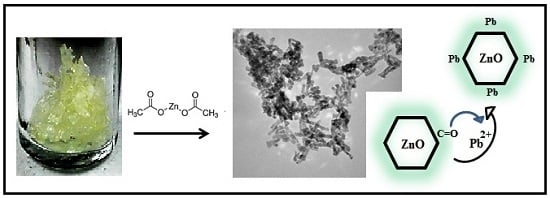
Green Synthesis of Zinc Oxide Nanoparticles for Enhanced Adsorption of Lead Ions from Aqueous Solutions: Equilibrium, Kinetic and Thermodynamic Studies
Abstract
:1. Introduction
2. Results and Discussion
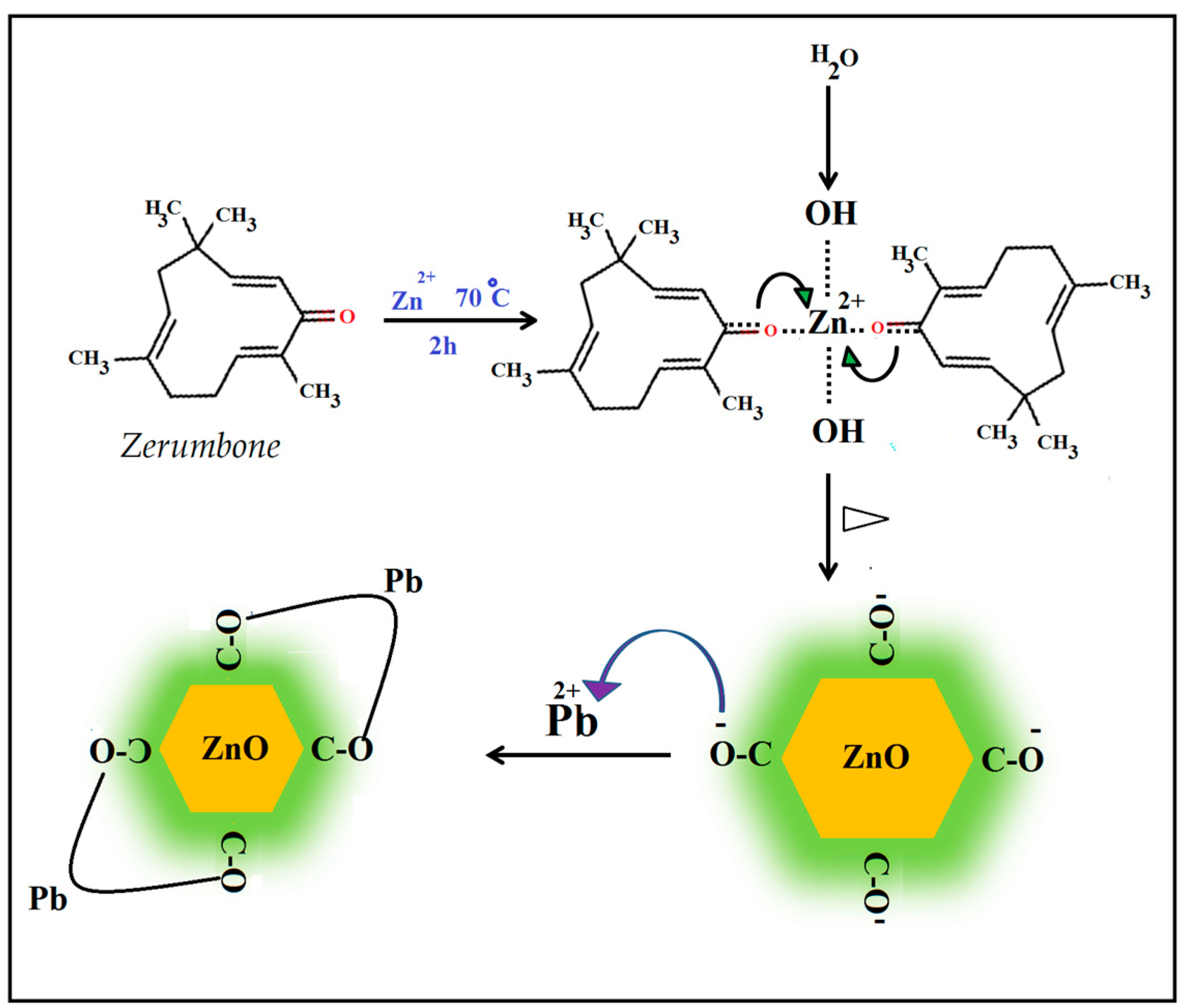
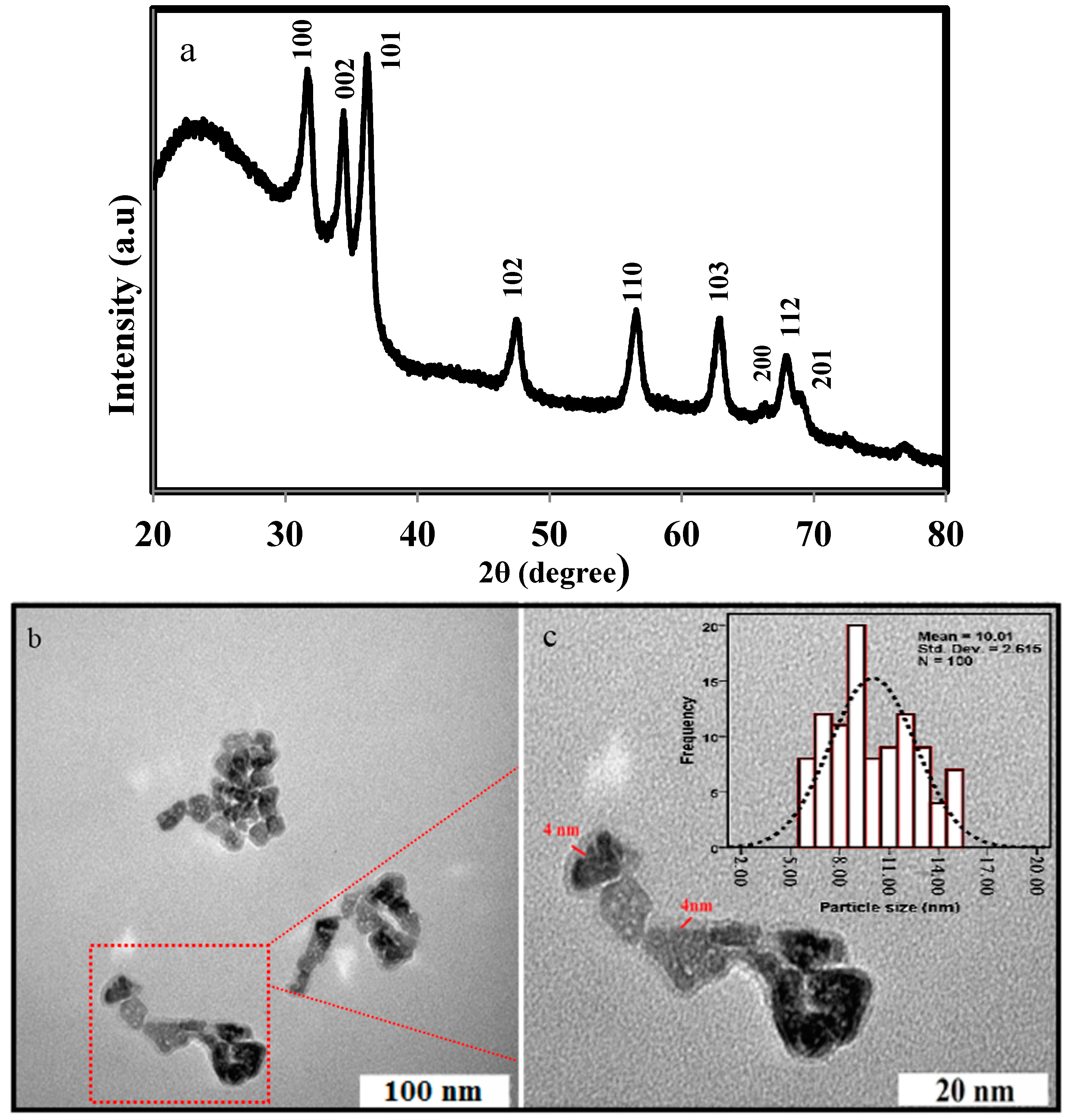
