Akanthopyrones A–D, α-Pyrones Bearing a 4-O-Methyl-β-d-glucopyranose Moiety from the Spider-Associated Ascomycete Akanthomyces novoguineensis
Abstract
:1. Introduction
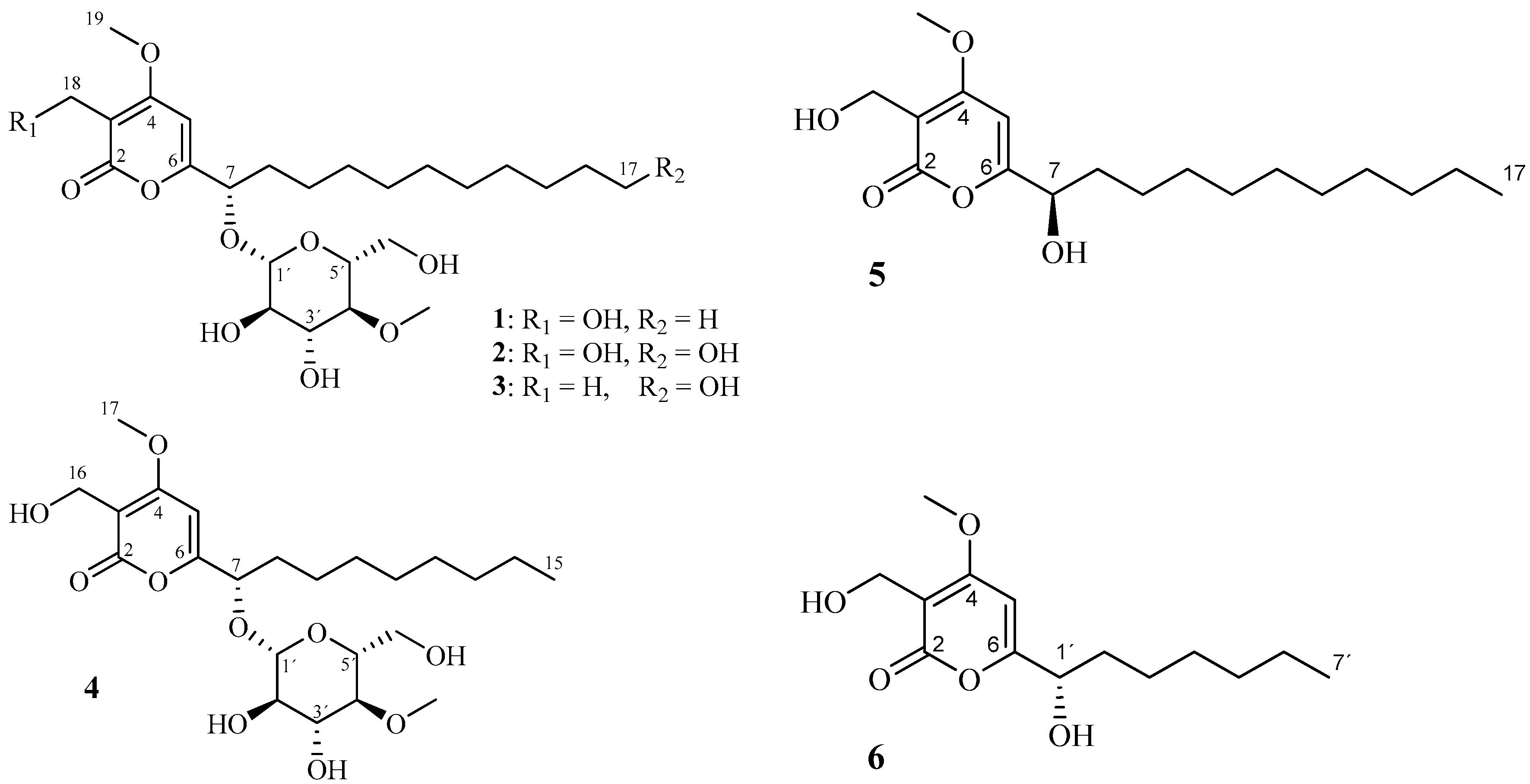
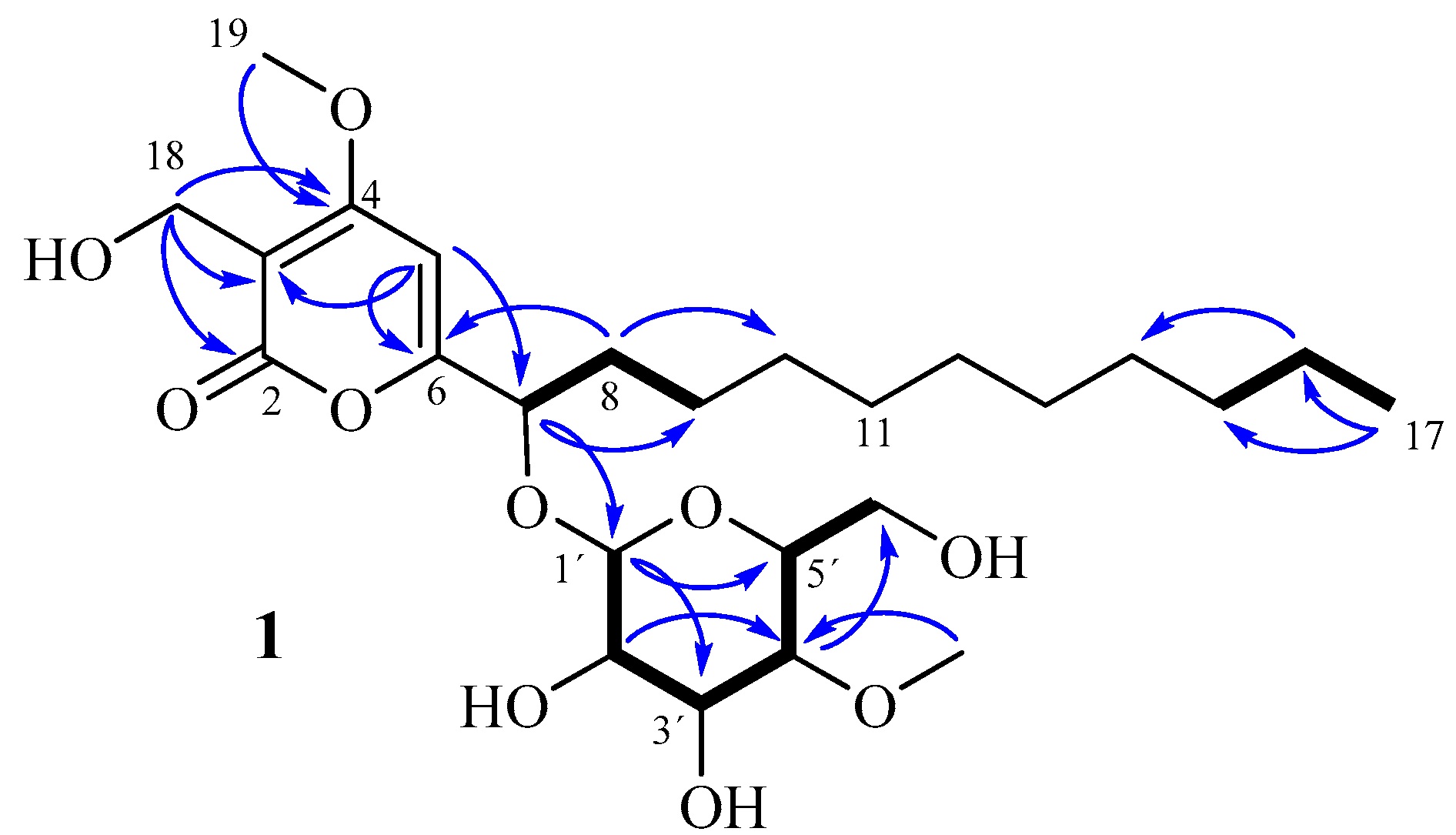
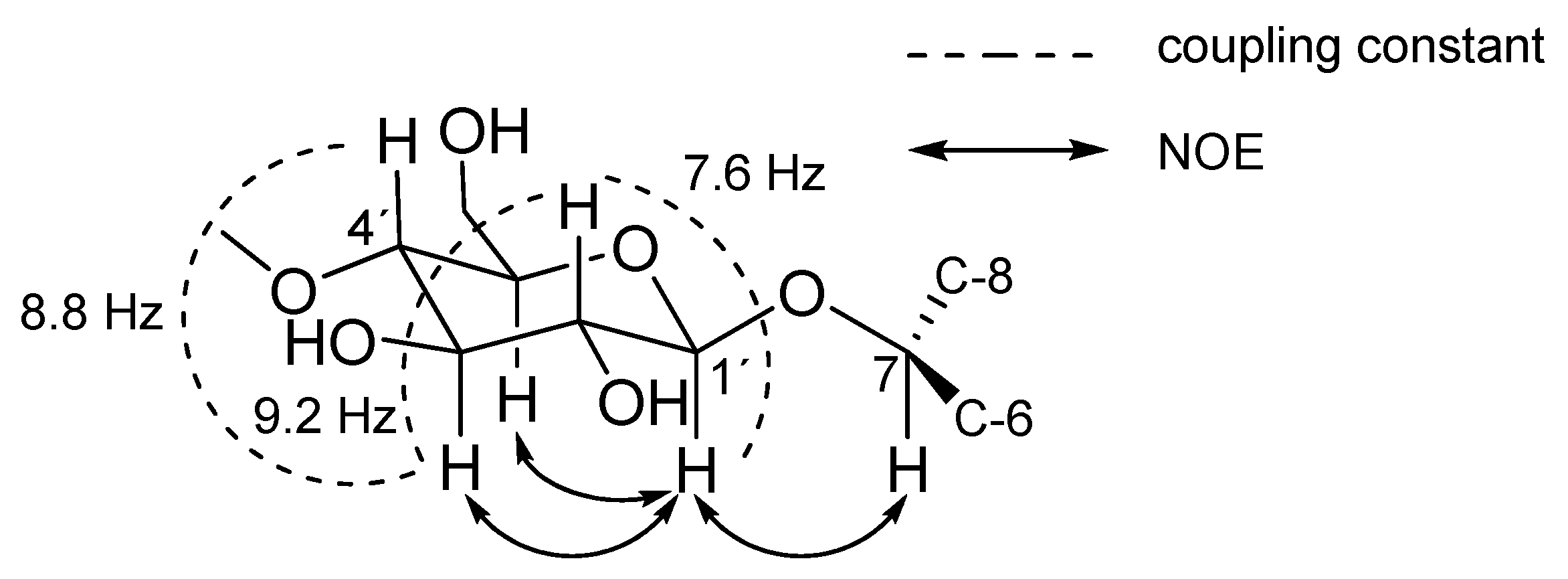
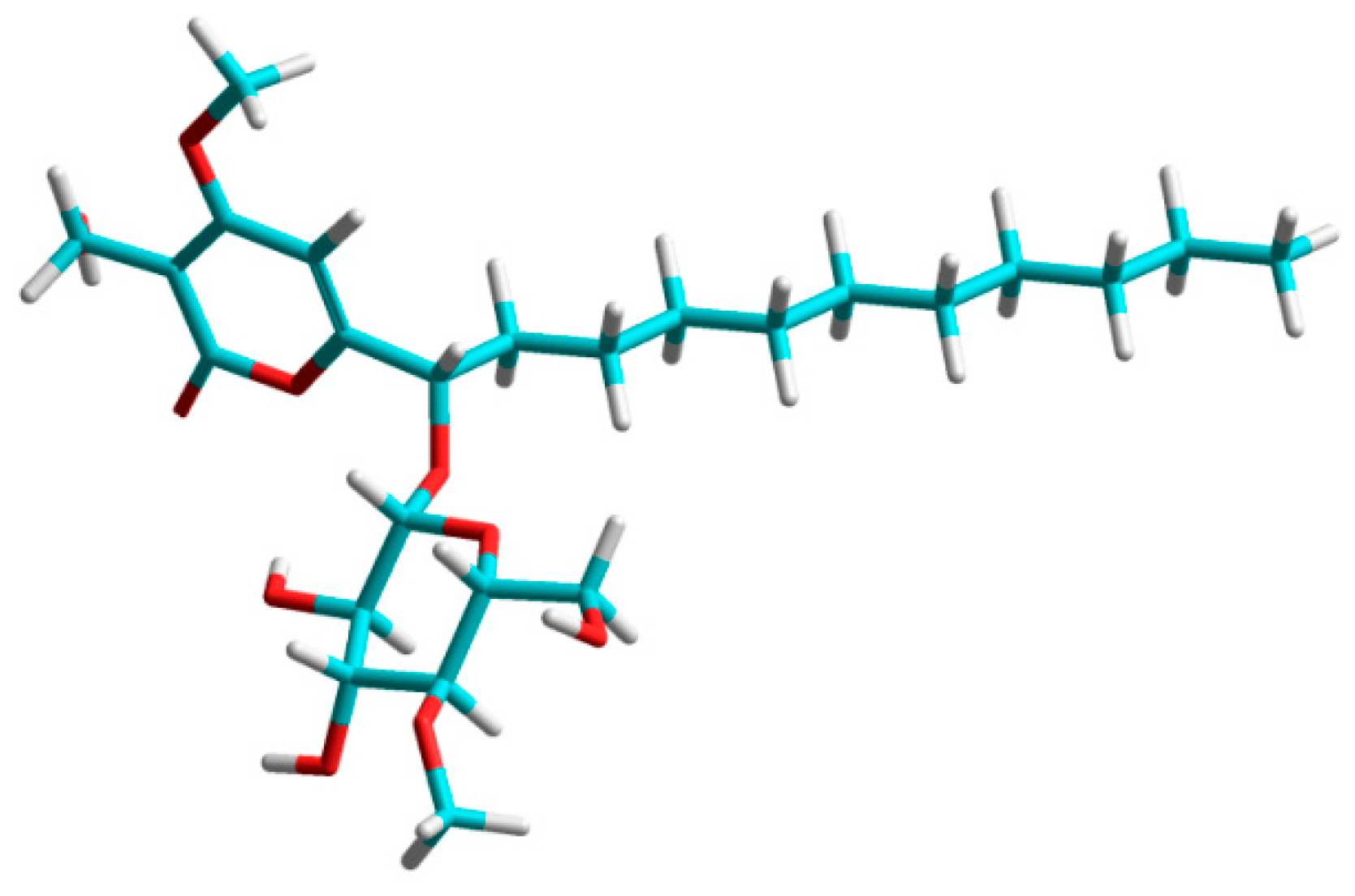
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Fungal Material
3.3. Fermentation and Extraction
3.4. Isolation of Compounds 1–4
3.5. Hydrolysis of Akanthopyrone A (1)
3.6. Biological Activities
3.6.1. Antimicrobial Activity Assay
3.6.2. Cytotoxicity Activity Assay
3.6.3. Anti-biofilm Activity Assay
3.6.4. Nematicidal Activity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Karwehl, S.; Stadler, M. Exploitation of Fungal Biodiversity for Discovery of Novel Antibiotics. Curr. Top. Microbiol. Immunol. 2016, 398, 303–338. [Google Scholar] [PubMed]
- Molnár, I.; Gibson, D.M.; Krasnoff, S.B. Secondary metabolites from entomopathogenic Hypocrealean fungi. Nat. Prod. Rep. 2010, 27, 1241–1275. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Kittakoop, P.; Kirtikara, K.; Hywel-Jones, N.L.; Thebtaranonth, Y. Bioactive substances from insect pathogenic fungi. Acc. Chem. Res. 2005, 38, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Humber, R.A. Evolution of entomopathogenicity in fungi. J. Invertebr. Pathol. 2008, 98, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Mains, E.B. Entomogenous Species of Akanthomyces, Hymenostilbe and Insecticola in North America. Mycol. Soc. Am. 1950, 42, 566–589. [Google Scholar] [CrossRef]
