Chem. 2011, 19, 7435–7440. [Google Scholar] [CrossRef] [PubMed]
- Berardi, F.; Abate, C.; Ferorelli, S.; Uricchio, V.; Colabufo, N.A.; Niso, M.; Perrone, R. Exploring the importance of piperazine N-atoms for σ2 receptor affinity and activity in a series of analogs of 1-cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl] piperazine (PB28). J. Med. Chem. 2009, 52, 7817–7828. [Google Scholar] [CrossRef] [PubMed]
- Berardi, F.; Abate, C.; Ferorelli, S.; Colabufo, N.A.; Perrone, R. 1-Cyclohexylpiperazine and 3,3-dimethylpiperidine derivatives as sigma-1 and sigma-2 receptor ligands: A review. Cent. Nerv. Syst. Agents Med. Chem. 2009, 9, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Bhat, R.; Mesangeau, C.; Poupaert, J.H.; McCurdy, C.R. Early development of sigma-receptor ligands. Future Med. Chem. 2010, 3, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alrokayan, S.A.; Kumar, S. Targeted anticancer therapy: Overexpressed receptors and nanotechnology. Clin. Chim. Acta 2014, 436, 78–92. [Google Scholar] [CrossRef] [PubMed]
- John, C.S.; Vilner, B.J.; Gulden, M.E.; Efange, S.M.; Langason, R.B.; Moody, T.W.; Bowen, W.D. Synthesis and pharmacological characterization of 4-[125I]-N-(N-benzylpiperidin-4-yl)- 4-iodobenzamide: A high affinity sigma receptor ligand for potential imaging of breast cancer. Cancer Res. 1995, 55, 3022–3027. [Google Scholar] [PubMed]
- Pati, M.L.; Groza, D.; Riganti, C.; Kopecka, J.; Niso, M.; Berardi, F.; Hager, S.; Heffeter, P.; Hirai, M.; Tsugawa, H.; et al. Sigma-2 receptor and progesterone receptor membrane component 1 (PGRMC1) are two different proteins: Proofs by fluorescent labeling and binding of sigma-2 receptor ligands to PGRMC1. Pharmacol. Res. 2017, 117, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Colabufo, N.A.; Abate, C.; Contino, M.; Inglese, C.; Niso, M.; Berardi, F.; Perrone, R. PB183, a sigma receptor ligand, as a potential PET probe for the imaging of prostate adenocarcinoma. Bioorg. Med. Chem. Lett. 2008, 18, 1990–1993. [Google Scholar] [CrossRef] [PubMed]
