Multifunctional Cinnamic Acid Derivatives
Abstract
:1. Introduction
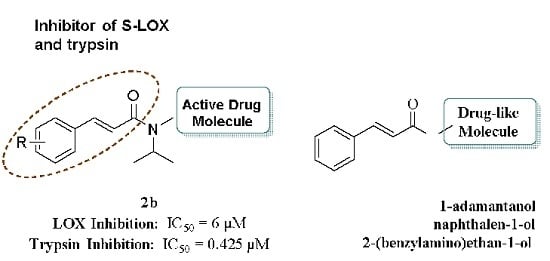
2. Results and Discussion
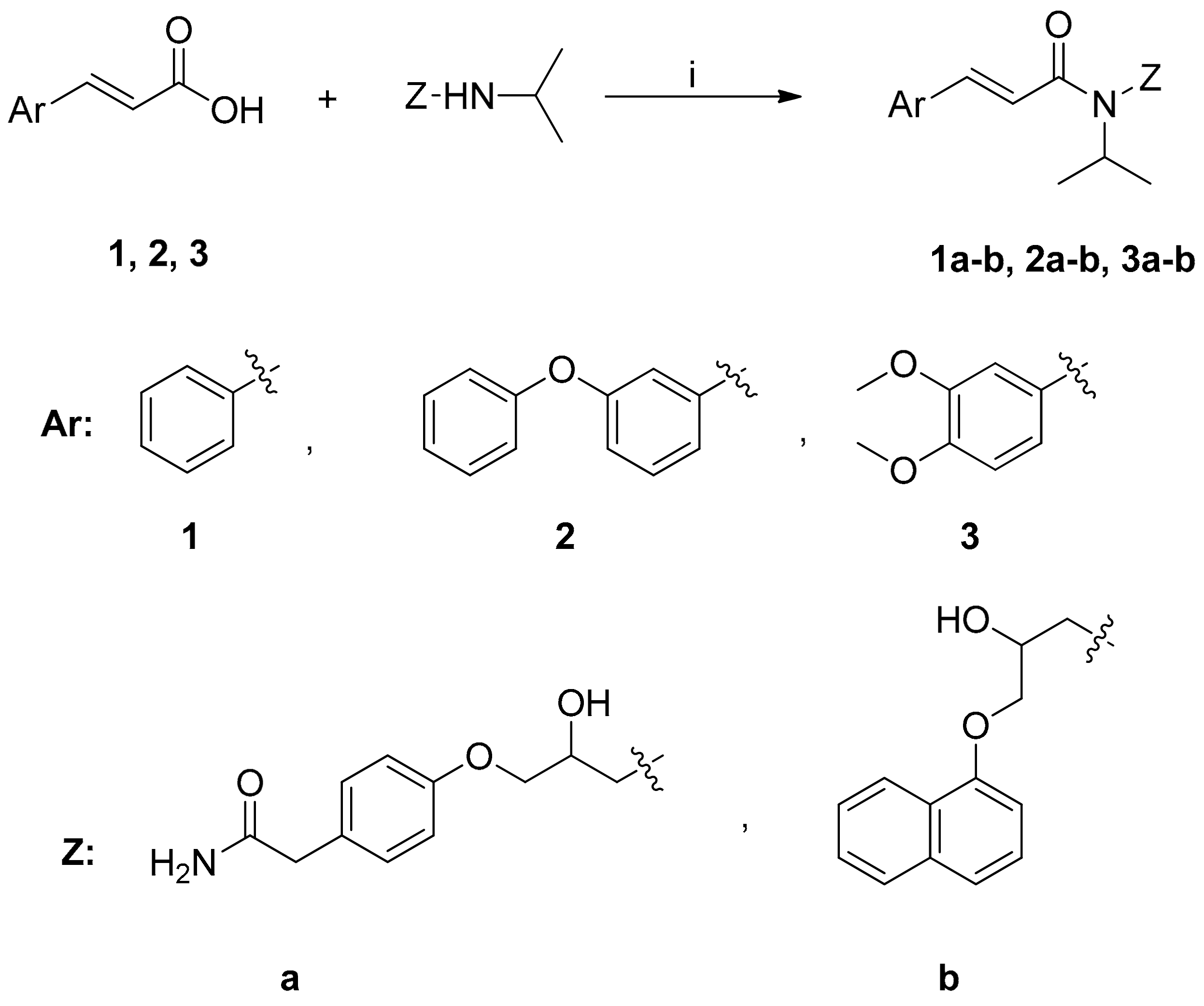
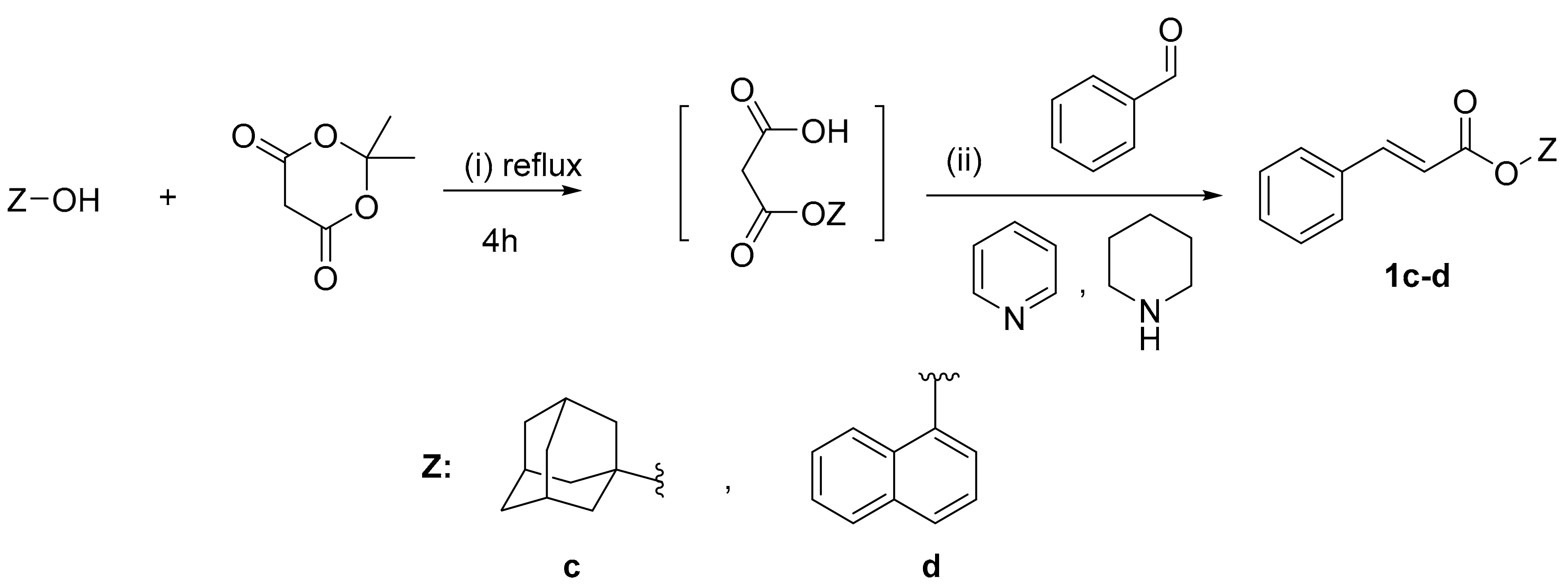
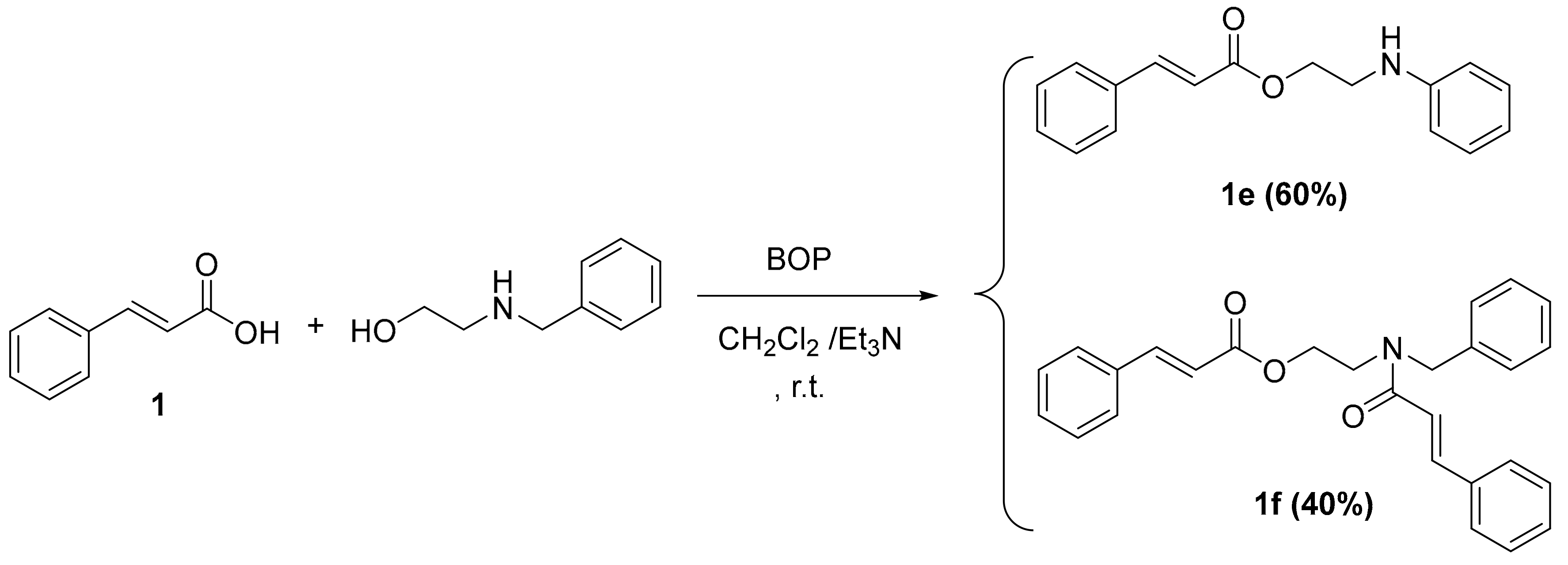
2.1. Chemistry
2.2. Physicochemical Studies
2.3. Biological Evaluation
2.4. Computational Studies—Docking Simulations on Soybean Lipoxygenase
3. Experimental Section
3.1. General Information
3.2. Chemistry General Procedure
3.2.1. General Procedure for One Pot Synthesis of Cinnamic Amides 1a–b, 2a–b and 3a–b
3.2.2. Synthesis of (S)-N-(2-hydroxy-3-(naphth-1-yloxy) propyl)-N-isopropyl-3-(3-phenoxyphenyl) acrylamide (S-2b)
3.2.3. General Procedure for the One Pot Synthesis of Cinnamic Esters Derivatives 1c–d
3.2.4. General Procedure for the One Pot Synthesis of Cinnamic Esters Derivatives 1e–f
3.3. Physicochemical Studies
Determination of RM Values
3.4. Biological In Vitro Assays
3.4.1. Inhibition of Linoleic Acid Lipid Peroxidation
3.4.2. Soybean Lipoxygenase Inhibition Study In Vitro
3.4.3. Inhibition of Trypsin Induced Proteolysis In Vitro
3.4.4. Evaluation of the Cytotoxicity
3.5. Computational Methods, Docking Simulations
Molecular Docking Studies on Soybean Lipoxygenase
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AAPH | 2,2-azobis (2-amidinopropane) dihydrochloride |
| ACE | Angiotensin converting enzyme |
| ACPYPE | AnteChamber PYthon Parser interface |
| BOP | O-(benzotriazol-1-yl)-N,N,N′,N″-tetramethyluronium hexafluorophosphate |
| CA | cinnamic acid |
| LOX | Lipoxygenase |
| NDGA | nordihydroguaiaretic acid |
| Prep TLC | Preparative Thin Layer Chromatography |
| RPTLC | Reverse-phase thin layer chromatography |
References