- Hsieh, L.S.; Tzean, S.S.; Wu, W.J. The genus Akanthomyces on spiders from Taiwan. Mycol. Soc. Am. 1997, 89, 319–324. [Google Scholar] [CrossRef]
- Hywel-Jones, N. Akanthomyces on spiders in Thailand. Mycol. Res. 1996, 100, 1065–1070. [Google Scholar] [CrossRef]
- Samson, R.A.; Brady, B.L. Akanthomyces novoguineensis sp. nov. Trans. Br. Mycol. Soc. 1982, 79, 571–572. [Google Scholar] [CrossRef]
- Samson, R.A.; Evans, H.C. Notes on entomogenous fungi from Ghana. II. The genus Akanthomyces. Plant Biol. 1974, 23, 28–35. [Google Scholar] [CrossRef]
- Kinoshita, H.; Wongsuntornpoj, S.; Ihara, F.; Nihira, T. Anti-Rhodotorula activity of mycophenolic acid enhanced in the presence of polyene-antibiotic nystatin. Lett. Appl. Microbiol. 2016, 64, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Kuephadungphan, W.; Phongpaichit, S.; Luangsa-ard, J.J.; Rukachaisirikul, V. Antimicrobial activity of invertebrate-pathogenic fungi in the genera Akanthomyces and Gibellula. Mycoscience 2013, 55, 127–133. [Google Scholar] [CrossRef]
- Lee, S.Y.; Nakajima, I.; Ihara, F.; Kinoshita, H.; Nihira, T. Cultivation of entomopathogenic fungi for the search of antibacterial compounds. Mycopathologia 2005, 160, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, M.M.; Gibson, D.M.; Clardy, J. Akanthomycin, a new antibiotic pyridone from the entomopathogenic fungus Akanthomyces gracilis. Org. Lett. 2002, 4, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Yamamoto, T.; Oshima, Y. Aromatic polyketide production in Cordyceps indigotica, an entomopathogenic fungus, induced by exposure to a histone deacetylase inhibitor. Org. Lett. 2012, 14, 2006–2009. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, H.; Takahashi, N.; Oshima, Y. Novel aromatics bearing 4-O-methylglucose unit isolated from the oriental crude drug Bombyx Batryticatus. Tetrahedron Lett. 2004, 45, 367–370. [Google Scholar] [CrossRef]
- Isaka, M.; Palasarn, S.; Supothina, S.; Komwijit, S.; Luangsa-Ard, J.J. Bioactive compounds from the scale insect pathogenic fungus Conoideocrella tenuis BCC 18627. J. Nat. Prod. 2011, 74, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Kornsakulkarn, J.; Thongpanchang, C.; Lapanun, S.; Srichomthong, K. Isocoumarin glucosides from the scale insect fungus Torrubiella tenuis BCC 12732. J. Nat. Prod. 2009, 72, 1341–1343. [Google Scholar] [CrossRef] [PubMed]
- Kornsakulkarn, J.; Saepua, S.; Srichomthong, K.; Supothina, S.; Thongpanchang, C. New mycotoxins from the scale insect fungus Aschersonia coffeae Henn. BCC 28712. Tetrahedron 2012, 68, 8480–8486. [Google Scholar] [CrossRef]
- Saepua, S.; Kornsakulkarn, J.; Choowong, W.; Supothina, S.; Thongpanchang, C. Bioxanthracenes and monomeric analogues from insect pathogenic fungus Conoideocrella luteorostrata Zimm. BCC 31648. Tetrahedron 2015, 71, 2400–2408. [Google Scholar] [CrossRef]
- Seephonkai, P.; Isaka, M.; Kittakoop, P.; Boonudomlap, U.; Thebtaranonth, Y. A novel ascochlorin glycoside from the insect pathogenic fungus Verticillium hemipterigenum BCC 2370. J. Antibiot. 2004, 57, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Helaly, S.E.; Kuephadungphan, W.; Phongpaichit, S.; Luangsa-ard, J.J.; Rukachaisirikul, V.; Stadler, M. Five unprecedented secondary metabolites from the spider parasitic fungus Akanthomyces novoguineensis. Molecules 2017, 22, 991. [Google Scholar] [CrossRef] [PubMed]
