Anti-Inflammatory Triterpene Glycosides from the Roots of Ilex dunniana Levl
Abstract
:1. Introduction
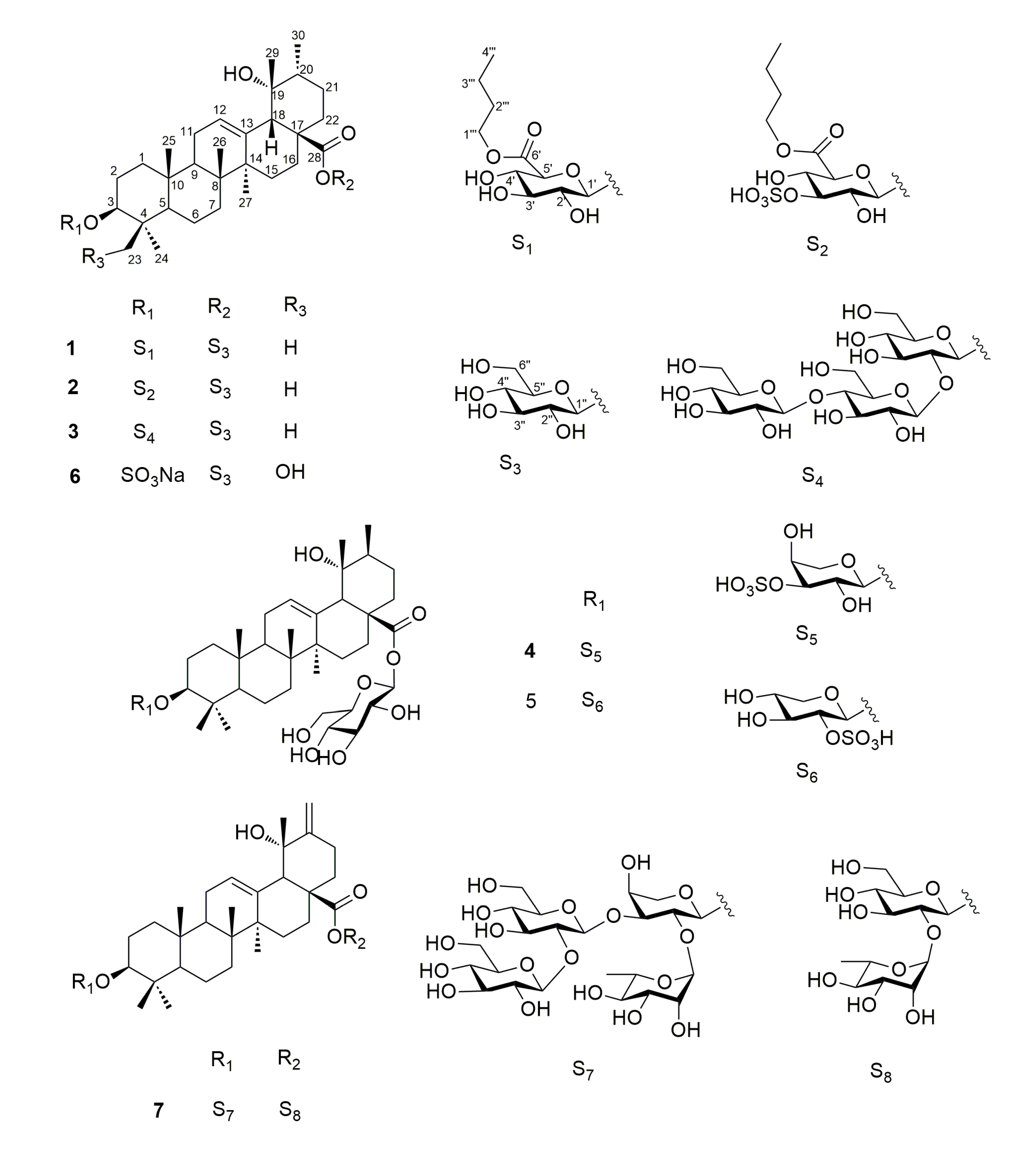
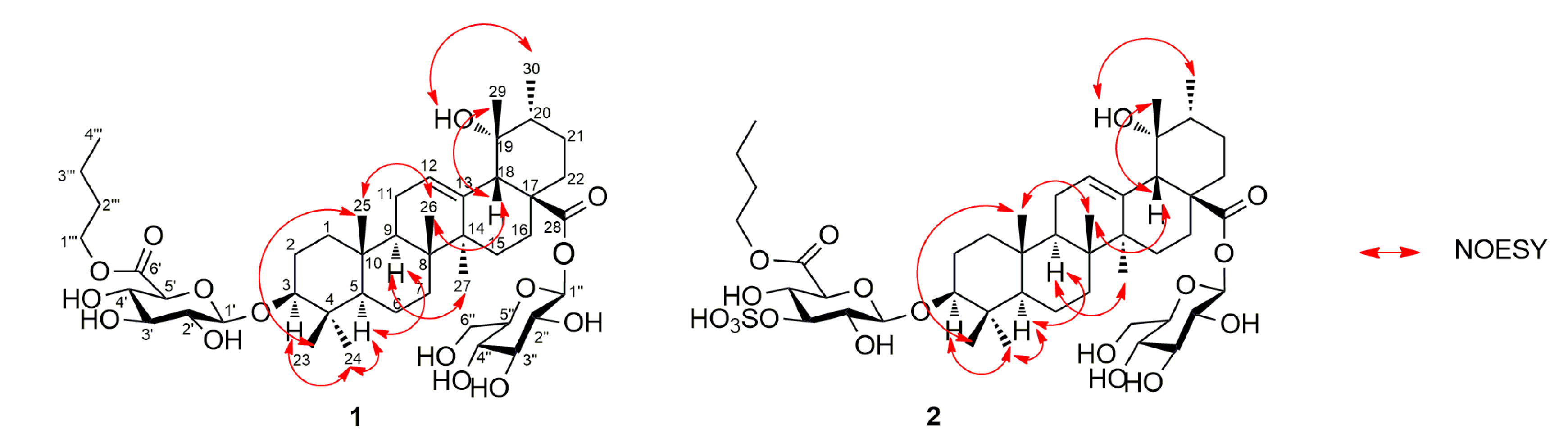
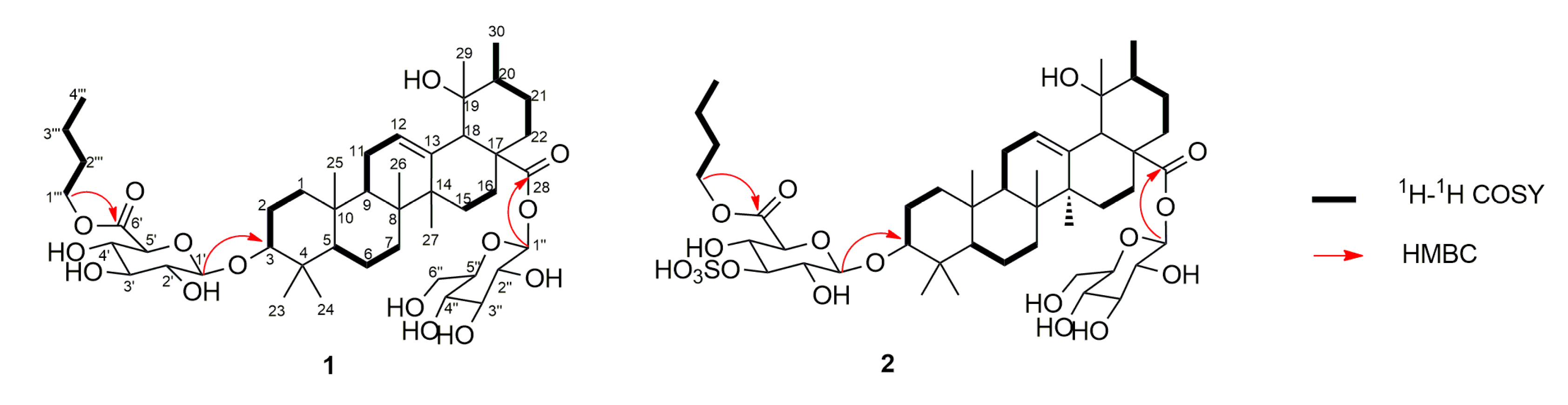
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Product Characterization
3.5. Acid Hydrolysis of Saponins
3.6. Detection of the Sulfate Group
3.7. In Vitro Anti-Inflammatory Activity Assays
3.8. Cytotoxicity Assays
3.9. In Vivo Anti-Inflammatory Assays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Y.; Li, L.; Zhang, P.; Pi, H.F.; Ruan, H.L.; Wu, J.Z. Anti-inflammatory and free radical scavenging activities of ethanol extracts of two plants of Ilex. Acta Med. Univ. Sci. Technol. Huazhong 2011, 40, 71–74. [Google Scholar]
- Song, J.L.; Yang, Y.J.; Qi, H.Y.; Li, Q. Chemical Constituents from Flowers of Gardenia jasminoides. J. Chin. Med. Mater. 2013, 36, 752–755. [Google Scholar]
- Ouyang, M.A. Glycosides from the leaves of Ilex hylonoma. Nat. Prod. Res. 2003, 17, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chai, X.Y.; Zeng, K.W.; Zhang, J.Y.; Li, N.; Jiang, Y.; Tu, P.F. Ilexpublesnins C-M, eleven new triterpene saponins from the roots of Ilex pubescens. Planta Med. 2013, 79, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Amimoto, K.; Yoshikawa, K.; Arihara, S. Triterpenes and triterpene glycosides from the leaves of Ilex rotunda. Phytochemistry 1993, 33, 1475–1480. [Google Scholar] [CrossRef]