- Pontiki, E.; Hadjipavlou-Litina, D. Antioxidant and anti-inflammatory activity of aryl-acetic and hydroxamic acids as novel lipoxygenase inhibitors. Med. Chem. 2006, 2, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D.; Geromichalos, G.; Papageorgiou, A. Anticancer activity and quantitative-structure activity relationship (QSAR) studies of a series of antioxidant/anti-inflammatory aryl-acetic and hydroxamic acids. Chem. Biol. Drug Des. 2009, 74, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D. Synthesis and pharmacochemical evaluation of novel aryl-acetic acid inhibitors of lipoxygenase, antioxidants, and anti-inflammatory agents. Bioorg. Med. Chem. 2007, 15, 5819–5827. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Ahmad, S.; Ajaz Rasool, S.; Asad Sayeed, S.; Siddiqi, R. Antibacterial activity directed isolation of compounds from Onosma hispidum. Microbiol. Res. 2006, 161, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; D’Aquino, M.; Tomassi, G.; Gentili, V.; Di-Felice, M.; Scaccini, C. Inhibition of human low-density lipoprotein oxidation by caffeic acid and other hydroxycinnamic acid derivatives. Free Radic. Biol. Med. 1995, 19, 541–552. [Google Scholar] [CrossRef]
- Yasuko, K.; Tomohiro, N.; Sei-Itsu, M.; Ai-Na, L.; Yasuo, F.; Takashi, T. Caffeic acid is a selective inhibitor for leukotriene biosynthesis. Biochim. Biophys. Acta 1984, 792, 92–97. [Google Scholar] [CrossRef]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The Neuroprotective Effects of Phenolic Acids: Molecular Mechanism of Action. Nutrients 2017. [Google Scholar] [CrossRef]
- Zang, L.Y.; Cosma, G.; Gardner, H.; Shi, X.; Castranova, V.; Vallyathan, V. Effect of Antioxidant Protection by P-coumaric acid on Low-density Lipoprotein Cholesterol Oxidation. Am. J. Physiol. Cell Physiol. 2000, 279, C954–C960. [Google Scholar] [PubMed]
- Mnafgui, K.; Derbali, A.; Sayadi, S.; Gharsallah, N.; Elfeki, A.S.; Allouche, N. Anti-obesity and cardioprotective effects of cinnamic acid in high fat diet- induced obese rats. J. Food Sci. Technol. 2015, 52, 4369–4377. [Google Scholar] [CrossRef] [PubMed]
- Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev. Med. Chem. 2012, 12, 749–7671. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Mincione, E.; Barontini, M.; Provenzano, G.; Setti, L. Obtaining 4-vinylphenols by 598 decarboxylation of natural 4-hydroxycinnamic acids under microwave irradiation. Tetrahedron 2007, 63, 9663–9667. [Google Scholar] [CrossRef]
- Paolini, G.; Shapland, R.; Van Hoorn, W.; Mason, J.; Hopkins, A. Global mapping of pharmacological space. Nat. Biotechnol. 2006, 24, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, M.; Goh, K.; Cusick, M.; Barabasi, A.; Vidal, M. Drug-target network. Nat. Biotechnol. 2007, 25, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Durrant, J.; Amaro, R.; Xie, L.; Urbaniak, M.D.; Ferguson, M.A.J.; Haapalainen, A.; Hijun Chen, Z.; Di Guilmi, A.M.; Wunder, F.; Bourne, P.E.; et al. A multidimensional strategy to detect polypharmacological targets in the absence of structural and sequence homology. PLoS Comput. Biol. 2010, 6, e1000648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oprea, T.; Mestres, J. Drug Repurposing: Far Beyond New Targets for Old Drugs. AAPS J. 2012, 14, 759–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boran, A.; Iyengar, R. Systems approaches to polypharmacology and drug discovery. Curr. Opin. Drug Discov. Dev. 2010, 13, 297–309. [Google Scholar]
- Bezerra, D.P.; Castro, F.O.; Alves, A.P.N.N.; Pessoa, C.; Moraes, M.O.; Silveira, E.R.; Lima, M.A.S.; Elmiro, F.J.M.; Costa-Lotufo, L.V. In vivo growth-inhibition of Sarcoma 180 by piplartine and piperine, two alkaloid amides from Piper. Braz. J. Med. Biol. Res. 2006, 39, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; Shin, J.C. Characterization of antioxidant alkaloids and phenolic acids from 601 anthocyanin-pigmented rice (Oryza sativa cv. Heugjinjubyeo). Food Chem. 2007, 104, 1670–1677. [Google Scholar] [CrossRef]