- Giner, J.L.; Feng, J.; Kiemle, D.J. NMR tube degradation method for sugar analysis of glycosides. J. Nat. Prod. 2016, 79, 2413–2417. [Google Scholar] [CrossRef] [PubMed]
- Smith, F. The constitution of mesquite gum. Part III. The structure of the monomethyl glucuronic acid component. J. Chem. Soc. 1951, 2646–2652. [Google Scholar] [CrossRef]
- Pažoutová, S.; Follert, S.; Bitzer, J.; Keck, M.; Surup, F.; Šrůtka, P.; Holuša, J.; Stadler, M. A new endophytic insect-associated Daldinia species, recognised from a comparison of secondary metabolite profiles and molecular phylogeny. Fungal Divers. 2013, 60, 107–123. [Google Scholar] [CrossRef]
- Chomcheon, P.; Wiyakrutta, S.; Sriubolmas, N.; Ngamrojanavanich, N.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Metabolites from the endophytic mitosporic Dothideomycete sp. LRUB20. Phytochemistry 2009, 70, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Tomita, Y.; Tori, K.; Yoshimura, Y. Determination of the absolute configuration of a secondary hydroxy group in a chiral secondary alcohol using glycosidation shifts in carbon-13 nuclear magnetic resonance spectroscopy. J. Am. Chem. Soc. 1978, 100, 3331–3339. [Google Scholar] [CrossRef]
- McGlacken, G.P.; Fairlamb, I.J.S. 2-Pyrone natural products and mimetics: Isolation, characterisation and biological activity. Nat. Prod. Rep. 2005, 22, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Fairlamb, I.J.S.; Marrison, L.R.; Dickinson, J.M.; Lu, F.J.; Schmidt, J.P. 2-Pyrones possessing antimicrobial and cytotoxic activities. Bioorg. Med. Chem. 2004, 12, 4285–4299. [Google Scholar] [CrossRef] [PubMed]