- Everaert, H.; Flamen, P.; Franken, P.R.; Verhaeghe, W.; Bossuyt, A. Sigma-receptor imaging by means of I123-IDAB scintigraphy: Clinical application in melanoma and non-small cell lung cancer. Anticancer Res. 1997, 17, 1577–1582. [Google Scholar] [PubMed]
- John, C.S.; Vilner, B.J.; Geyer, B.C.; Moody, T.; Bowen, W.D. Targeting sigma receptor-binding benzamides as in vivo diagnostic and therapeutic agents for human prostate tumors. Cancer Res. 1999, 59, 4578–4583. [Google Scholar] [PubMed]
- Caveliers, V.; Everaert, H.; John, C.S.; Lahoutte, T.; Bossuyt, A. Sigma receptor scintigraphy with N-[2-(1′-piperidinyl)ethyl]-3-(123)I-iodo-4-methoxybenzamide of patients with suspected primary breast cancer: First clinical results. J. Nucl. Med. 2002, 43, 1647–1649. [Google Scholar] [PubMed]
- Michelot, J.M.; Moreau, M.-F.C.; Labarre, P.G.; Madelmont, J.-C.; Veyre, A.J.; Papon, J.M.; Parry, D.F.; Bonafous, J.F.; Boire, J.-Y.P.; Desplanches, G.G.; et al. Synthesis and evaluation of new Iodine-125 radiopharmaceuticals as potential tracers for malignant melanoma. J. Nucl. Med. 1991, 32, 1573–1580. [Google Scholar] [PubMed]
- John, C.S.; Bowen, W.D.; Saga, T.; Kinuya, S.; Vilner, B.J.; Baumgold, J.; Paik, C.H.; Reba, R.C.; Neumann, R.D.; Varma, V.M.; et al. A malignant melanoma imaging agent: Synthesis, characterization, in vitro binding and biodistribution of iodine-125-(2-piperidinylaminoethyl)-4-iodobenzamide. J. Nucl. Med. 1993, 34, 2169–2175. [Google Scholar] [PubMed]
- Banerjee, R.; Tyagi, P.; Li, S.; Huang, L. Anisamide-targeted stealth liposomes: A potent carrier for targeting doxorubicin to human prostate cancer cells. Int. J. Cancer 2004, 112, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Li, S.D.; Huang, L. Targeted delivery of antisense oligodeoxynucleotide and small interference RNA into lung cancer cells. Mol. Pharm. 