- Yee, E.M.H.; Pasquier, E.; Iskander, G.; Wood, K.; StC Black, D.; Kumar, N. Synthesis of novel isoflavene—Propranolol hybrids as anti-tumor agents. Biorgan. Med. Chem. 2013, 21, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Soda, M.; Hu, D.; Endo, S.; Takemura, M.; Li, J.; Wada, R.; Ifuku, S.; Zhao, H.T.; El-Kabbani, O.; Ohta, S.; et al. Design, synthesis and evaluation of caffeic acid phenethyl ester-based inhibitors targeting a selectivity pocket in the active site of human aldo-keto reductase 1B10. Eur. J. Med. Chem. 2012, 48, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Maji, D.; Samanta, S.; Sinha, R.K. Design, Synthesis and Antidiabetic, Cardiomyopathy Studies of Cinnamic Acid-Amino Acid Hybrid Analogs. Med. Chem. 2014, 4, 2–8. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Nicolotti, O.; Carotti, A. Design, synthesis and pharmacobiological evaluation of novel acrylic acid derivatives acting as lipoxygenase and cyclooxygenase-1 inhibitors with antioxidant and anti-inflammatory activities. Eur. J. Med. Chem. 2011, 46, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Geromichalos, G. Novel cinnamic acid derivatives as antioxidant and anticancer agents: Design, synthesis and modeling studies. Molecules 2014, 19, 9655–9674. [Google Scholar] [CrossRef] [PubMed]
- Peperidou, A.; Kapoukranidou, D.; Kontogiorgis, C.; Hadjipavlou-Litina, D. Multitarget molecular hybrids of cinnamic acids. Molecules 2014, 19, 20197–20226. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, E.; Fakhri, A.; Joshaghania, M. Coumarins: Facile and Expeditious Synthesis via 648 Keggin-Type Heteropolycompounds under Solvent-Free Condition. J. Heterocycl. Chem. 2013, 50, 1121–1128. [Google Scholar]
