Hemi-Synthesis and Anti-Oomycete Activity of Analogues of Isocordoin
Abstract
:1. Introduction
2. Results and Discussion
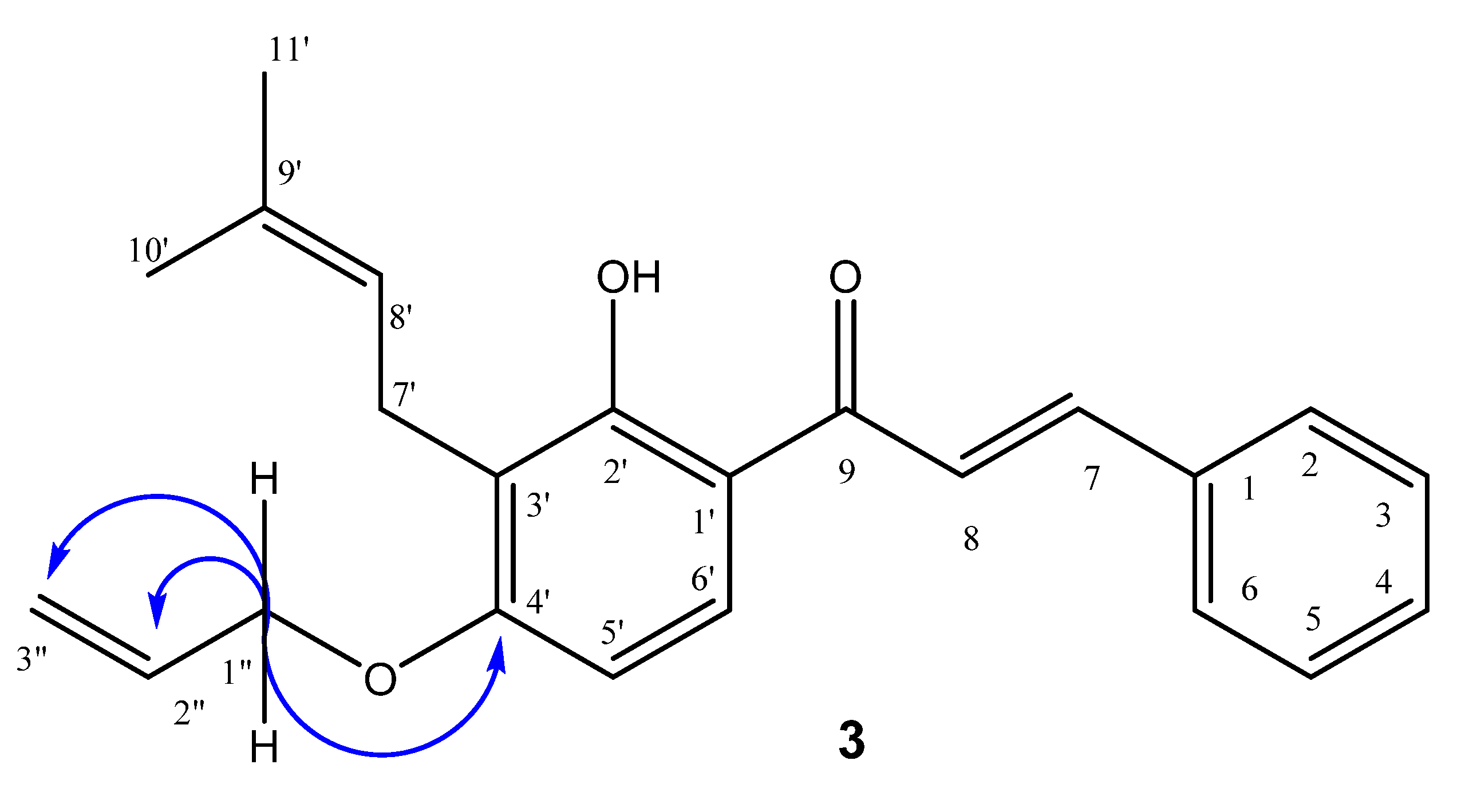
2.1. Synthesis and Characterization
2.2. Anti-Oomycete Activity Assays of Compounds 1–8
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Isolation and Characterization of Isocordoin (1)
3.4. General Procedure for the Synthesis of 4-Oxyalkyl-isocordoin Analogues (2–8)
3.5. Oomycete Strain
3.6. Minimum Inhibitory Concentration Evaluation
3.7. Spores Germination Inhibition Assay
3.8. Mycelial Growth Inhibition Assay
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sugamoto, K.; Matsusita, Y.; Matsui, K.; Kurogi, C.; Matsui, T. Synthesis and antibacterial activity of chalcones bearing prenyl or geranyl groups from Angelica keiskei. Tetrahedron 2011, 67, 5346–5359. [Google Scholar] [CrossRef]
- Yazaki, K.; Sasaki, K.; Tsurumaru, Y. Prenylation of aromatic compounds, a key diversification of plant secondary metabolites. Phytochemistry 2009, 70, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Barron, D.; Ibrahim, R. Isoprenylated flavonoids—A survey. Phytochemistry 1996, 43, 921–982. [Google Scholar] [CrossRef]
- Botta, B.; Vitali, A.; Menendez, P.; Misitia, D.; Delle Monache, G. Prenylated flavonoids: Pharmacology and biotechnology. Curr. Med. Chem. 2005, 12, 713–739. [Google Scholar] [CrossRef]
- Chen, X.; Mukwaya, E.; Wong, M.S.; Zhang, Y. A systematic review on biological activities of prenylated flavonoids. Pharm. Biol. 2014, 52, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Barragan, E.; Patil, S.A.; Patil, S.; Bugarin, A. Direct Synthesis and antimicrobial evaluation of structurally complex chalcones. Chem. Sel. 2016, 1, 3647–3650. [Google Scholar] [CrossRef]
- Delle Monach, F.; Cuca Suarez, L.E.; Marini-Bettolo, G.B. Flavonoids from the seeds of six Lonchocarpus species. Phytochemistry 1978, 27, 1812–1813. [Google Scholar] [CrossRef]
- Borges-Argáez, R.; Peña-Rodríguez, L.M.; Waterman, P. Flavonoids from two Lonchocarpus species of the Yucatan Peninsula. Phytochemistry 2002, 60, 533–540. [Google Scholar] [CrossRef]
- Caamal-Fuentes, E.E.; Peraza-Sánchez, S.R.; Torres-Tapia, L.W.; Moo-Puc, R.E. Isolation and identification of cytotoxic compounds from Aeschynomene fascicularis, a Mayan medicinal plant. Molecules 2015, 20, 13563–13574. [Google Scholar] [CrossRef] [PubMed]