- Nishimura, K.; Miyase, T.; Noguchi, H. Triterpenoid saponins from Ilex kudincha. J. Nat. Prod. 1999, 62, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Choi, Y.H.; Kim, N.D.; Park, Y.M.; Kim, G.Y. Anti-Inflammatory effects of beta-lapachone in lipopolysaccharide-stimulated BV2 microglia. Int. Immunopharmacol. 2007, 7, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, J.H.; Zhao, Q.; Liao, C.R.; Ding, L.Q.; Chen, L.X.; Zhao, F.; Qiu, F. Guaiane-type sesquiterpenes from Curcuma phaeocaulis and their inhibitory effects on nitric oxide production. J. Nat. Prod. 2013, 76, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, J.F.; Heller, M. The arginine-nitric oxide pathway: A target for new drugs. Med. Res. Rev. 1994, 14, 23–74. [Google Scholar] [CrossRef] [PubMed]
- Konturek, S.; Konturek, P.C. Role of nitric oxide in the digestive system. Digestion 1995, 56, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, K.; Shichiri, M.; Marumo, F.; Hirata, Y. NO inhibits cytokine-induced iNOS expression and NF-κB activation by interfering with phosphorylation and degradation of IκB-α. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L. Nitric oxide as a unique signaling molecule in vascular system: A historical overview. J. Physiol. Pharmacol. 2002, 53, 503–514. [Google Scholar] [PubMed]
- Wallace, J.L. Nitric oxide as a regulator of inflammatory processes. Mem. Inst. Oswaldo Cruz 2005, 100, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedula, V.S.P.; Schilling, J.K.; Miller, J.S.; Andriantsiferana, R.; Rasamison, V.E.; Kingston, D.G.I. New cytotoxic oleanane saponins from the infructescences of Polyscias amplifolia from the Madagascar rainforest. Planta Med. 2003, 69, 440–444. [Google Scholar] [PubMed]
- Elbandy, M.; Miyamoto, T.; Delaude, C.; Lacaille-Dubois, M.A. Acylated preatroxigenin glycosides from Atroxima congolana. J. Nat. Prod. 2003, 66, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Akai, E.; Takeda, T.; Kobayashi, Y.; Ogihara, Y. Minor triterpenoid saponins from the leaves of Bupleurum rotundifolium L. Chem. Pharm. Bull. 1985, 33, 3715–3723. [Google Scholar] [CrossRef]