2006, 3, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Li, S.D.; Chono, S.; Huang, L. Efficient oncogene silencing and metastasis inhibition via systemic delivery of siRNA. Mol. Ther. 2008, 16, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Bacin, F.; Michelot, J.; Bonafous, J.; Veyre, A.; Moreau, M.-F.; Kemeny, J.-L.; Chossat, F.; Bekhechi, D. Clinical study of [123I] N-(2-diethylaminoethyl)-4-iodobenzamide in the diagnosis of primary and metastatic ocular melanoma. Acta Ophthalmol. Scand. 1998, 76, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Cachin, F.; Miot-Noirault, E.; Gillet, B.; Isnardi, V.; Labeille, B.; Payoux, P.; Meyer, N.; Cammilleri, S.; Gaudy, C.; Razzouk-Cadet, M.; et al. 123I-BZA2 as a melanin-targeted radiotracer for the identification of melanoma metastases: Results and perspectives of a multicenter Phase III clinical trial. J. Nucl. Med. 2014, 55, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Megalizzi, V.; Mathieu, V.; Mijatovic, T.; Gailly, P.; Debeir, O.; De Neve, N.; Van Damme, M.; Bontempi, G.; Haibe-Kains, B.; Decaestecker, C.; et al. 4-IBP, a sigma1 receptor agonist, decreases the migration of human cancer cells, including glioblastoma cells, in vitro and sensitizes them in vitro and in vivo to cytotoxic insults of proapoptotic and proautophagic drugs. Neoplasia 2007, 9, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Megalizzi, V.; Decaestecker, C.; Debeir, O.; Spiegl-Kreinecker, S.; Berger, W.; Lefranc, F.; Kast, R.E.; Kiss, R. Screening of anti-glioma effects induced by sigma-1 receptor ligands: Potential new use for old anti-psychiatric medicines. Eur. J. Cancer 2009, 45, 2893–2905. [Google Scholar] [CrossRef] [PubMed]
- Achison, M.; Boylan, M.T.; Hupp, T.R.; Spruce, B.A. HIF-1alpha contributes to tumour-selective killing by the sigma receptor antagonist rimcazole. Oncogene 2007, 26, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Wang, S.; Zhao, Y.; Zhu, R.; Wang, W.; Sun, Y. Haloperidol, a sigma receptor 1 antagonist, promotes ferroptosis in hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2017. [Google Scholar] [CrossRef] [PubMed]
