Rational Design of Cyclic Antimicrobial Peptides Based on BPC194 and BPC198
Abstract
:1. Introduction
2. Results and Discussion
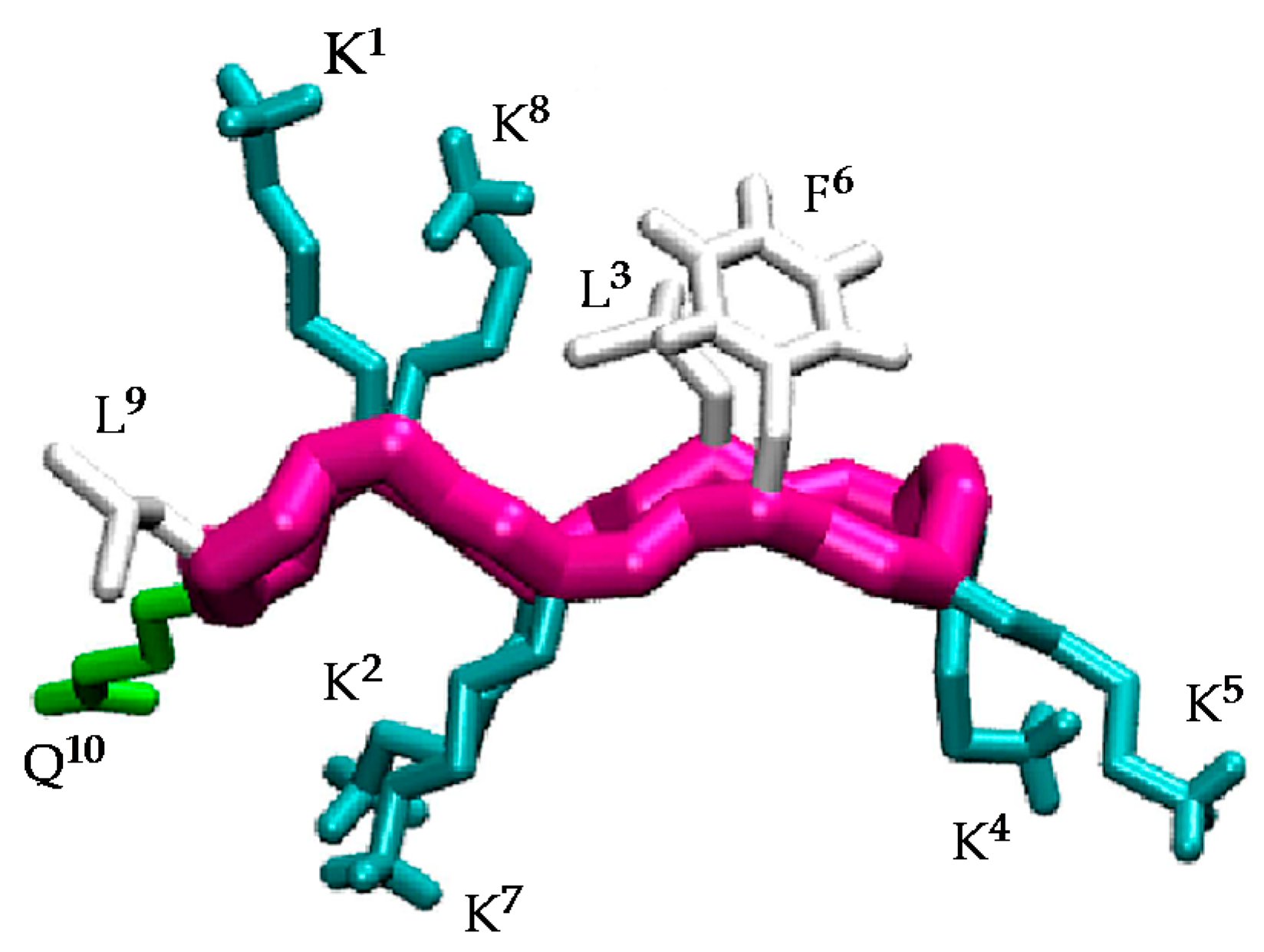
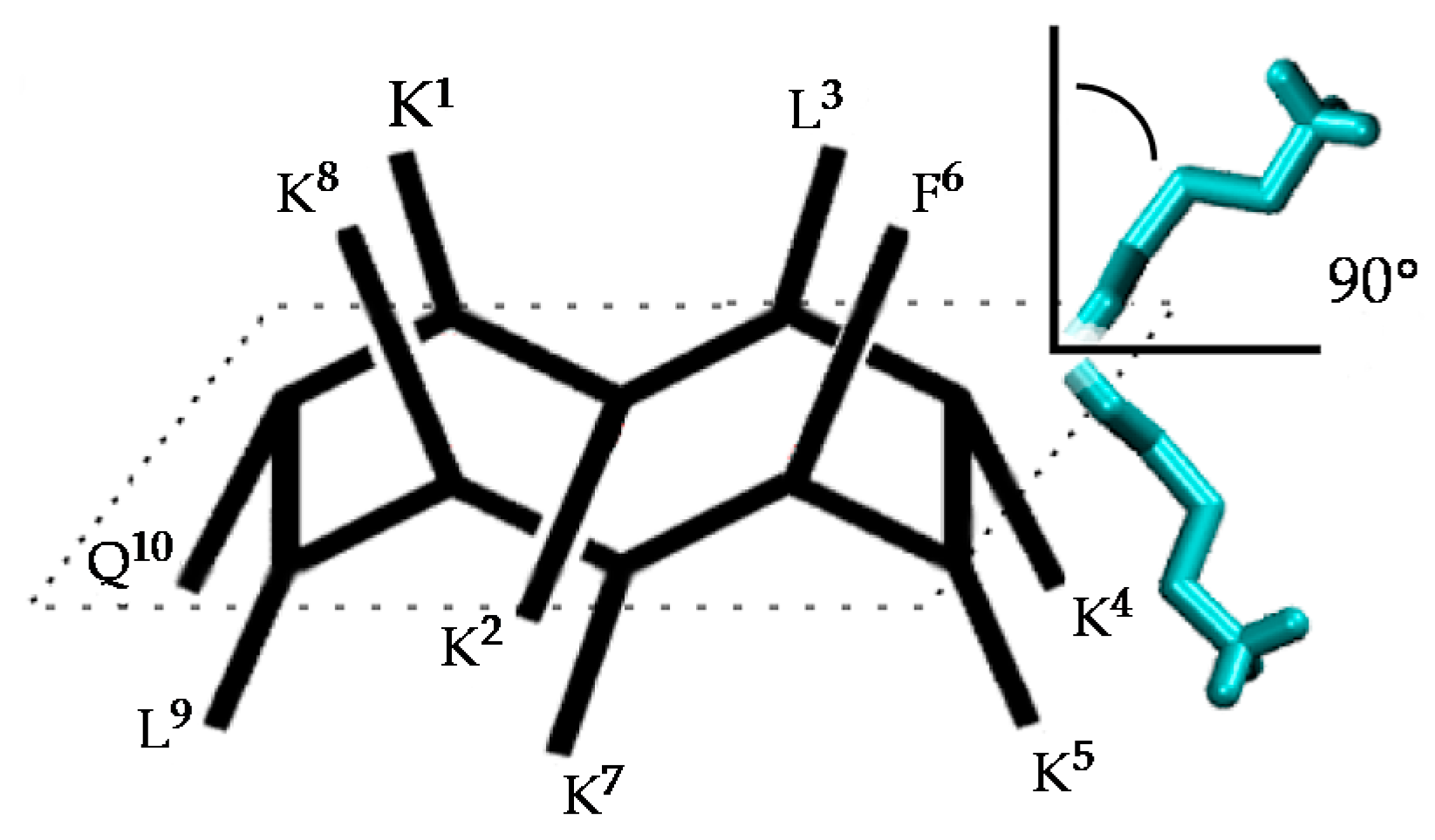
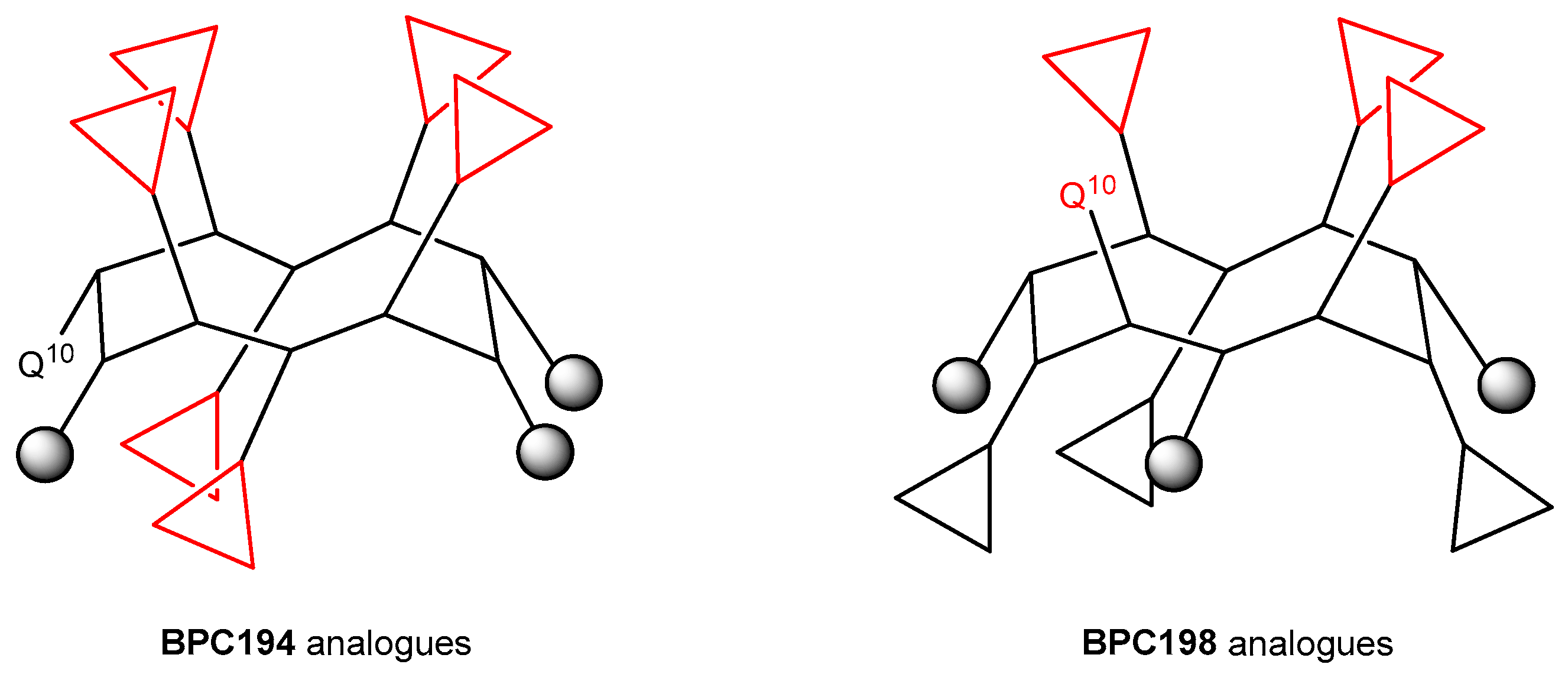
2.1. Structural Factors Governing the Activity of Cyclic Peptides BPC194 and BPC198
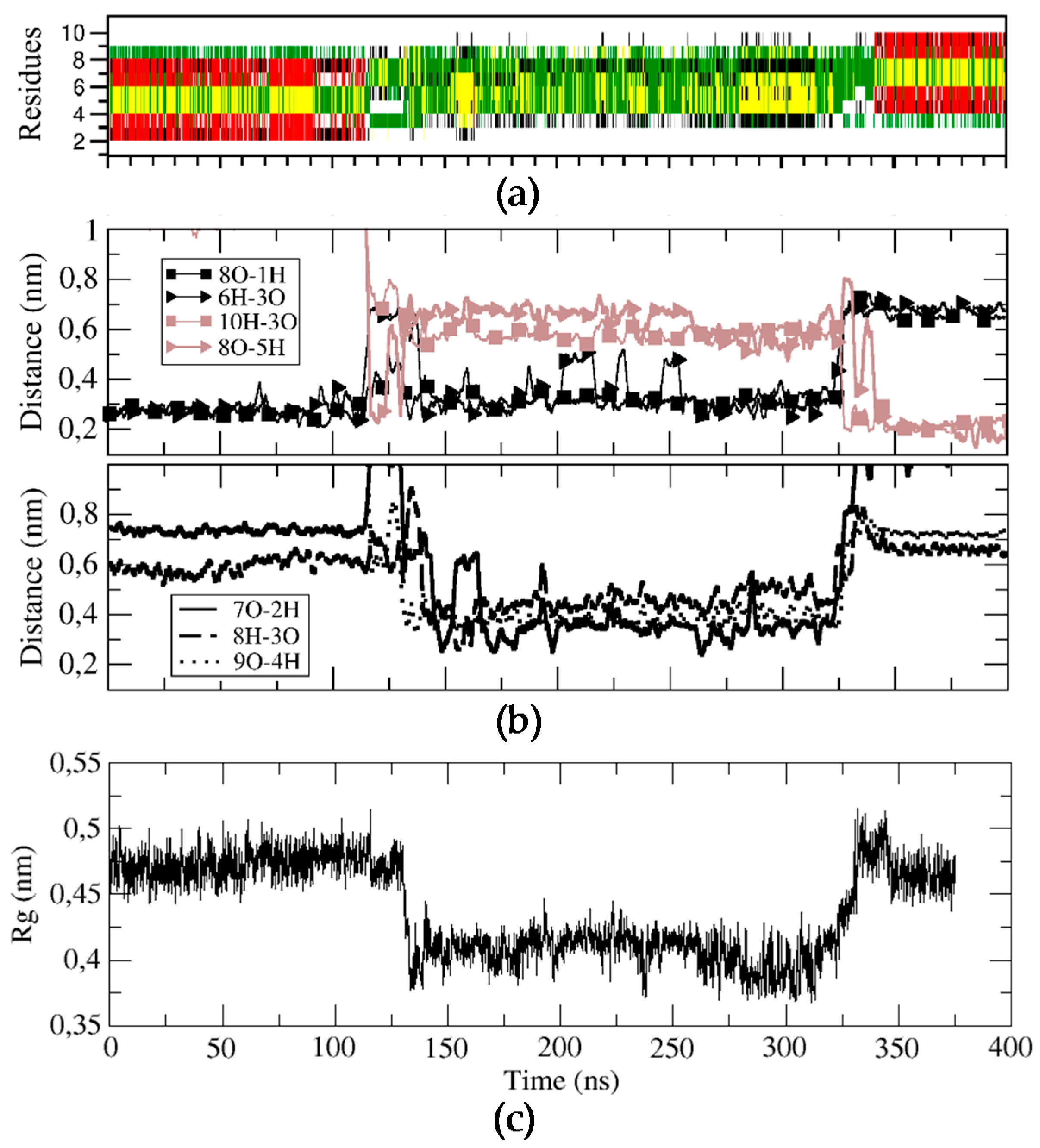
2.2. Stable Secondary Structure of BPC194 and BPC198
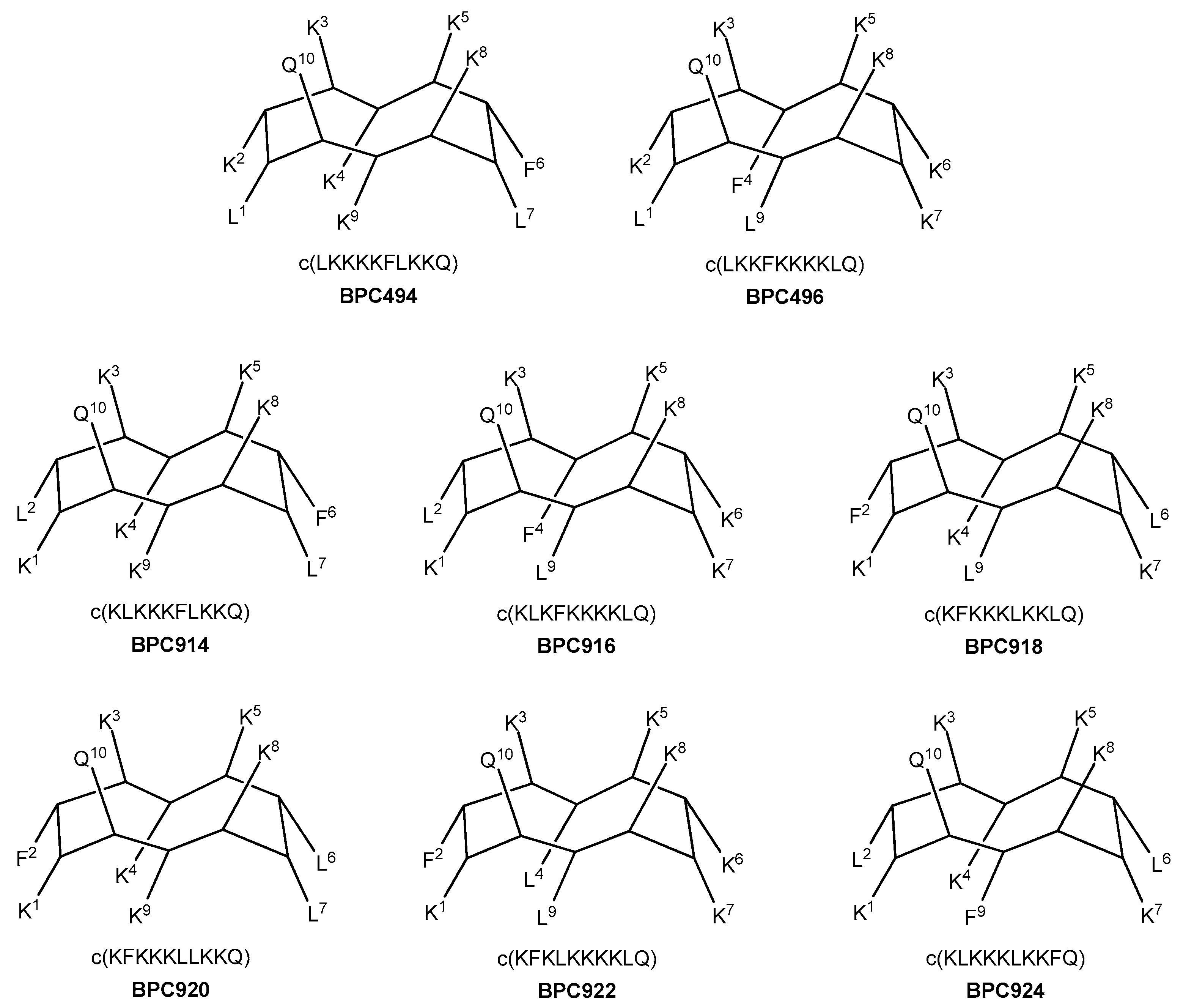
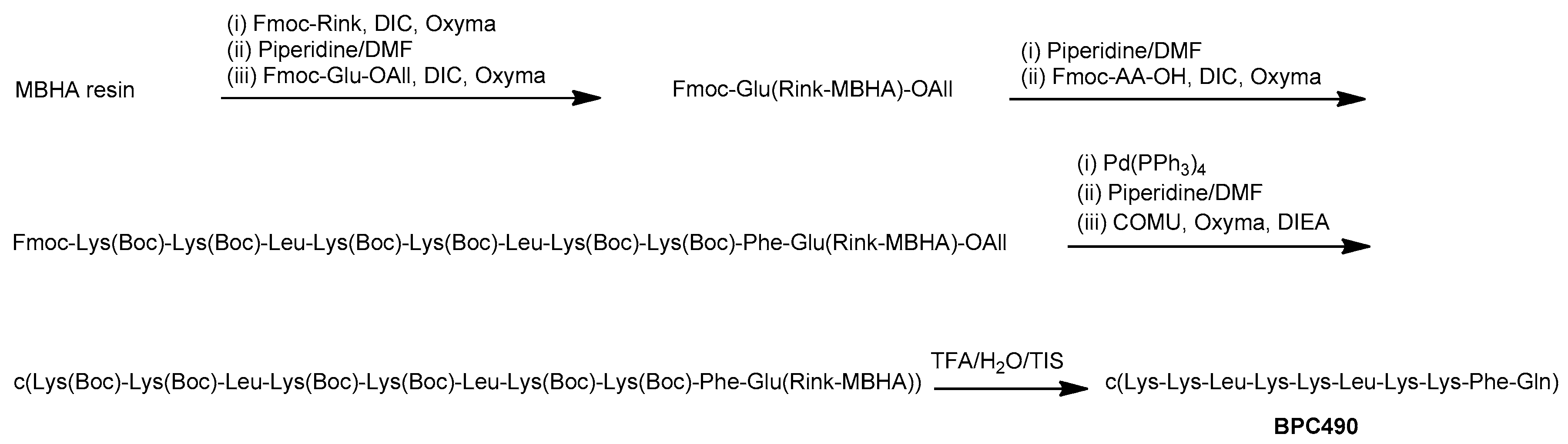
2.3. Design and Synthesis of BPC194 and BPC198 Analogues
2.4. Antibacterial Activity of the Designed Cyclic Decapeptides
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Synthesis of the Cyclic Peptides
3.3. Bacterial Strains and Growth Conditions
3.4. Antibacterial Activity
3.5. Molecular Dynamics Simulations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Agrios, G.N. Plant Pathology, 5th ed.; Academic Press: San Diego, CA, USA, 2005. [Google Scholar]
- Vidaver, A.K. Uses of antimicrobials in plant agriculture. Clin. Infect. Dis. 2002, 34, 107–110. [Google Scholar] [CrossRef] [PubMed]
- McManus, P.S.; Stockwell, V.O.; Sundin, V.O.; Jones, A.L. Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 2002, 46, 443–465. [Google Scholar] [CrossRef] [PubMed]