- Sacco, R.E.; Waters, W.R.; Rudolph, K.M.; Drew, M.L. Comparative nitric oxide production by LPS-stimulated monocyte-derived macrophages from Ovis canadensis and Ovis aries. Comp. Immunol. Microbiol. Infect. Dis. 2006, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- García-Argáez, A.N.; Ramírez Apan, T.O.; Delgado, H.P.; Velázquez, G.; Martínez-Vázquez, M. Anti-inflammatory activity of coumarins from Decatropis bicolor on TPA ear mice model. Planta Med. 2000, 66, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Mencherini, T.; Cau, A.; Bianco, G.; Loggia, R.D.; Aquino, R.P.; Autore, G. An extract of Apium graveolens var. dulce leaves: Structure of the major constituent, apiin, and its anti-inflammatory properties. J. Pharm. Pharmacol. 2007, 59, 891–897. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–7 are available from the authors. |
| Position | Compound 1 | Compound 2 | ||
|---|---|---|---|---|
| δC | δH a (J in Hz) | δC | δH a (J in Hz) | |
| 1 | 38.7 | 0.84, 1.46 | 38.2 | 0.76, 1.37 |
| 2 | 26.9 | 1.85, 2.12 | 26.4 | 1.88, 1.74 |
| 3 | 89.2 | 3.32 dd (11.7, 4.5) | 88.9 | 3.23 dd (12.0, 4.5) |
| 4 | 39.4 | 39.0 | ||
| 5 | 55.7 | 0.80 | 55.2 | 0.71 |
| 6 | 18.6 | 1.44, 1.28 | 18.1 | 1.37, 1.21 |
| 7 | 33.4 | 1.41, 1.56 | 32.9 | 1.49, 1.31 |
| 8 | 40.4 | 40.0 | ||
| 9 | 47.6 | 1.74 | 47.1 | 1.65 |
| 10 | 36.8 | 36.4 | ||
| 11 | 23.9 | 2.00 | 23.4 | 1.89 |
| 12 | 128.3 | 5.53 br s | 127.8 | 5.41 br s |
| 13 | 139.2 | 138.7 | ||
| 14 | 42.0 | 41.5 | ||
| 15 | 29.1 | 2.44 m, 1.22 | 28.7 | 2.31 m, 1.13 |