- Alsharif, W.; Shen, B.; Park, J.H.; Scatliffe, S.; Chin, F.; McCurdy, C. Highly Selective sigma-2 receptor PET radioligand [11C]WF197: Radiosynthesis and pilot PET imaging study in mice. J. Nucl. Med. 2017, 58, 724. [Google Scholar]
- Hellewell, S.B.; Bowen, W.D. A sigma-like binding site in rat pheochromocytoma (PC12) cells: Decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain. Brain Res. 1990, 527, 244–253. [Google Scholar] [CrossRef]
- Hellewell, S.B.; Bruce, A.; Feinstein, G.; Orringer, J.; Williams, W.; Bowen, W.D. Rat liver and kidney contain high densities of sigma-1 and sigma-2 receptors: Characterization by ligand binding andphotoaffinity labeling. Eur. J. Pharmacol. 1994, 268, 9–18. [Google Scholar] [CrossRef]
- Wang, L.; Ye, J.; He, Y.; Deuther-Conrad, W.; Zhang, J.; Zhang, X.; Cui, M.; Steinbach, J.; Huang, Y.; Brust, P.; et al. 18F-Labeled indole-based analogs as highly selective radioligands for imaging sigma-2 receptors in the brain. Bioorg. Med. Chem. 2017, 25, 3792–3802. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Xu, J.; Jones, L.A.; Li, S.; Dumstorff, C.; Vangveravong, S.; Chen, D.L.; Wheeler, K.T.; Welch, M.J.; Mach, R.H. Fluorine-18-labeled benzamide analogues for imaging the sigma-2 receptor status of solid tumors with positron emission tomography. J. Med. Chem. 2007, 50, 3194–3204. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03057743 (accessed on 21 August 2017).
- Vilner, B.J.; Bowen, W.D. Sigma receptor active neurolepticsare cytotoxic to C6 glioma cells in culture. Eur. J. Pharmacol. 1993, 244, 199–201. [Google Scholar] [CrossRef]
- Vilner, B.J.; de Costa, B.R.; Bowen, W.D. Cytotoxic effects of sigma ligands: Sigma receptor-mediated alterations in cellular morphology and viability. J. Neurosci. 1995, 15, 117–134. [Google Scholar] [PubMed]