- Sundin, G.W.; Bender, C.L. Ecological and genetic analysis of copper and streptomycin resistance in Pseudomonas syringae pv. syringae. Appl. Environ. Microbiol. 1993, 59, 1018–1024. [Google Scholar] [PubMed]
- Montesinos, E. Antimicrobial peptides and plant disease control. FEMS Microbiol. Lett. 2007, 270, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Marcos, J.F.; Muñoz, A.; Pérez-Payá, E.; Misra, S.; López-García, B. Identification and rational design of novel antimicrobial peptides for plant protection. Annu. Rev. Phytopathol. 2008, 46, 271–301. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, E.; Bardají, E. Synthetic antimicrobial peptides as agricultural pesticides for plant-disease control. Chem. Biodivers. 2008, 5, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Keymanesh, K.; Soltani, S.; Sardari, S. Application of antimicrobial peptides in agriculture and food industry. World J. Microbiol. Biotechnol. 2009, 25, 933–944. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, B.; Gorr, S.-U. Antimicrobial peptides: Mechanisms of action and resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, N.B.; Cobacho, N.B.; Viana, J.F.C.; Lima, L.A.; Sampaio, K.B.O.; Dohms, S.S.M.; Ferreira, A.C.R.; de la Fuente-Núñez, C.; Costa, F.F.; Franco, O.L.; et al. The next generation of