- Helaly, S.; Schneider, K.; Nachtigall, J.; Vikineswary, S.; Tan, G.Y.A.; Zinecker, H.; Imhoff, J.F.; Süssmuth, R.D.; Fiedler, H.-P. Gombapyrones, new α-pyrone metabolites produced by Streptomyces griseoruber Acta 3662. J. Antibiot. 2009, 62, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.; Su, Y.-S.; Cheng, M.-J.; Liu, T.-W.; Chen, I.-S.; Wu, M.-D.; Chang, H.-S.; Yuan, G.-F. Chemical constituents from a soil-derived Actinomycete, Actinomadura miaoliensis BCRC 16873, and their inhibitory activities on lipopolysaccharide-induced tumor necrosis factor production. Chem. Biodivers. 2013, 10, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Noumeur, S.R.; Helaly, S.E.; Jansen, R.; Gereke, M.; Stradal, T.E.B.; Harzallah, D.; Stadler, M. Preussilides A–F, bicyclic polyketides from the endophytic fungus Preussia similis with antiproliferative activity. J. Nat. Prod. 2017, 80, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Luangsa-ard, J.J.; Mongkolsamrit, S.; Thanakitpipattana, D.; Khonsanit, A.; Tasanathai, K.; Noisripoom, W.; Humber, R.A. Clavicipitaceous entomopathogens: New species in Metarhizium and a new genus Nigelia. Mycol. Prog. 2017, 16, 369–391. [Google Scholar] [CrossRef]