- Borges-Argáez, R.; Poot-Díaz, M.E.; Waterman, P.G.; Peña-Rodríguez, L.M. Cytotoxic and antiprotozoal activity of flavonoids from Lonchocarpus spp. Phytomedicine 2007, 14, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Borges-Argáez, R.; Vela-Catzín, T.; Yam-Puc, A.; Chan-Bacab, M.J.; Moo-Puc, R.E.; Cáceres-Farfán, M. Antiprotozoal and cytotoxic studies on some isocordoin derivatives. Planta Med. 2009, 75, 1336–1338. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.S.J.; Blaney, W.M.; Delle Monache, F.; Marini-Bettolo, G.B. Insect antifeedant activity associated with compounds isolated from species of Lonchocarpus and Tephrosia. J. Chem. Ecol. 1990, 16, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Avila-Villarreal, G.; Hernández-Abreu, O.; Hidalgo-Figueroa, S.; Navarrete-Vázquez, G.; Escalante-Erosa, F.; Peña-Rodríguez, L.M.; Villalobos-Molina, R.; Estrada-Soto, S. Antihypertensive and vasorelaxant effects of dihydrospinochalcone-A isolated from Lonchocarpus xuul Lundell by NO production: Computational and ex vivo approaches. Phytomedcine 2013, 20, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.H.; Wang, R.F.; Guo, S.Z.; Liu, B. An update on antitumor activity of naturally occurring chalcones. Evid. Based Complement Altern. Med. 2013, 2013, 815621. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.H.; Shi, S.C.; Ma, X.J.; Zhou, B.N. Total synthesis of two prenylated flavanonols from Maackia tenuifolia. Nat. Prod. Lett. 1992, 1, 99–102. [Google Scholar] [CrossRef]
- Vitali, A.; Ferrari, F.; Monache, G.D.; Bombardelli, E.; Botta, B. Synthesis and biosynthesis of isocordoin. Planta Med. 2001, 67, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Bolasco, A.; Fioravanti, R.; Rossi, F.; Rossi, P.; Vitali, A. Use of cyclodextrins in biotransformation reactions with cell cultures of Morus nigra: Biosynthesis of prenylated chalcone isocordoin. Biotechnol. Appl. Biochem. 2010, 56, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Basabe, P.; de Román, M.; Díez, D.; Marcos, I.S.; Blanco, A.; Bodero, O.; Mollinedo, F.; Sierra, B.G.; Urones, J.G. Prenylflavonoids and prenyl/alkyl-phloroacetophenones: Synthesis and antitumour biological evaluation. Eur. J. Med. Chem. 2010, 45, 4258–4269. [Google Scholar] [CrossRef] [PubMed]
- Seyedi, S.; Jafari, Z.; Attaran, N.; Sadeghian, H.; Saberi, M.; Riazi, M. Design, synthesis and SAR studies of 4-allyoxyaniline amides as potent 15-lipoxygensae inhibitors. Bioorg. Med. Chem. 2009, 17, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Flores, S.; Montenegro, I.; Villena, J.; Cuellar, M.; Werner, E.; Godoy, P.; Madrid, A. Synthesis and Evaluation of novel oxyalkylated derivatives of 2′,4′-dihydroxychalcone as anti-oomycete agents against bronopol resistant strains of Saprolegnia sp. Int. J. Mol. Sci. 2016, 17, 1366. [Google Scholar] [CrossRef] [PubMed]
- Modak, M.; Rojas, M.; Torres, R. Chemical analysis of the resinous exudate isolated from Heliotropium taltalense and evaluation of the antioxidant activity of the phenolics components and the resin in homogeneous and heterogeneous systems. Molecules 2009, 14, 1980–1989. [Google Scholar] [CrossRef] [PubMed]