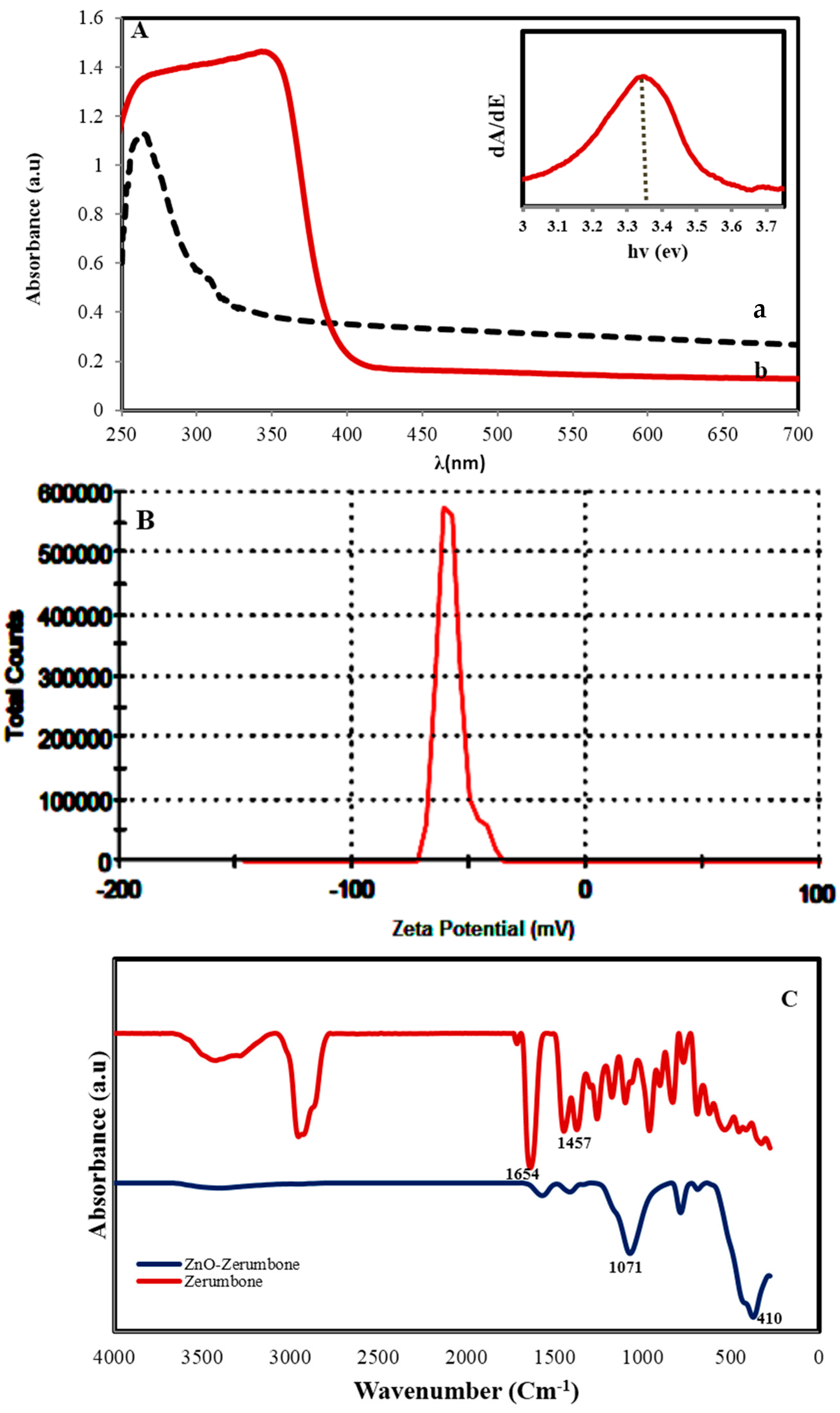
2.1. Characterization of ZnO NPs
2.2. Evaluation of Adsorption Mechanism