- Phainuphong, P.; Rukachaisirikul, V.; Saithong, S.; Phongpaichit, S.; Bowornwiriyapan, K.; Muanprasat, C.; Srimaroeng, C.; Duangjai, A.; Sakayaroj, J. Lovastatin analogues from the soil-derived fungus Aspergillus sclerotiorum PSU-RSPG178. J. Nat. Prod. 2016, 79, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Helaly, S.E.; Thongbai, B.; Hyde, K.D.; Stadler, M. Pyristriatins A and B: Pyridino-cyathane antibiotics from the Basidiomycete Cyathus cf. striatus. J. Nat. Prod. 2016, 79, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Rahme, L.G.; Stevens, E.J.; Wolfort, S.F.; Shao, J.; Tompkins, R.G.; Ausubel, F.M. Common virulence factors for bacterial pathogenicity in plants and animals. Science 1995, 268, 1899–1902. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.; Anke, H.; Arendholz, W.-R.; Hansske, F.; Anders, U.; Sterner, O.; Bergquist, K.-E. Lachnumon and lachnumol A, new metabolites with nematicidal and antimicrobial activities from the ascomycete Lachnum papyraceum (Karst.) Karst. J. Antibiot. 1993, 46, 961–967. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–4 are available from the authors. |
| Pos | 1 a | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | |
| 2 | - | 165.1, C | - | 167.1, C | - | 167.9, C | - | 167.8, C |
| 3 | - | 104.4, C | - | 105.1, C | - | 102.1, C | - | 105.1, C |
| 4 | - | 167.4, C | - | 170.8, C | - | 169.0, C | - | 170.8, C |
| 5 | 6.76, s | 94.9, CH | 7.06, s | 96.6, CH | 7.00, s | 96.8, CH | 7.06, s | 96.6, CH |
| 6 | - | 165.3, C | - | 167.8, C | - | 164.9, C | - | 167.0, C |
| 7 | 4.51, b dd (7.3, 4.7) | 76.4, CH | 4.67, dd (7.3, 4.6) | 77.1, CH | 4.65, dd (7.5, 4.9) | 77.0, CH | 4.66, dd (7.3, 4.7) | 77.1, CH |
| 8 | 1.74, m; 1.84, m | 34.1, CH2 | 1.76, m; 1.86, m | 35.4, CH2 | 1.76, m; 1.85, m | 35.4, CH2 | 1.76, m; 1.86, m | 35.4, CH2 |