- Spitzer, D.; Simon, P.O.; Kashiwagi, H.; Xu, J.; Zeng, C.; Vangveravong, S.; Zhou, D.; Chang, K.; McDunn, J.E.; Hornick, J.R.; et al. Use of multifunctional sigma-2 receptor ligand conjugates to trigger cancer selective cell death signaling. Cancer Res. 2012, 72, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Hornick, J.R.; Xu, J.; Vangveravong, S.; Tu, Z.; Mitchem, J.B.; Spitzer, D.; Goedegebuure, P.; Mach, R.H.; Hawkins, W.G. The novel sigma-2 receptor ligand SW43 stabilizes pancreas cancer progression in combination with gemcitabine. Mol. Cancer. 2010, 9. [Google Scholar] [CrossRef] [PubMed]
- Casellas, P.; Galiegue, S.; Bourrie, B.; Ferrini, J.B.; Jbilo, O.; Vidal, H. SR31747A: A peripheral σ ligand with potent antitumor activities. Anti Cancer Drugs 2004, 15, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, A.; Prezzavento, O.; Pappalardo, M.S.; Bousquet, E.; Iadanza, M.; Pike, V.W.; Ronsisvalle, G. Synthesis of (+)- and (−)-cis-2-[(1-adamantylamino)-methyl]-1-phenyl-cyclopropane derivatives as high affinity probes for σ1 and σ 2 binding sites. IL Farmaco 2002, 57, 45–53. [Google Scholar] [CrossRef]
- Franchini, S.; Battisti, U.M.; Prandi, A.; Tait, A.; Borsari, C.; Cichero, E.; Fossa, P.; Cilia, A.; Prezzavento, O.; Ronsisvalle, S.; et al. Scouting new sigma receptor ligands: Synthesis, pharmacological evaluation and molecular modeling of 1,3-dioxolane-based structures and derivatives. Eur. J. Med. Chem. 2016, 112, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Comeau, A.; Bowen, W.D.; Mach, R.H.; Ross, B.D.; Hong, H.; Van Dort, M.E. Design and investigation of a [18F]-labeled benzamide derivative as a high affinity dual sigma receptor subtype radioligand for prostate tumor imaging. Mol. Pharm. 2017, 14, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Lever, J.R.; Litton, T.P.; Fergason-Cantrell, E.A. Characterization ofpulmonary sigma receptors by radioligand binding. Eur. J. Pharm. 2015, 762, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Wanka, L.; Iqbal, K.; Schreiner, P.R. The lipophilic bullet hits the targets: Medicinal chemistry of adamantane derivatives. Chem. Rev. 2013, 113, 3516–3604. [Google Scholar] [CrossRef] [PubMed]