- Madrid, A.; Espinoza, L.; González, C.; Mellado, M.; Villena, J.; Santander, S.; Silva, V.; Montenegro, I. Antifungal study of the resinous exudate and of meroterpenoids isolated from Psoralea glandulosa (Fabaceae). J. Ethnopharmacol. 2012, 144, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Kito, T.; Yamamoto, K.; Hirao, I. The O-alkylation of phenols of esters II. The steric hindrance and electronic effect in the O-alkylation of phenols by esters. Bull. Chem. Soc. Jpn. 1977, 50, 706–709. [Google Scholar] [CrossRef]
- Lei, T.; Wang, J.Y.; Pei, J. Roles of Flexible Chains in Organic Semiconducting Materials. Chem. Mater. 2014, 26, 594–603. [Google Scholar] [CrossRef]
- Da Silva, G.; da Silva, M.; Souza, E.; Barison, A.; Simões, S.; Varotti, F.; Barbosa, L.; Viana, G.; Villar, J. Design and Synthesis of New Chalcones Substituted with Azide/Triazole Groups and Analysis of Their Cytotoxicity Towards HeLa Cells. Molecules 2012, 17, 10331–10343. [Google Scholar] [CrossRef] [PubMed]
- Do nascimento, M.C.; Mors, W.B. Chalcones of the root bark of Derris sericea. Phytochemistry 1972, 11, 3023–3028. [Google Scholar] [CrossRef]
- Tse, B.; Balkovec, J.M.; Blazey, C.M.; Hsu, M.J.; Nielsen, J.; Schmatz, D. Alkyl side-chain derivatives of sordaricin as potent antifungal agents against yeast. Bioorg. Med. Chem. Lett. 1998, 8, 2269–2272. [Google Scholar] [CrossRef]
- Schreier, S.; Malheiros, S.V.P.; Paula, E. Surface active drugs: Self-association and interaction with membranes and surfactants. Physicochemical and biological aspects. Biochim. Biophys. Acta. 2000, 1508, 210–234. [Google Scholar] [CrossRef]
- Mousa, W.K.; Raizada, M.N. The Diversity of Anti-Microbial Secondary Metabolites Produced by Fungal Endophytes: An Interdisciplinary Perspective. Front. Microbiol. 2013, 4, 65. [Google Scholar] [CrossRef] [PubMed]
- Warrilow, A.G.S.; Hull, C.; Rolley, N.J.; Parker, E.; Nes, W.N.; Smith, S.N.; Kelly, D.E.; Kelly, S.T. Clotrimazole as a Potent Agent for Treating the Oomycete Fish Pathogen Saprolegnia parasitica through Inhibition of Sterol 14α-Demethylase (CYP51). Appl. Environ. Microbiol. 2014, 80, 6154–6166. [Google Scholar] [CrossRef] [PubMed]
- Kandaswamy, N.; Raveendiran, N. Synthesis, Characterization, and In Vitro Antimicrobial and Anticancer Evaluation of Copolyester Bearing 4-Arylidene Curcumin in the Main Chain. Int. Sch. Res. Not. 2014, 495927. [Google Scholar] [CrossRef]
- Madrid Villegas, A.; Espinoza Catalán, L.; Montenegro Venegas, I.; Villena García, J.; Carrasco Altamirano, H. New Catechol derivatives of safrole and their antiproliferative activity towards breast cancer cells. Molecules 2011, 16, 4632–4641. [Google Scholar] [CrossRef] [PubMed]