| 9 | 1.38, m | 24.9, CH2 | 1.44, m | 25.9, CH2 | 1.43, m | 25.9, CH2 | 1.43, m | 25.9, CH2 |
| 10 | 1.29, b m | 29.2, CH2 | 1.35, b m | 30.6, CH2 | 1.35, b m | 30.5, CH2 | 1.35, b m | 30.5, CH2 |
| 11 | 1.27, b m | 29.3,c CH2 | 1.32, b m | 30.7, c CH2 | 1.32, b m | 30.6,c CH2 | 1.32, b m | 30.6, c CH2 |
| 12 | 1.25, b m | 29.4,c CH2 | 1.30, b m | 30.9, c CH2 | 1.30, b m | 30.7,c CH2 | 1.30, b m | 30.7, c CH2 |
| 13 | 1.25, b m | 29.5,c CH2 | 1.30, b m | 30.8, c CH2 | 1.30, b m | 30.9,c CH2 | 1.30, b m | 33.2, CH2 |
| 14 | 1.25, b m | 29.6,c CH2 | 1.30, b m | 30.7, c CH2 | 1.30, b m | 30.8,c CH2 | 1.30, b m | 23.9, CH2 |
| 15 | 1.25, b m | 31.8, CH2 | 1.34, b m | 27.1, CH2 | 1.35, b m | 27.1, CH2 | 0.90, t (7.1) | 14.6, CH3 |
| 16 | 1.29, b m | 22.6, CH2 | 1.52, m | 33.8, CH2 | 1.53, m | 33.8, CH2 | 4.44, s | 54.2, CH2 |
| 17 | 0.87, t (6.9) | 14.1, CH3 | 3.54, t (6.7) | 63.2, CH2 | 3.54, t (6.8) | 63.2, CH2 | 3.99, s | 58.0, CH3 |
| 18 | 4.51, b s | 54.3, CH2 | 4.44, s | 54.2, CH2 | 1.87, s | 8.6, CH3 | - | - |
| 19 | 3.93, s | 56.9, CH3 | 3.99, s | 58.1, CH3 | 3.96, s | 57.7, CH3 | - | - |
| β-4-O-methyl-d-glucopyranose | ||||||||
| 1′ | 4.29, d (7.6) | 100.6, CH | 4.26, d (7.9) | 102.7, CH | 4.25, d (7.9) | 102.6, CH | 4.25, d (7.9) | 102.7, CH |
| 2′ | 3.43, dd (7.6, 9.2) | 73.6, CH | 3.29, dd (7.9, 9.2) | 75.3, CH | 3.28, dd (7.9, 9.2) | 75.4, CH | 3.28, dd (7.9, 9.2) | 75.4, CH |
| 3′ | 3.54, dd (9.2, 8.8) | 76.6, CH | 3.43, dd (9.2, 8.8) | 78.3, CH | 3.43, dd (9.1, 9.1) | 78.3, CH | 3.43, dd (9.1, 9.1) | 78.4, CH |