| 16 | 26.0 | 3.09 m, 1.99 | 25.5 | 2.95 m, 1.97 |
| 17 | 48.6 | 48.1 | ||
| 18 | 54.3 | 2.90 s | 53.9 | 2.78 s |
| 19 | 72.5 | 72.0 | ||
| 20 | 42.0 | 1.33 | 41.6 | 1.25 |
| 21 | 26.6 | 2.00, 1.84 | 26.0 | 1.86, 1.99 |
| 22 | 37.6 | 2.03, 1.82 | 37.2 | 1.70, 1.89 |
| 23 | 28.0 | 1.33 s | 27.5 | 1.17 s |
| 24 | 16.8 | 0.96 s | 16.3 | 0.88 s |
| 25 | 15.5 | 0.86 s | 15.0 | 0.72 s |
| 26 | 17.3 | 1.15 s | 16.8 | 1.02 s |
| 27 | 24.5 | 1.69 s | 24.0 | 1.58 s |
| 28 | 176.9 | 176.5 | ||
| 29 | 26.9 | 1.39 s | 26.1 | 1.29 s |
| 30 | 16.6 | 1.06 d (8.0) | 16.1 | 0.98 d (6.4) |
| 1′ | 107.3 | 4.95 d (7.7) | 106.2 | 4.89 d (7.6) |
| 2′ | 75.3 | 4.06 | 73.4 | 4.02 |
| 3′ | 77.9 | 4.26 | 83.6 | 5.16 |
| 4′ | 73.0 | 4.48 | 72.0 | 4.40 t (9.1) |
| 5′ | 77.2 | 4.57 | 76.1 | 4.48 d (9.5) |
| 6′ | 170.3 | 169.3 | ||
| 1′′ | 95.7 | 6.26 d (8.0) | 95.3 | 6.13 d (8.0) |
| 2′′ | 73.9 | 4.20 | 71.2 | 4.10 |
| 3′′ | 78.8 | 4.28 | 78.2 | 4.19 |
| 4′′ | 71.1 | 4.31 | 70.6 | 4.20 |
| 5′′ | 79.1 | 4.02 | 78.7 | 3.91 m |
| 6′′ | 62.2 | 4.44, 4.38 | 61.6 | 4.32, 4.27 |
| 1′′′ | 64.9 | 4.26 | 64.6 | 4.19 |
| 2′′′ | 30.8 | 1.56 | 30.3 | 1.48 |
| 3′′′ | 19.2 | 1.30 | 18.7 | 1.22 |
| 4′′′ | 13.7 | 0.75 t (7.3) | 13.2 | 0.68 t (7.1) |
| Extracts | Dose (mg/kg) | Edema Degree (mg) | Inhibitation Rate (%) |
|---|---|---|---|
| 75% Ethanol extract | 50.0 | 15.31 ± 2.01 * | 23.5 |
| n-Butanol extract | 50.0 | 12.50 ± 1.50 ** | 37.5 |
| Dexamethasone a | 1.0 | 5.80 ± 0.90 ** | 71.0 |
| Control group | - | 20.01 ± 2.31 | - |
| Compounds | IC50 (μM) | Compounds | IC50 (μM) |
|---|---|---|---|
| 1 | 11.60 ± 0.89 | 5 | 50.7 ± 3.25 |
| 2 | 12.30 ± 1.21 | 6 | 22.3 ± 2.23 |
| 3 | 9.70 ± 0.86 | 7 | 55.2 ± 3.26 |