antimicrobial peptides (AMPs) as molecular therapeutic tools for the treatment of diseases with social and economic impacts. Drug Discov. Today 2017, 22, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Koh, J.-J.; Liu, S.; Lakshminarayanan, R.; Verma, C.S.; Beuerman, R.W. Membrane active antimicrobial peptides: Translating mechanistic insights to design. Front. Neurosci. 2017, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Lohner, K. Membrane-active antimicrobial peptides as template structures for novel antibiotic agents. Curr. Top. Med. Chem. 2017, 17, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Mansour, S.C.; Hancock, R.E. Antimicrobial peptides: An introduction. Methods Mol. Biol. 2017, 1548, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Wang, Y.; Li, G.; Liu, S.; Cui, N.; Liu, S.; Langford, P.R.; Wang, C. The SapA protein is involved in resistance to antimicrobial peptide PR-39 and virulence of Actinobacillus pleuropneumoniae. Front. Microbiol. 2017, 8, 811. [Google Scholar] [CrossRef] [PubMed]
- Raja, Z.; André, S.; Abbassi, F.; Humblot, V.; Lequin, O.; Bouceba, T.; Correia, I.; Casale, S.; Foulon, T.; Sereno, D.; et al. Insight into the mechanism of action of temporin-SHa, a new broad-spectrum antiparasitic and antibacterial agent. PLoS ONE 2017, 12, e0174024. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Le, C.-F.; Fang, C.-M.; Sekaran, S.D. Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-16. [Google Scholar] [CrossRef] [PubMed]
- Frecer, V.; Ho, B.; Ding, J.L. De novo design of potent antimicrobial peptides. Antimicrob. Agents Chemother. 2004, 48, 3349–3357. [Google Scholar] [CrossRef] [PubMed]
- Dimarcq, J.-L.; Bulet, P.; Hetru, C.; Hoffmann, J. Cysteine-rich antimicrobial peptides in invertebrates. Biopolymers 1998, 47, 465–477. [Google Scholar] [CrossRef]
- Lee, D.L.; Hodges, R.S. Structure activity relationships of de novo designed cyclic antimicrobial peptides based on gramicidin S. Biopolymers 2003, 71, 28–48. [Google Scholar] [CrossRef] [PubMed]
- Egberts, E.; Berendsen, H.J.C. Molecular dynamics simulation of a smectic liquid crystal with atomic detail. J. Chem. Phys. 1988, 89, 3718–3732. [Google Scholar] [CrossRef]
- Wendoloski, J.J.; Kimatian, S.J.; Schutt, C.E.; Salemme, F.R. Molecular dynamics simulation of a phospholipid micelle. Science 1989, 243, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Leontiadou, H.; Mark, A.E.; Marrink, S.J. Antimicrobial peptides in action. J. Am. Chem. Soc. 2006, 128, 12156–12161. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, S.K.; Larson, R.G. Binding and insertion of alpha-helical antimicrobial peptides in POPC bilayers studied by molecular dynamics simulations. Chem. Phys. Lipids 2004, 132, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Appelt, C.; Eisenmenger, F.; Kühne, R.; Schmieder, P.; Söderhäll, J.A. Interaction of the antimicrobial peptide cyclo(RRWWRF) with membranes by molecular dynamics simulations. Biophys. J. 2005, 89, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Ma, B.; Nussinov, R. Conformational study of the protegrin-1 (PG-1) dimer interaction with lipid bilayers and its effect. BMC Struct. Biol. 2007, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.; Leontiadou, H.; Mark, A.E.; Marrink, S.J. Toroidal pores formed by antimicrobial peptides show significant disorder. Biochim. Biophys. Acta 2008, 1778, 2308–2317. [Google Scholar] [CrossRef] [PubMed]
- Orioni, B.; Bocchinfuso, G.; Kim, J.Y.; Palleschi, A.; Grande, G.; Bobone, S.; Park, Y.; Kim, J.I.; Hahm, K.S.; Stella, L. Membrane perturbation by the antimicrobial peptide PMAP-23: A fluorescence and molecular dynamics study. Biochim. Biophys. Acta 2009, 1788, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Cirac, A.D.; Moiset, G.; Mika, J.T.; Koçer, A.; Salvador, P.; Poolman, B.; Marrink, S.J.; Sengupta, D. The molecular basis for antimicrobial activity of pore-forming cyclic peptides. Biophys. J. 2011, 100, 2422–2431. [Google Scholar] [CrossRef] [PubMed]