| 4′ | 3.21, dd (8.8, 9.5) | 79.1, CH | 3.09, dd (9.2, 9.5) | 81.1, CH | 3.09, dd (9.1, 9.5) | 81.1, CH | 3.09, dd (9.1, 9.5) | 81.1, CH |
| 5′ | 3.26, m | 75.4, CH | 3.23, m | 77.4, CH | 3.22, m | 77.4, CH | 3.22, m | 77.4, CH |
| 6′ | 3.74, dd (12.2, 4.3) 3.89, dd (12.2, 2.4) | 61.8, CH2 | 3.68, dd (11.8, 5.3) 3.84, dd (11.8, 2.0) | 62.5, CH2 | 3.68, dd (11.8, 5.4) 3.83, dd (11.8, 2.0) | 62.5, CH2 | 3.68, dd (11.8, 5.4) 3.83, dd (11.8, 2.0) | 62.5, CH2 |
| OMe | 3.57, s | 60.8, CH3 | 3.56, s | 60.9, CH3 | 3.55, s | 60.9, CH3 | 3.55, s | 61.0, CH3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuephadungphan, W.; Helaly, S.E.; Daengrot, C.; Phongpaichit, S.; Luangsa-ard, J.J.; Rukachaisirikul, V.; Stadler, M. Akanthopyrones A–D, α-Pyrones Bearing a 4-O-Methyl-β-d-glucopyranose Moiety from the Spider-Associated Ascomycete Akanthomyces novoguineensis. Molecules 2017, 22, 1202. https://doi.org/10.3390/molecules22071202
Kuephadungphan W, Helaly SE, Daengrot C, Phongpaichit S, Luangsa-ard JJ, Rukachaisirikul V, Stadler M. Akanthopyrones A–D, α-Pyrones Bearing a 4-O-Methyl-β-d-glucopyranose Moiety from the Spider-Associated Ascomycete Akanthomyces novoguineensis. Molecules. 2017; 22(7):1202. https://doi.org/10.3390/molecules22071202
Chicago/Turabian StyleKuephadungphan, Wilawan, Soleiman E. Helaly, Charuwan Daengrot, Souwalak Phongpaichit, Janet Jennifer Luangsa-ard, Vatcharin Rukachaisirikul, and Marc Stadler. 2017. "Akanthopyrones A–D, α-Pyrones Bearing a 4-O-Methyl-β-d-glucopyranose Moiety from the Spider-Associated Ascomycete Akanthomyces novoguineensis" Molecules 22, no. 7: 1202. https://doi.org/10.3390/molecules22071202