| 4 | 33.5 ± 2.11 | dexamethasone a | 0.03 |
| Compounds | Cell Viability | ||||
|---|---|---|---|---|---|
| 5.0 μM | 10.0 μM | 20.0 μM | 40.0 μM | 80.0 μM | |
| 1 | 99.74 ± 1.21 | 99.28 ± 1.32 | 99.35 ± 1.26 | 99.62 ± 1.89 | 99.70 ± 1.36 |
| 2 | 98.32 ± 2.05 | 98.25 ± 1.87 | 98.16 ± 1.25 | 98.56 ± 1.37 | 98.26 ± 1.88 |
| 3 | 91.74 ± 1.11 * | 90.28 ± 1.82 * | 89.35 ± 1.35 * | 88.62 ± 1.21 * | 88.50 ± 1.32 * |
| 4 | 99.36 ± 1.31 | 99.37 ± 1.21 | 99.67 ± 1.32 | 99.57 ± 1.53 | 99.33 ± 1.30 |
| 5 | 98.21 ± 1.93 | 99.33 ± 1.54 | 99.32 ± 1.32 | 99.21 ± 1.66 | 99.13 ± 1.28 |
| 6 | 102.21 ± 1.03 | 102.28 ± 1.56 | 103.35 ± 1.58 | 103.26 ± 1.28 | 103.22 ± 1.89 |
| 7 | 101.55 ± 1.42 | 101.68 ± 1.36 | 101.76 ± 1.47 | 101.38 ± 1.58 | 101.68 ± 1.38 |
| Control group | 100.00 ± 1.51 | 100.00 ± 1.26 | 100.00 ± 1.56 | 100.00 ± 1.78 | 100.00 ± 1.55 |
| Dexamethasone a | 101.02 ± 1.01 | 99.98 ± 1.32 | 100.25 ± 1.67 | 101.37 ± 1.83 | 99.78 ± 1.63 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.-S.; Zhang, Y.; Hu, W.-Z.; Chen, X.; Fu, X.; Lv, X.; Zhang, L.-H.; Zhang, N.; Li, G. Anti-Inflammatory Triterpene Glycosides from the Roots of Ilex dunniana Levl. Molecules 2017, 22, 1206. https://doi.org/10.3390/molecules22071206
Shi Y-S, Zhang Y, Hu W-Z, Chen X, Fu X, Lv X, Zhang L-H, Zhang N, Li G. Anti-Inflammatory Triterpene Glycosides from the Roots of Ilex dunniana Levl. Molecules. 2017; 22(7):1206. https://doi.org/10.3390/molecules22071206
Chicago/Turabian StyleShi, Yu-Sheng, Yan Zhang, Wen-Zhong Hu, Xi Chen, Xin Fu, Xia Lv, Li-Hong Zhang, Ning Zhang, and Guang Li. 2017. "Anti-Inflammatory Triterpene Glycosides from the Roots of Ilex dunniana Levl" Molecules 22, no. 7: 1206. https://doi.org/10.3390/molecules22071206