- Hadjipavlou-Litina, D.; Bariamis, S.; Militsopoulou, M.; Athanassopoulos, C.M.; Papaioannou, D. Trioxsalen derivatives with lipoxygenase inhibitory activity. J. Enzym. Inhib. Med. Chem. 2009, 24, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Shanbag, V.R.; Crider, M.A.; Gohkale, R.; Harpalani, A.; Dick, R.M. Ester and amide prodrugs 654 of ibuprofen and naproxen: Synthesis, anti-inflammatory activity, and gastrointestinal toxicity. J. Pharm. Sci. 1992, 81, 149–154. [Google Scholar] [CrossRef]
- Bate-Smith, E.C.; Westall, R.G. Chromatographic behavior and chemical structure in some naturally occurring phenolic substances. Biochim. Biophys. Acta 1950, 4, 427–440. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Packer, L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996, 10, 709–720. [Google Scholar] [PubMed]
- Suzuki, Y.I.; Forman, H.J.; Sevanian, A. Oxidants as stimulators of signal transduction. Free Radic. Biol. Med. 1997, 22, 269–285. [Google Scholar] [CrossRef]
- Esposito, F.; Ammendola, R.; Faraonio, R.; Russo, T.; Cimino, F. Redox control of signal transduction, gene expression and cellular senescence. Neurochem. Res. 2004, 29, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Do antioxidants impair signaling by reactive oxygen species and lipid oxidation products? FEBS Lett. 2012, 586, 3767–3770. [Google Scholar] [CrossRef] [PubMed]
- Liegois, C.; Lermusieau, G.; Colin, S. Measuring antioxidant efficiency of wort, malt, and hops against the 2,2-azobis(2-amidinopropane) dihydrochloride-induced oxidation of an aqueous dispersion of linoleic acid. J. Agric. Food Chem. 2000, 48, 1129–1134. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Crooks, S.W.; Stockley, R.A. Leukotriene B4. Int. J. Biochem. Cell Biol. 1998, 30, 173–178. [Google Scholar] [CrossRef]