- Spilovsk, K.; Zemek, F.; Korabecny, J.; Nepovimova, E.; Soukup, O.; Windisch, M.; Kuca, K. Adamantane—A Lead Structure for Drugs in Clinical Practice. Curr. Med. Chem. 2016, 23, 1–22. [Google Scholar] [CrossRef]
- Kornhuber, J.; Schoppmeyer, K.; Riederer, P. Affinity of 1-aminoadamantanes for the binding site in post-mortem human frontal cortex. Neurosci. Lett. 1993, 163, 129–131. [Google Scholar] [CrossRef]
- Ronsisvalle, G.; Marrazzo, A.; Prezzavento, O.; Pasquinucci, L.; Falcucci, B.; Di Toro, R.; Spampinato, S. Substituted 1-phenyl-2-cyclopropylmethylamines with high affinity and selectivity for sigma sites. Bioorg. Med. Chem. 2000, 8, 1503–1513. [Google Scholar] [CrossRef]
- Bourrie, B.; Bribes, E.; De Nys, N.; Esclangon, M.; Garcia, L.; Galiegue, S.; Lair, P.; Paul, R.; Thomas, C.; Vernieres, J.-C.; et al. SSR125329A, a high affinity sigma receptor ligand with potent anti-inflammatory properties. Eur. J. Pharmacol. 2002, 456, 123–131. [Google Scholar] [CrossRef]
- Bucolo, C.; Marrazzo, A.; Ronsisvalle, S.; Ronsisvalle, G.; Cuzzocrea, S.; Mazzon, E.; Caputi, A.; Drago, F. A novel adamantane derivative attenuates retinal ischemia–reperfusion damage in the rat retina through σ1 receptors. Eur. J. Pharmacol. 2006, 536, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Banister, S.D.; Yoo, D.T.; Wern Chua, S.; Cui, J.; Mach, R.H.; Kassiou, M. N-Arylalkyl-2-azaadamantanes as cage-expanded polycarbocyclic sigma (σ) receptor ligands. Bioorg. Med. Chem. Lett. 2011, 21, 5289–5292. [Google Scholar] [CrossRef] [PubMed]