- Monroc, S.; Badosa, E.; Besalú, E.; Planas, M.; Bardají, E.; Montesinos, E.; Feliu, L. Improvement of cyclic decapeptides against plant pathogenic bacteria using a combinatorial chemistry approach. Peptides 2006, 27, 2575–2584. [Google Scholar] [CrossRef] [PubMed]
- Mika, J.T.; Moiset, G.; Cirac, A.D.; Feliu, L.; Bardají, E.; Planas, M.; Sengupta, D.; Marrink, S.J.; Poolman, B. Structural basis for the enhanced activity of cyclic antimicrobial peptides: The case of BPC194. Biochim. Biophys. Acta 2011, 1808, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.C.; Ho, M.H.; Wang, W.H.; Sun, Y.C. Molecular dynamics simulation of folding of a short helical peptide with many charged residues. J. Phys. Chem. B 2005, 109, 19980–19986. [Google Scholar] [CrossRef] [PubMed]
- Güell, I.; Vilà, S.; Badosa, E.; Montesinos, E.; Feliu, L.; Planas, M. Design, synthesis, and biological evaluation of cyclic peptidotriazoles derived from BPC194 as novel agents for plant protection. Pept. Sci. 2017, 108, e23012. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, E.; Colescott, R.L.; Bossinger, C.D.; Cook, P. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef]