- Laham, F.; Kälsch, A.-I.; Heinrich, L.; Birck, R.; Kallenberg, C.G.M.; Heeringa, P.; Yard, B. Inhibition of neutrophil-mediated production of reactive oxygen species (ROS) by endothelial cells is not impaired in anti-neutrophil cytoplasmic autoantibodies (ANCA)-associated vasculitis patients. Clin. Exp. Immunol. 2010, 161, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Kayama, Y.; Minamino, T.; Toko, H.; Sakamoto, M.; Shimizu, I.; Takahashi, H.; Okada, S.; Tateno, K.; Moriya, J.; Yokoyama, M.; et al. Cardiac 12/15 lipoxygenase-induced inflammation is involved in heart failure. J. Exp. Med. 2009, 206, 1565. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D. Lipoxygenase inhibitors: A comparative QSAR study review and evaluation of new QSARs. Med. Res. Rev. 2008, 28, 39–117. [Google Scholar] [CrossRef] [PubMed]
- Muller, K. 5-Lipoxygenase and 12-lipoxygenase: Attractive targets for the development of novel antipsoriatic drugs. Arch. Pharm. 1994, 327, 3–19. [Google Scholar] [CrossRef]
- Dengler, W.A.; Schulte, J.; Berger, D.P.; Mertelsmann, R.; Fiebig, H.H. Development of a propidium iodide fluorescence assay for proliferation and cytotoxicity assays. Anti Cancer Drugs 1995, 6, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Yamazaki, C.; Yamazaki, M. Luteolin as an Anti-inflammatory and Anti-allergic Constituent of Perilla frutescens. Biol. Pharm. Bull. 2002, 25, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.-Y.; Lim, S.; Han, S.-M.; Park, H.-P.; Shin, I.; Kim, J.; Kim, N.; Lee, J.; Kim, K.; Kim, C. Harpagophytum procumbens Suppresses Lipopolysaccharide-Stimulated Expressions of Cyclooxygenase-2 and Inducible Nitric Oxide Synthase in Fibroblast Cell Line L929. J. Pharmacol. Sci. 2003, 93, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Rekker, R.F. The Hydrophobic Fragmental Constant. Pharmacochem. Libr. 1977, 1, 19. [Google Scholar]
- CambridgeSoft, USA, ChemDraw ChemOffice2016. 1986–2009. Available online: http://www.cambridgesoft.com/software/chemDraw/ (accessed on 26 February 2013).