- Riganas, S.; Papanastasiou, I.; Foscolos, G.B.; Tsotinis, A.; Serin, G.; Mirjolet, J.-F.; Dimas, K.; Kourafalos, V.N.; Eleutheriades, A.; Moutsos, V.I.; et al. New adamantane phenylalkylamines with σ-receptor binding affinity and anticancer activity, associated with putative antagonism of neuropathic pain. J. Med. Chem. 2012, 55, 10241–10261. [Google Scholar] [CrossRef] [PubMed]
- Riganas, S.; Papanastasiou, I.; Foscolos, G.B.; Tsotinis, A.; Bourguignon, J.J.; Serin, G.; Mirjolet, J.F.; Dimas, K.; Kourafalos, V.N.; Eleutheriades, A.; et al. Synthesis, σ1, σ2-receptors binding affinity and antiproliferative action of new C1-substituted adamantanes. Bioorg. Med. Chem. 2012, 20, 3323–3331. [Google Scholar] [CrossRef] [PubMed]
- Riganas, S.; Papanastasiou, I.; Foscolos, G.B.; Tsotinis, A.; Dimas, K.; Kourafalos, V.N.; Eleutheriades, A.; Moutsos, V.I.; Khan, H.; Margarita, P.; et al. New adamantane derivatives with sigma affinity and antiproliferative activity. Med. Chem. 2012, 8, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Fytas, C.; Zoidis, G.; Tsotinis, A.; Fytas, G.; Khan, M.A.; Akhtar, S.; Rahman, K.M.; Thurston, D.E. Novel 1-(2-aryl-2-adamantyl)piperazine derivatives with antiproliferative activity. Eur. J. Med. Chem. 2015, 93, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Ferle-Vidović, A.; Kaštelan, M.; Petrović, D.; Šuman, L.; Kaselj, M.; Škare, D.; Mlinarić-Majerski, K. Synthesis and biological activity of phencyclidine and its adamantylamine derivatives. Eur. J. Med. Chem. 1993, 28, 243–250. [Google Scholar] [CrossRef]
- Papanastasiou, I.; Riganas, S.; Foscolos, G.B.; Tsotinis, A.; Akhtar, S.A.; Khan, M.A.; Rahman, K.M.; Thurston, D.E. Synthesis and Cytotoxicity of 4-(2-Adamantyl)phenylalkylamines. Lett. Org. Chem. 2015, 12, 319–323. [Google Scholar] [CrossRef]
- Koperniku, A.; Foscolos, A.-S.; Papanastasiou, I.; Foscolos, G.B.; Tsotinis, A.; Schols, D. 4-(1-Adamantyl)phenylalkylamines with Potential Antiproliferative Activity. Lett. Org. Chem. 2016, 13, 171–176. [Google Scholar] [CrossRef]