- Van der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Van Gunsteren, W.F.; Krüger, P.; Billeter, S.R.; Mark, A.E.; Eising, A.A.; Scott, W.R.P.; Hüneberger, P.H.; Tironi, I.G. Biomolecular Simulation: The GROMOS96 Manual and User Guide; Biomos: Zurich, Switzerland, 1996. [Google Scholar]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; Hermans, J. Interaction models for water in relation to protein hydration. Intermol. Forces, 1981, 14, 331–342. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1471. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; Nola, A.D.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Kabsch, W.; Sander, C. Dictionary of protein secondary structure: Pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 1983, 22, 2577–2637. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |



| Code | MIC Intervals (μM) | ||
|---|---|---|---|
| Pss a | Xav a | Ea a | |
| BPC194 | 3.1-6.2 | 3.1–6.2 | 6.2–12.5 |
| BPC198 | 3.1–6.2 | 3.1–6.2 | 12.5–25 |
| BPC480 | 12.5–25 | 12.5–25 | >50 |
| BPC482 | 6.2–12.5 | 6.2–12.5 | >50 |
| BPC484 | 12.5–25 | 25–50 | >50 |
| BPC486 | 12.5–25 | >50 | >50 |
| BPC488 | 6.2–12.5 | 6.2–12.5 | >50 |
| BPC490 | 3.1–6.2 | 3.1–6.2 | 12.5–25 |
| BPC492 | 12.5–25 | 12.5–25 | >50 |
| BPC494 | 6.2–12.5 | 6.2–12.5 | >50 |