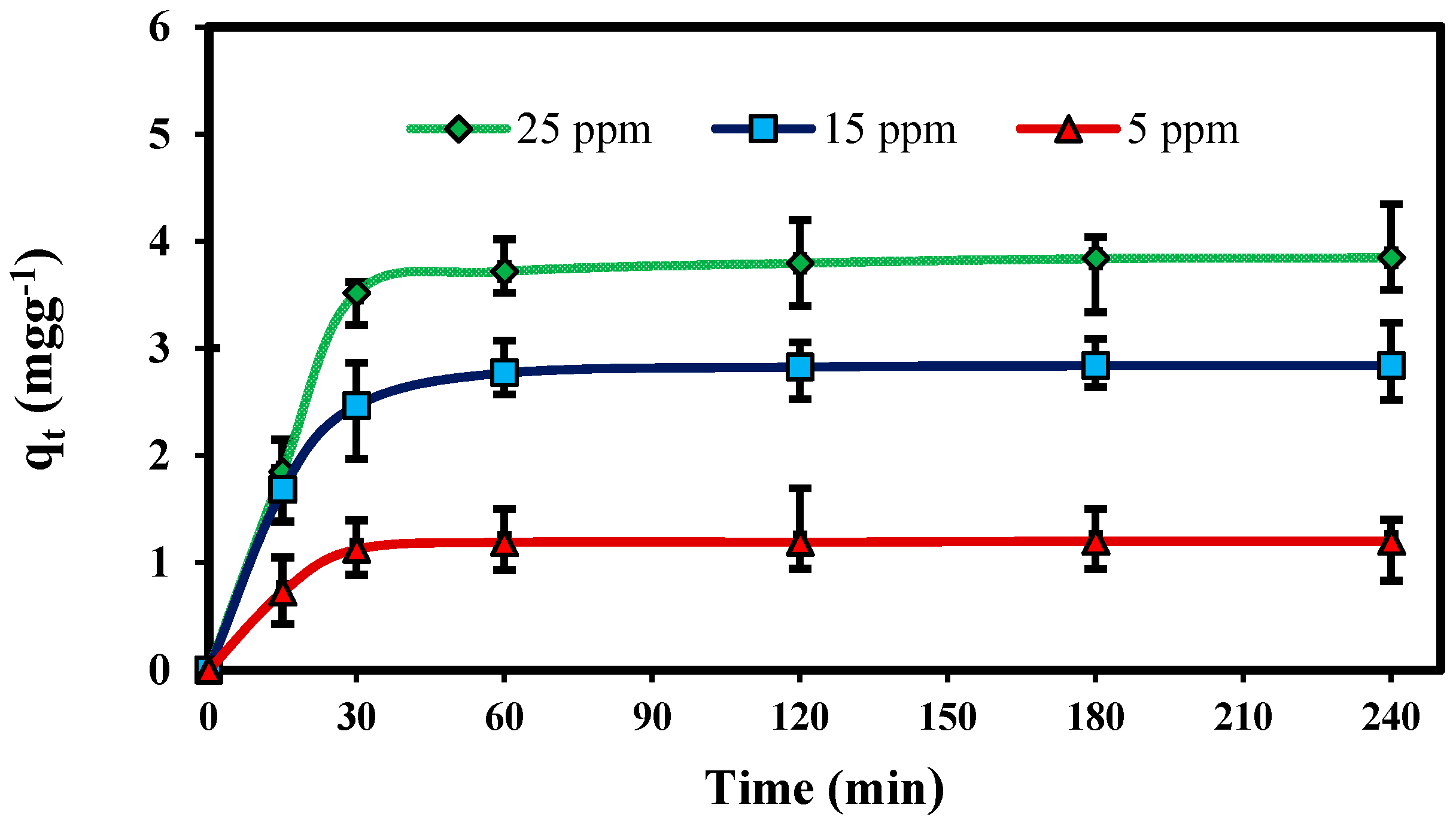
2.2.1. Effect of Contact Time
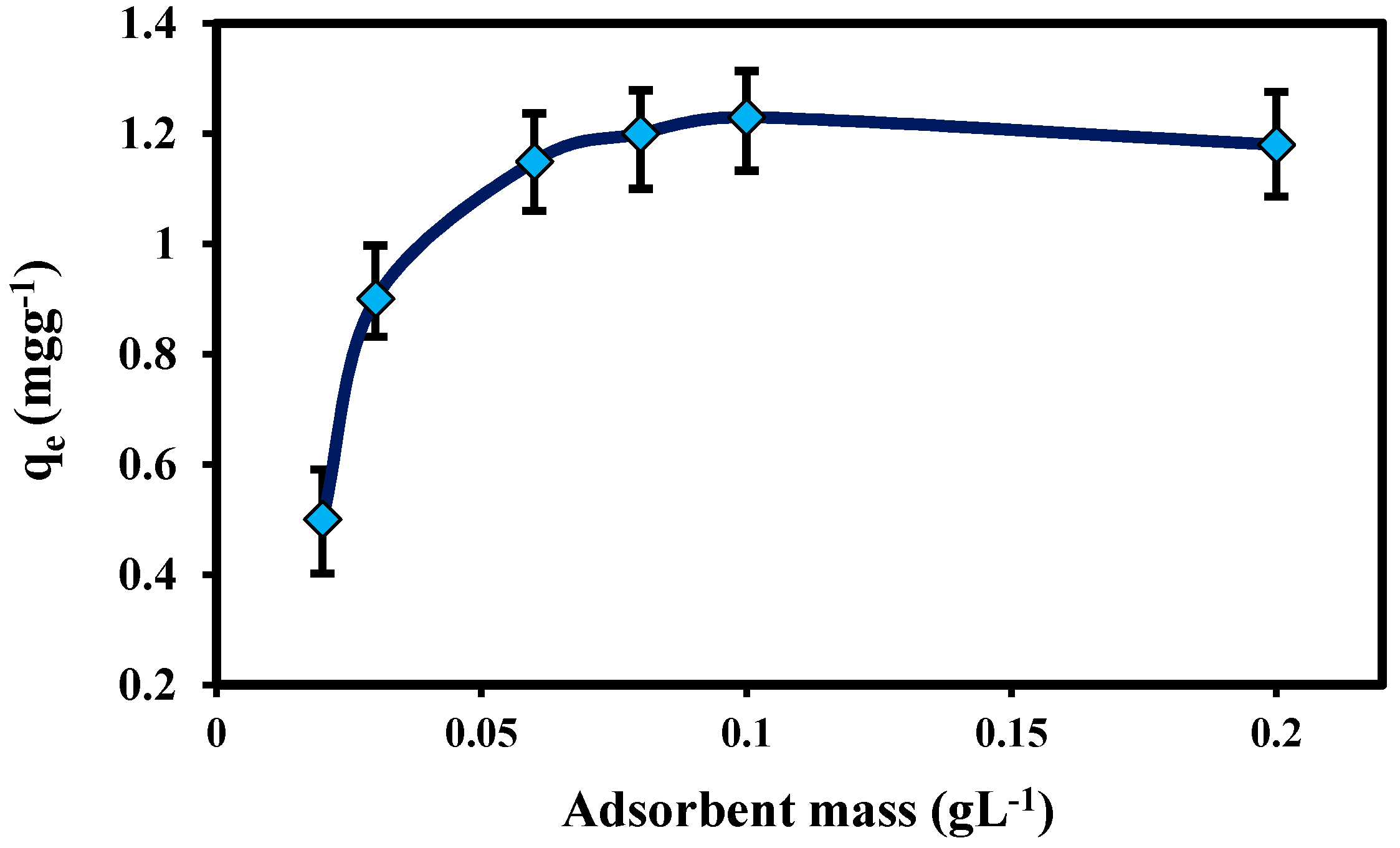
2.2.2. Effect of Mass of Adsorbent