- Papanastasiou, I.; Tsotinis, A.; Kolocouris, N.; Nikas, S.P.; Vamvakides, A. New aminoadamantane derivatives with antiproliferative activity. Med. Chem. Res. 2014, 23, 1966–1975. [Google Scholar] [CrossRef]
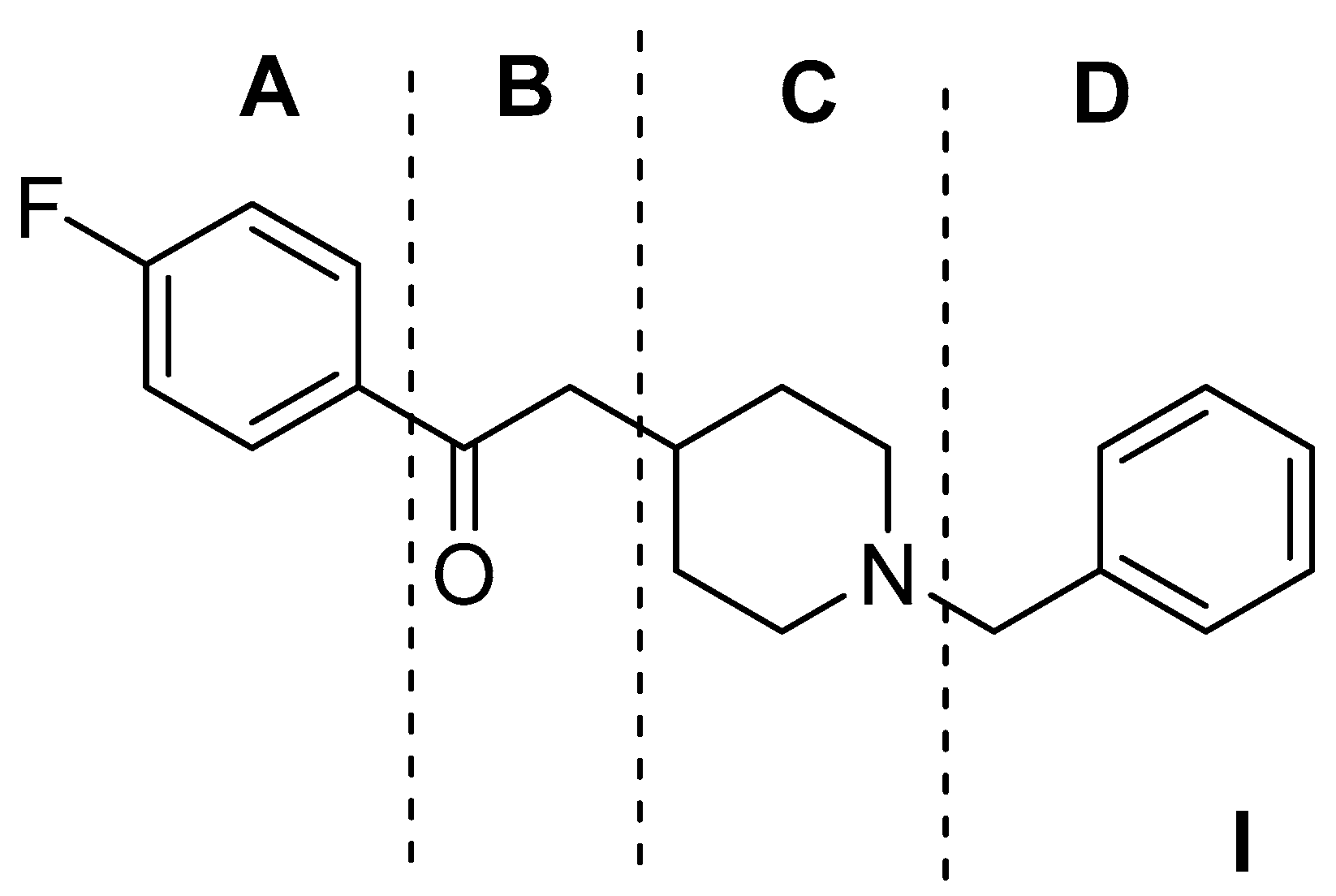


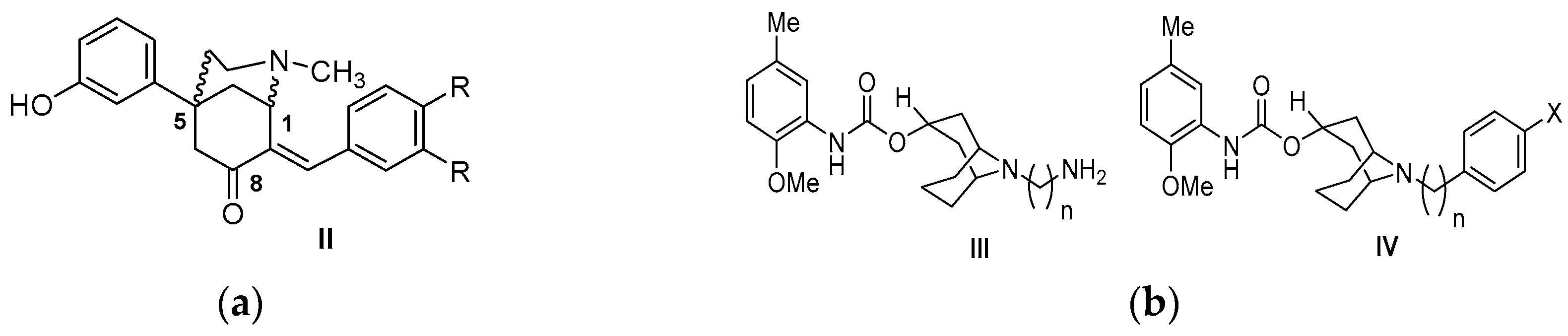
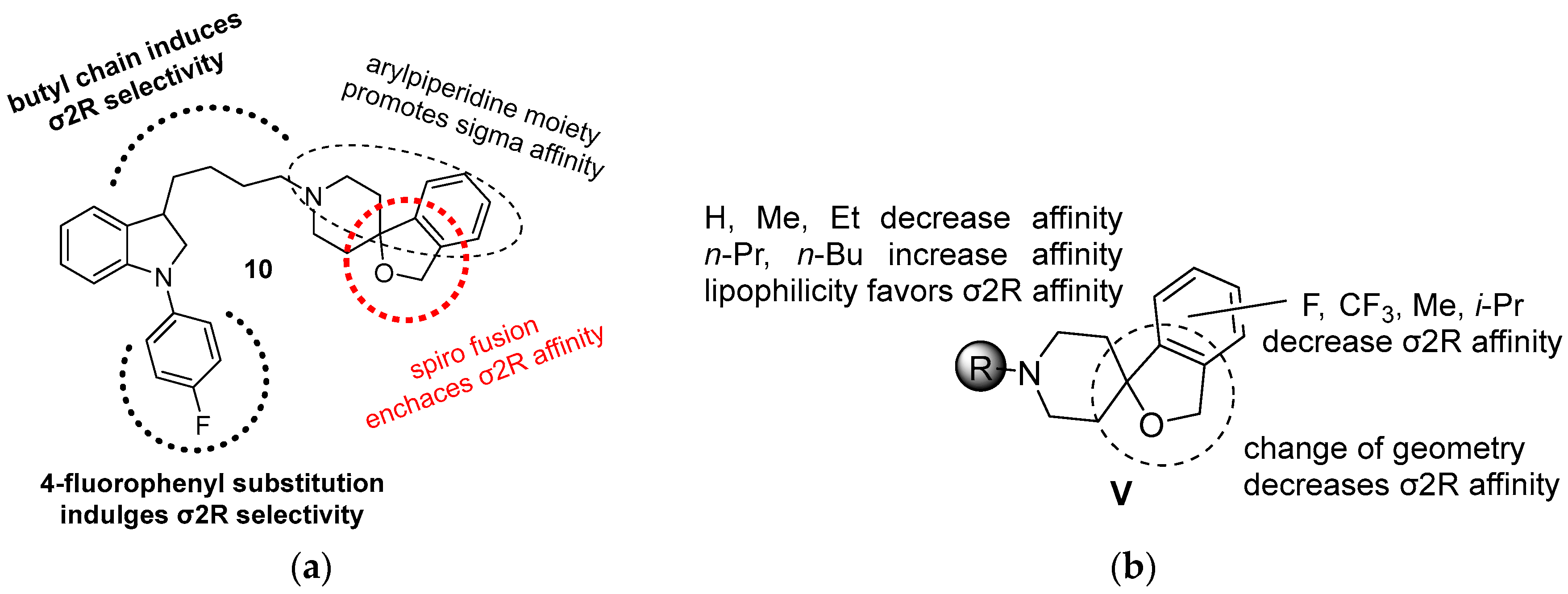
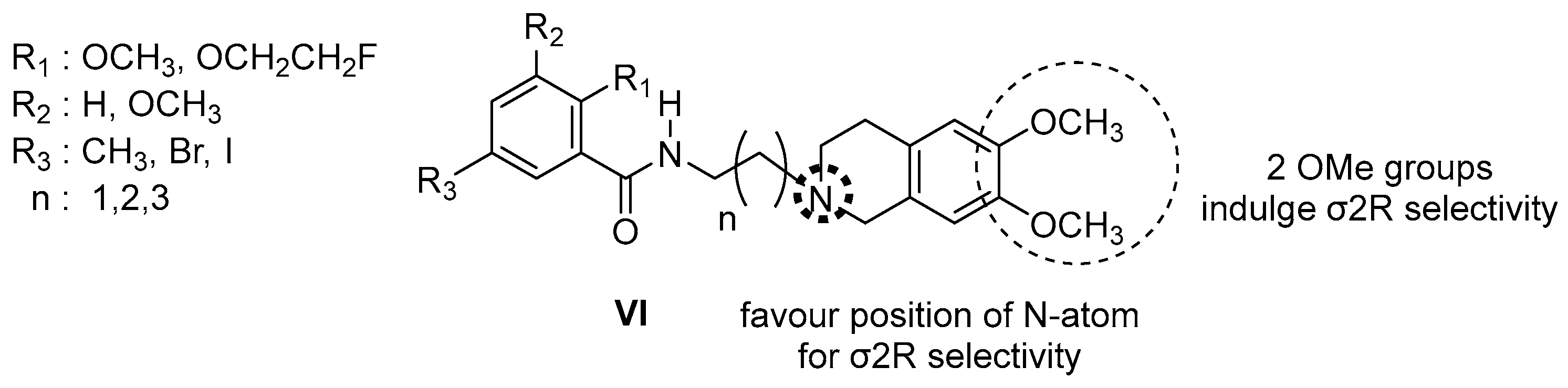
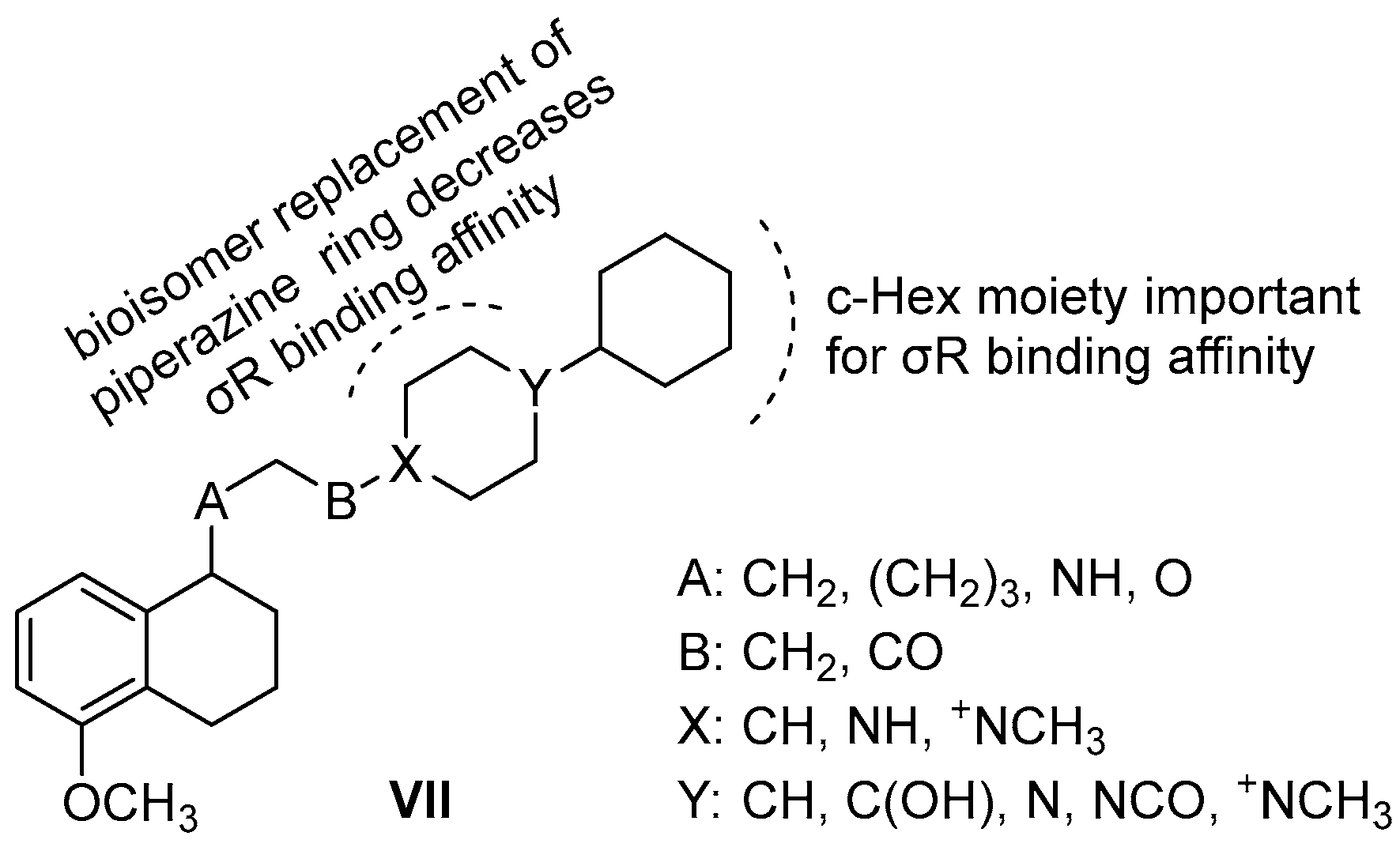
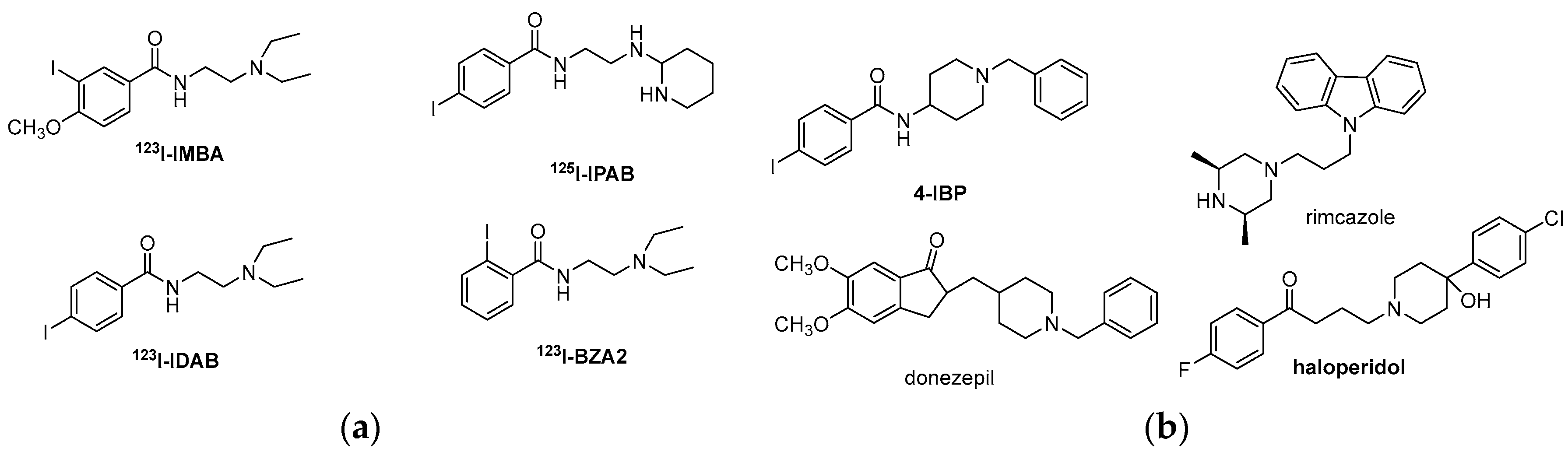
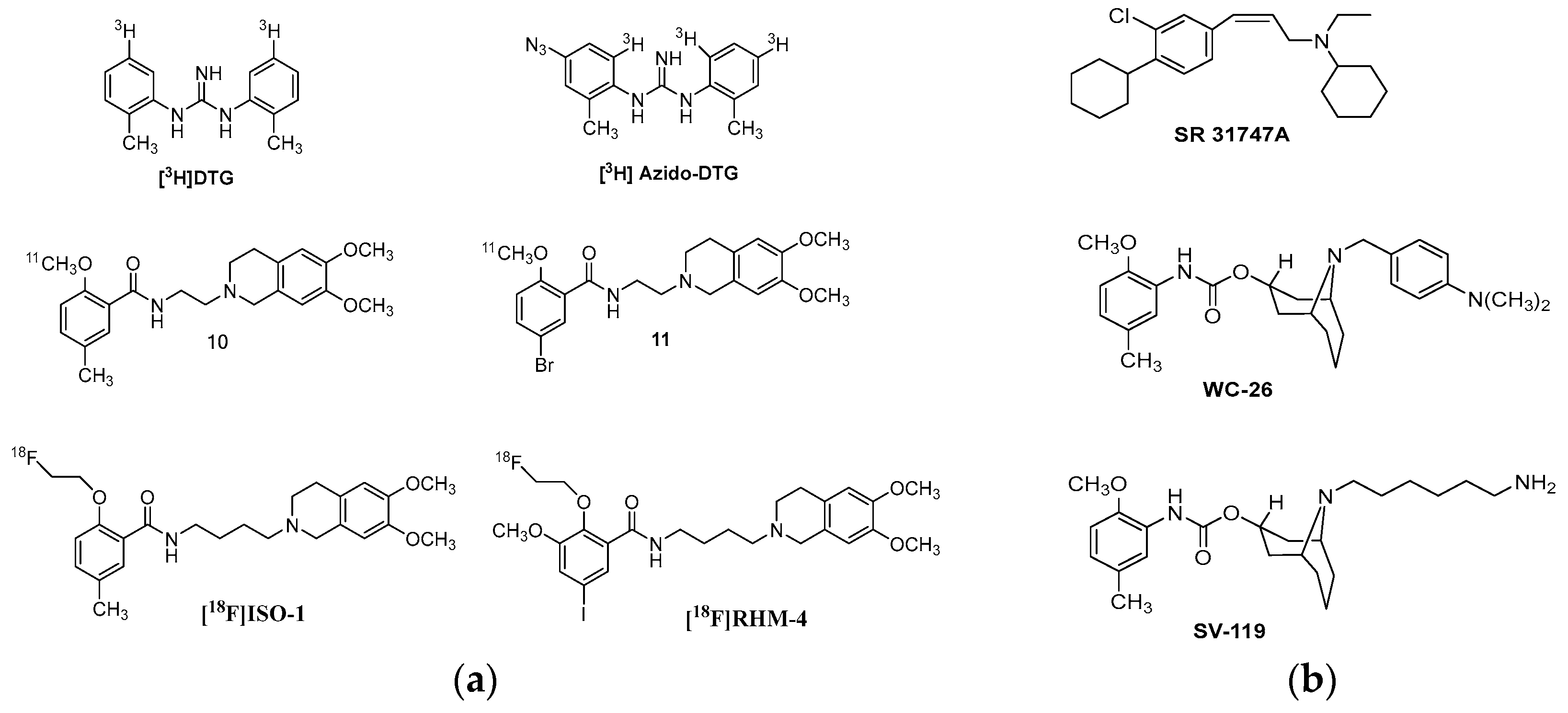
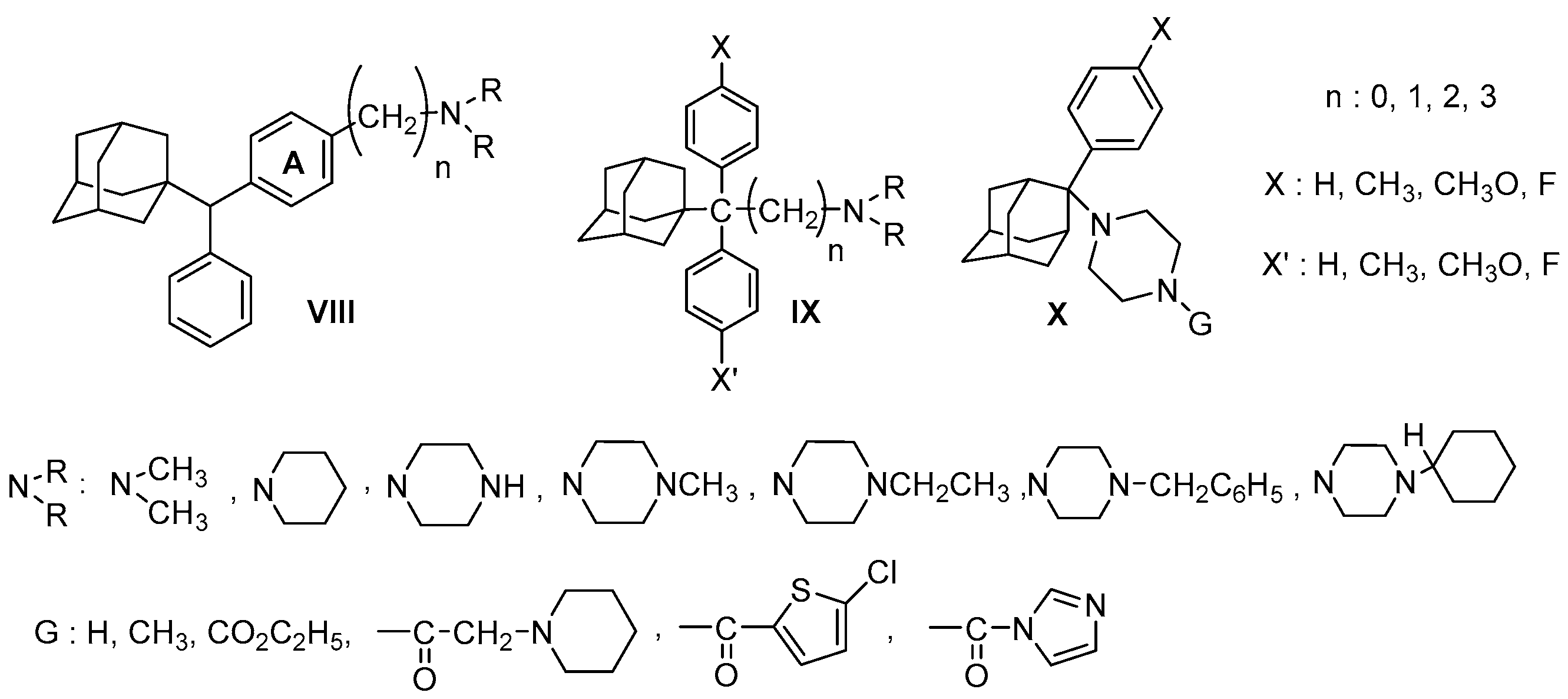
| Origin | Species | Cell Line | σ2R Ligand |
|---|---|---|---|
| breast cancer | human | T47 D | [3H]DTG |
| human | MCF7 | [3H]DTG | |
| mouse | EMT-6 | [3H]DTG,WC-26,SV-119 | |
| colon cancer | human | primary tumor | [3H]DTG |
| leukemia | human | Th-P1 | [3H]DTG |
| lung | human | NCI-H727 | [3H]DTG |
| melanoma | human | A375 | [3H]DTG |
| human | MDA MB-435 | WC-26,SV-119 | |
| neurologic | human | U-138MG | [3H]DTG |
| human | primary tumor | [3H]DTG | |
| mouse | NB41A3 | [3H]DTG | |
| mouse | N1E-115 | [3H]DTG | |
| rat | C6 | [3H]DTG | |
| pancreas cancer | mouse | Panc-02 | SV-119 |
| human | Panc-01 | SV-119 | |
| human | AsPc-1 | SV-119 | |
| human | CFPAC | SV-119 | |
| prostate | human | LNCaP | [3H]DTG |
| sarcoma | human | primary tumor | [3H]DTG |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiadis, M.-O.; Karoutzou, O.; Foscolos, A.-S.; Papanastasiou, I. Sigma Receptor (σR) Ligands with Antiproliferative and Anticancer Activity. Molecules 2017, 22, 1408. https://doi.org/10.3390/molecules22091408
Georgiadis M-O, Karoutzou O, Foscolos A-S, Papanastasiou I. Sigma Receptor (σR) Ligands with Antiproliferative and Anticancer Activity. Molecules. 2017; 22(9):1408. https://doi.org/10.3390/molecules22091408
Chicago/Turabian StyleGeorgiadis, Markos-Orestis, Olga Karoutzou, Angeliki-Sofia Foscolos, and Ioannis Papanastasiou. 2017. "Sigma Receptor (σR) Ligands with Antiproliferative and Anticancer Activity" Molecules 22, no. 9: 1408. https://doi.org/10.3390/molecules22091408