- OpenBabel, Version 2.2.3. 2006. Available online: http://sourceforge.net/projects/openbabel (accessed on 10 February 2010).
- UCSF Chimera Software. Available online: http://www.cgl.ucsf.edu/chimera (accessed on 10 February 2010).
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model 2006, 25, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 2010, 78, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- AutoDock Vina, version 1.1.1; The Scripps Research Institute: San Diego, CA, USA, 2010.
- Sargis Pallakyan, PyRx-Python Prescription, v.0.5. The Scripps Research Institute, 2008–2010. Available online: http://pyrx.scripps.edu/ (accessed on 10 February 2010).
- Minor, W.; Steczko, J.; Stec, B.; Otwinowski, Z.; Bolin, J.T.; Walter, R.; Axelrod, B. Crystal structure of soybean lipoxygenase L-1 at 1.4 Å resolution. Biochemistry 1996, 35, 10687–10701. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Mol. Biol. 2003, 10, 980. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–3, 1a–f, 2a–b, 3a–b and S-2b are available from the authors. |

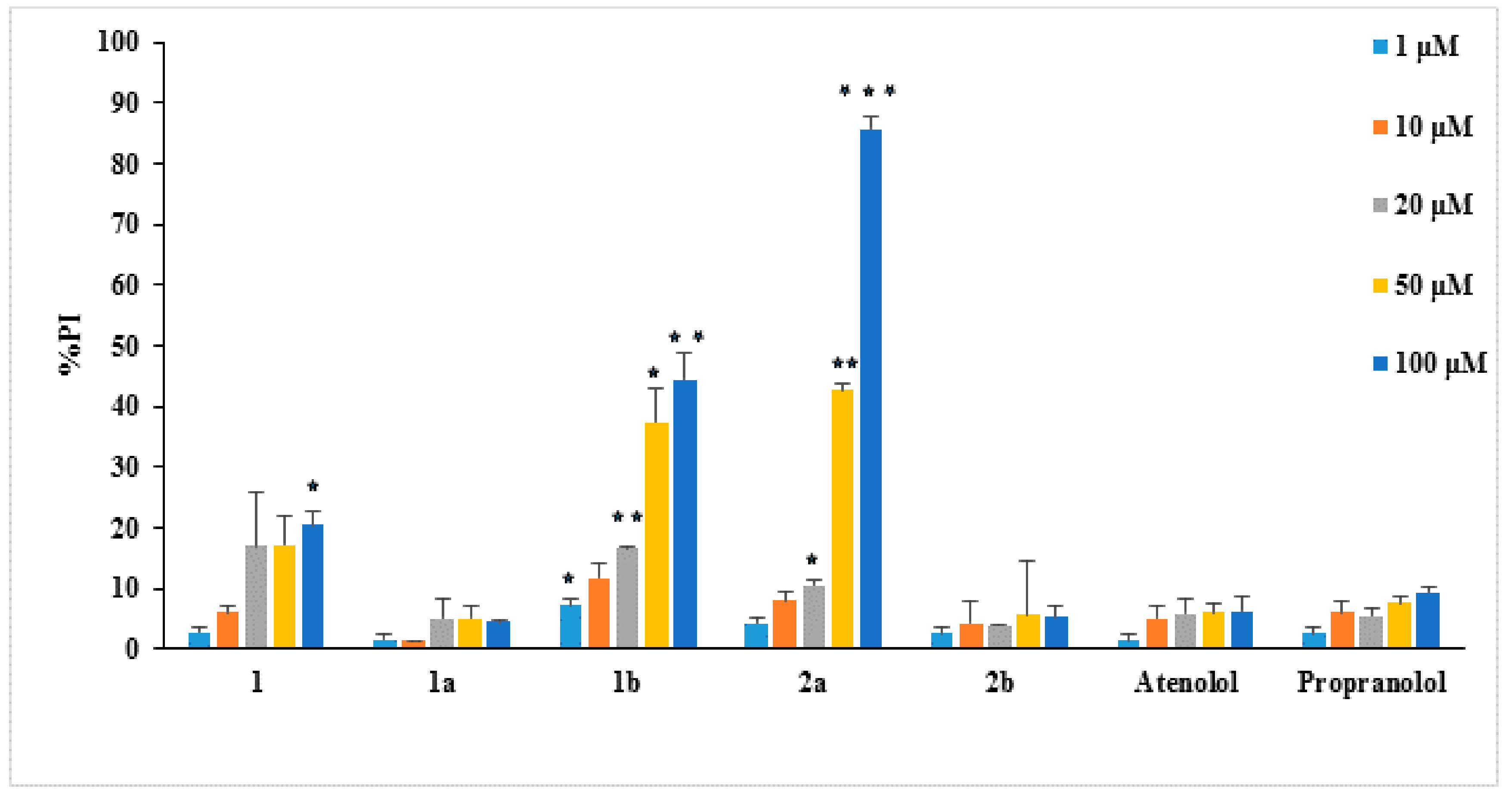


| Compounds | RM a (±SD) b | ILP% @100 μΜ b,c | IC50 μΜ or LOX Inh. % @ 100 μΜ b,c | IC50 μΜ or Trypsin Inh. % @100 μM b,c |
|---|---|---|---|---|
| 1 d | −0.485 ± 0.044 | 78 | 56 μM | 55 μM |
| 2 d | −0.41 ± 0.016 | 84 | 66 μM | na |