| BPC496 | 6.2–12.5 | 6.2–12.5 | >50 |
| BPC914 | 12.5–25 | 12.5–25 | >50 |
| BPC916 | 6.2–12.5 | 12.5–25 | >50 |
| BPC918 | 3.1–6.2 | 1.6–3.1 | 12.5–25 |
| BPC920 | 12.5–25 | 25–50 | >50 |
| BPC922 | 12.5–25 | 12.5–25 | >50 |
| BPC924 | 3.1–6.2 | 1.6–3.1 | 12.5–25 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirac, A.D.; Torné, M.; Badosa, E.; Montesinos, E.; Salvador, P.; Feliu, L.; Planas, M. Rational Design of Cyclic Antimicrobial Peptides Based on BPC194 and BPC198. Molecules 2017, 22, 1054. https://doi.org/10.3390/molecules22071054
Cirac AD, Torné M, Badosa E, Montesinos E, Salvador P, Feliu L, Planas M. Rational Design of Cyclic Antimicrobial Peptides Based on BPC194 and BPC198. Molecules. 2017; 22(7):1054. https://doi.org/10.3390/molecules22071054
Chicago/Turabian StyleCirac, Anna D., Maria Torné, Esther Badosa, Emilio Montesinos, Pedro Salvador, Lidia Feliu, and Marta Planas. 2017. "Rational Design of Cyclic Antimicrobial Peptides Based on BPC194 and BPC198" Molecules 22, no. 7: 1054. https://doi.org/10.3390/molecules22071054
APA StyleCirac, A. D., Torné, M., Badosa, E., Montesinos, E., Salvador, P., Feliu, L., & Planas, M. (2017). Rational Design of Cyclic Antimicrobial Peptides Based on BPC194 and BPC198. Molecules, 22(7), 1054. https://doi.org/10.3390/molecules22071054