- Zaror, L.; Collado, L.; Bohle, H.; Landskron, E.; Montaña, J.; Avendaño, F. Saprolegnia parasitica en salmones y truchas del sur de Chile. Arch. Med. Vet. 2004, 36, 71–78. [Google Scholar] [CrossRef]
- Madrid, A.; Godoy, P.; González, S.; Zaror, L.; Moller, A.; Werner, E.; Cuellar, M.; Villena, J.; Montenegro, I. Chemical Characterization and Anti-Oomycete Activity of Laureliopsis philippianna Essential Oils against Saprolegnia parasitica and S. australis. Molecules 2015, 20, 8033–8047. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.G.; Liu, L.; Hu, K.; Yang, X.L.; Wang, G.X. In vitro screening of fungicidal chemicals for antifungal activity against Saprolegnia. J. World Aquacult. Soc. 2013, 44, 528–535. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–8 are available from the authors. |

| Compounds | MIC (µg/mL) | |
|---|---|---|
| S. parasitica | S. australis | |
| 1 | >200 | 200 |
| 2 | 75 | 50 |
| 3 | 100 | 75 |
| 4 | 125 | 125 |
| 5 | 100 | 75 |
| 6 | 150 | 125 |
| 7 | >200 | >200 |
| 8 | 150 | 75 |
| Bronopol | >200 | 175 |
| Clotrimazole | 125 | 150 |
| Itraconazole | 100 | 100 |
| Compounds | MOC (µg/mL) | |
|---|---|---|
| S. parasitica | S. australis | |
| 1 | >200 | >200 |
| 2 | 75 | 75 |
| 3 | 100 | 100 |
| 4 | 125 | 125 |
| 5 | 125 | 100 |
| 6 | 150 | 150 |
| 7 | >200 | >200 |
| 8 | 150 | 100 |
| Bronopol | >200 | 200 |
| Clotrimazole | 150 | 175 |
| Itraconazole | 100 | 100 |
| Compounds (200 µg/mL) | MIG (%) | |
|---|---|---|
| S. parasitica | S. australis | |
| 1 | 0 | 0 |
| 2 | 52 | 58 |
| 3 | 38 | 42 |
| 4 | 35 | 38 |
| 5 | 25 | 30 |
| 6 | 28 | 30 |
| 7 | 0 | 0 |
| 8 | 31 | 33 |
| Bronopol | 0 | 33 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar, B.; Montenegro, I.; Villena, J.; Werner, E.; Godoy, P.; Olguín, Y.; Madrid, A. Hemi-Synthesis and Anti-Oomycete Activity of Analogues of Isocordoin. Molecules 2017, 22, 968. https://doi.org/10.3390/molecules22060968
Escobar B, Montenegro I, Villena J, Werner E, Godoy P, Olguín Y, Madrid A. Hemi-Synthesis and Anti-Oomycete Activity of Analogues of Isocordoin. Molecules. 2017; 22(6):968. https://doi.org/10.3390/molecules22060968
Chicago/Turabian StyleEscobar, Beatriz, Iván Montenegro, Joan Villena, Enrique Werner, Patricio Godoy, Yusser Olguín, and Alejandro Madrid. 2017. "Hemi-Synthesis and Anti-Oomycete Activity of Analogues of Isocordoin" Molecules 22, no. 6: 968. https://doi.org/10.3390/molecules22060968
APA StyleEscobar, B., Montenegro, I., Villena, J., Werner, E., Godoy, P., Olguín, Y., & Madrid, A. (2017). Hemi-Synthesis and Anti-Oomycete Activity of Analogues of Isocordoin. Molecules, 22(6), 968. https://doi.org/10.3390/molecules22060968