| 3 d | −0.869 ± 0.0614 | 86 | na | na |
| 1a | −0.720 ± 0.012 | 97 | 66 μΜ | 0.315 μΜ |
| 1b | 0.411 ± 0.061 | 69 | 50 μΜ | 10 μΜ |
| 2a | 0.128 ± 0.0204 | 86 | 35 μΜ | 0.325 μΜ |
| 2b | 0.907 ± 0.008 | 84 | 6 μΜ | 0.425 μΜ |
| 3a | −0.841 ± 0.0014 | 86 | 10 μΜ | 1 μΜ |
| 3b | 0.0819 ± 0.01 | 71 | 10 μΜ | 0.516 μΜ |
| 1c | 0.911 ± 0.0112 | 20 | na | 49 μΜ |
| 1d | 0.0889 ± 0.002 | na | 27.5 μΜ | na |
| 1e | −0.374 ± 0.0176 | 60 | 100 μΜ | na |
| 1f | 0.1402 ± 0.0227 | na | 20% | 100 μΜ |
| S-2b | 86 | 6 μM | 2.75 μM | |
| NDGA | 0.45 μM | |||
| Trolox | 93 | |||
| Atenolol | na | 22.5 μΜ | na | |
| Propranolol | na | 42 μΜ | na | |
| Salicylic acid | 53.6% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peperidou, A.; Pontiki, E.; Hadjipavlou-Litina, D.; Voulgari, E.; Avgoustakis, K. Multifunctional Cinnamic Acid Derivatives. Molecules 2017, 22, 1247. https://doi.org/10.3390/molecules22081247
Peperidou A, Pontiki E, Hadjipavlou-Litina D, Voulgari E, Avgoustakis K. Multifunctional Cinnamic Acid Derivatives. Molecules. 2017; 22(8):1247. https://doi.org/10.3390/molecules22081247
Chicago/Turabian StylePeperidou, Aikaterini, Eleni Pontiki, Dimitra Hadjipavlou-Litina, Efstathia Voulgari, and Konstantinos Avgoustakis. 2017. "Multifunctional Cinnamic Acid Derivatives" Molecules 22, no. 8: 1247. https://doi.org/10.3390/molecules22081247