2.2.3. Effect of pH
2.3. Metal Adsorption Characteristics
2.3.1. Adsorption Kinetics Modeling Study
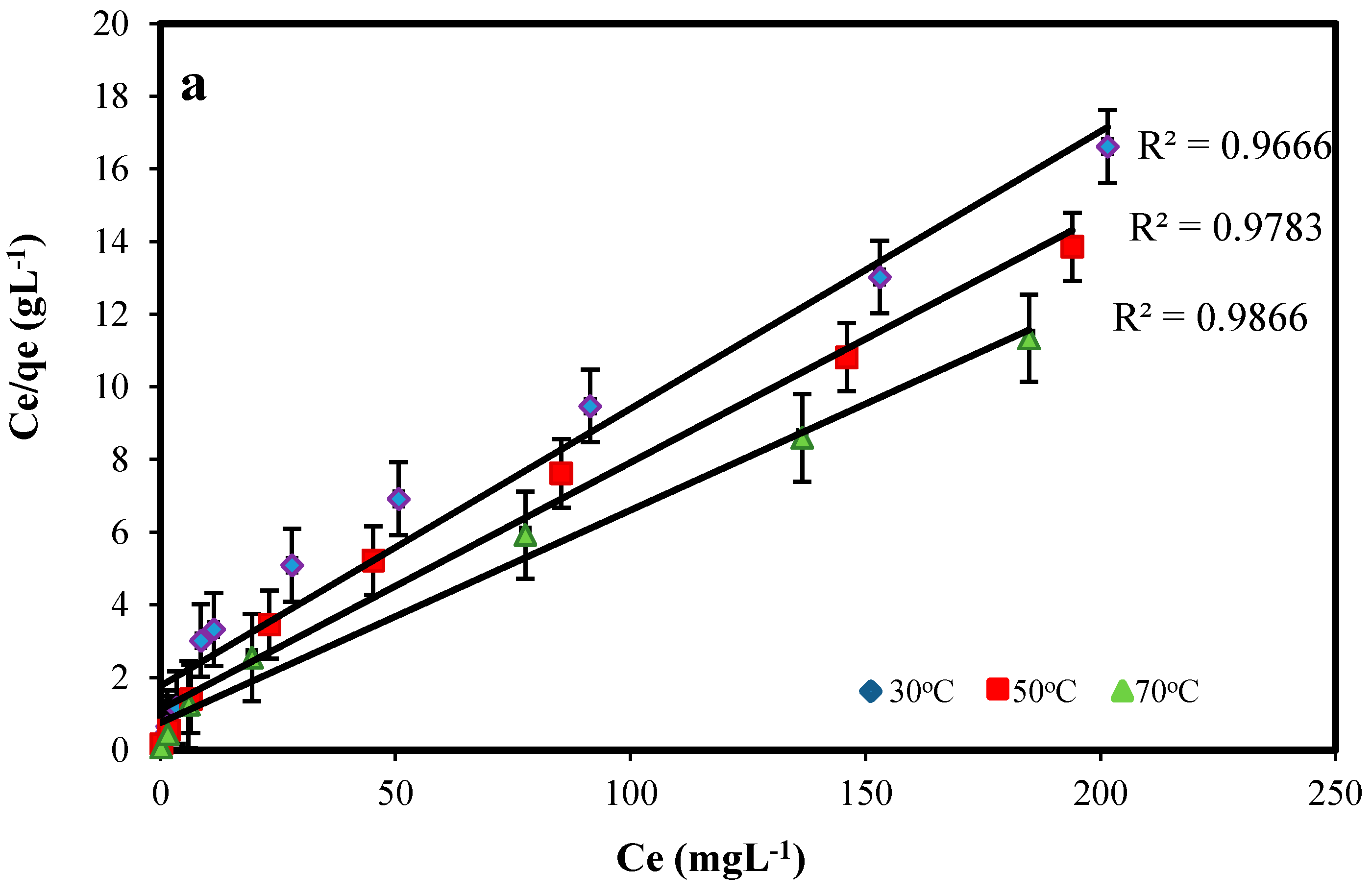
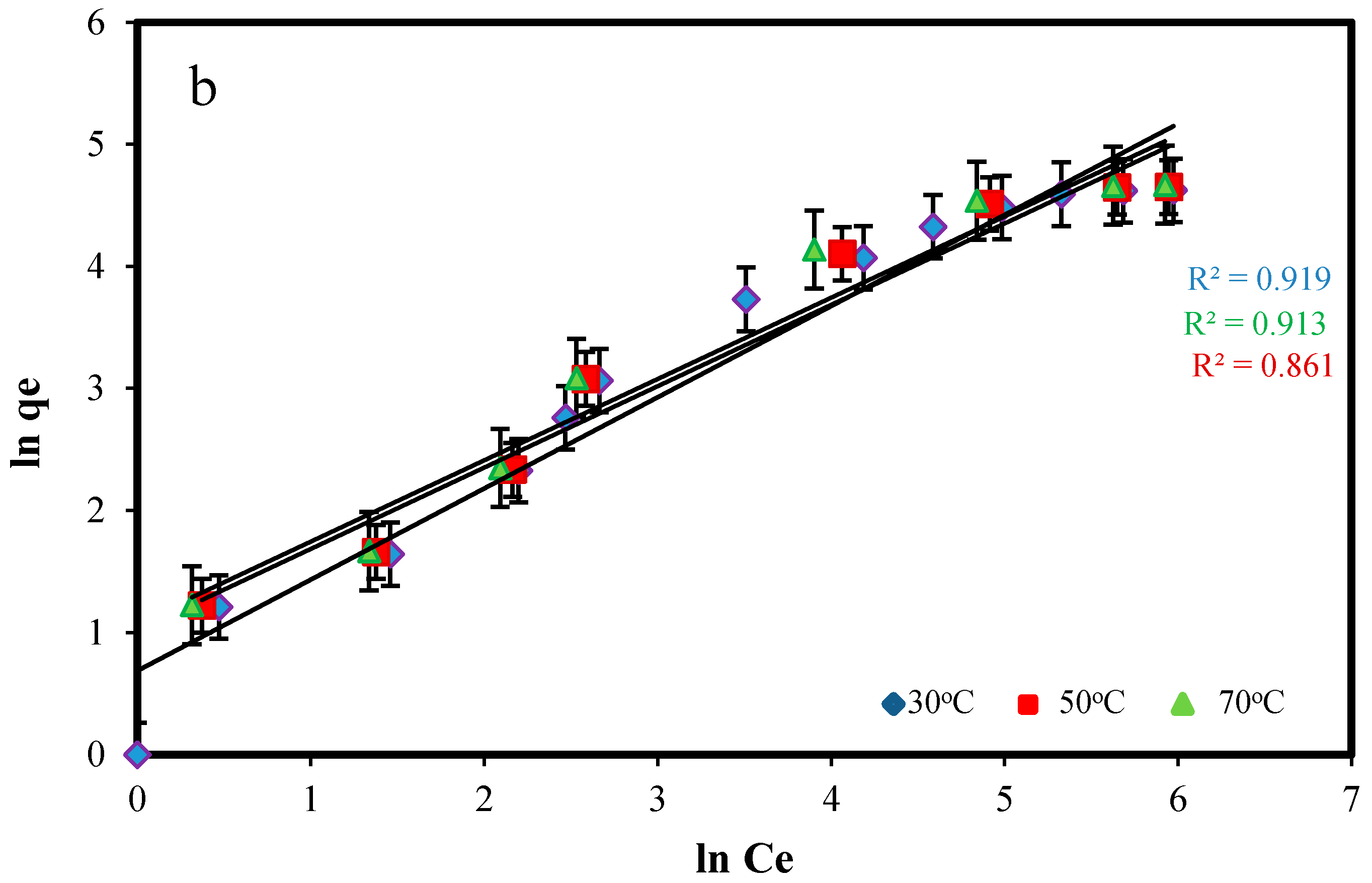
2.3.2. Adsorption Isotherm Modeling
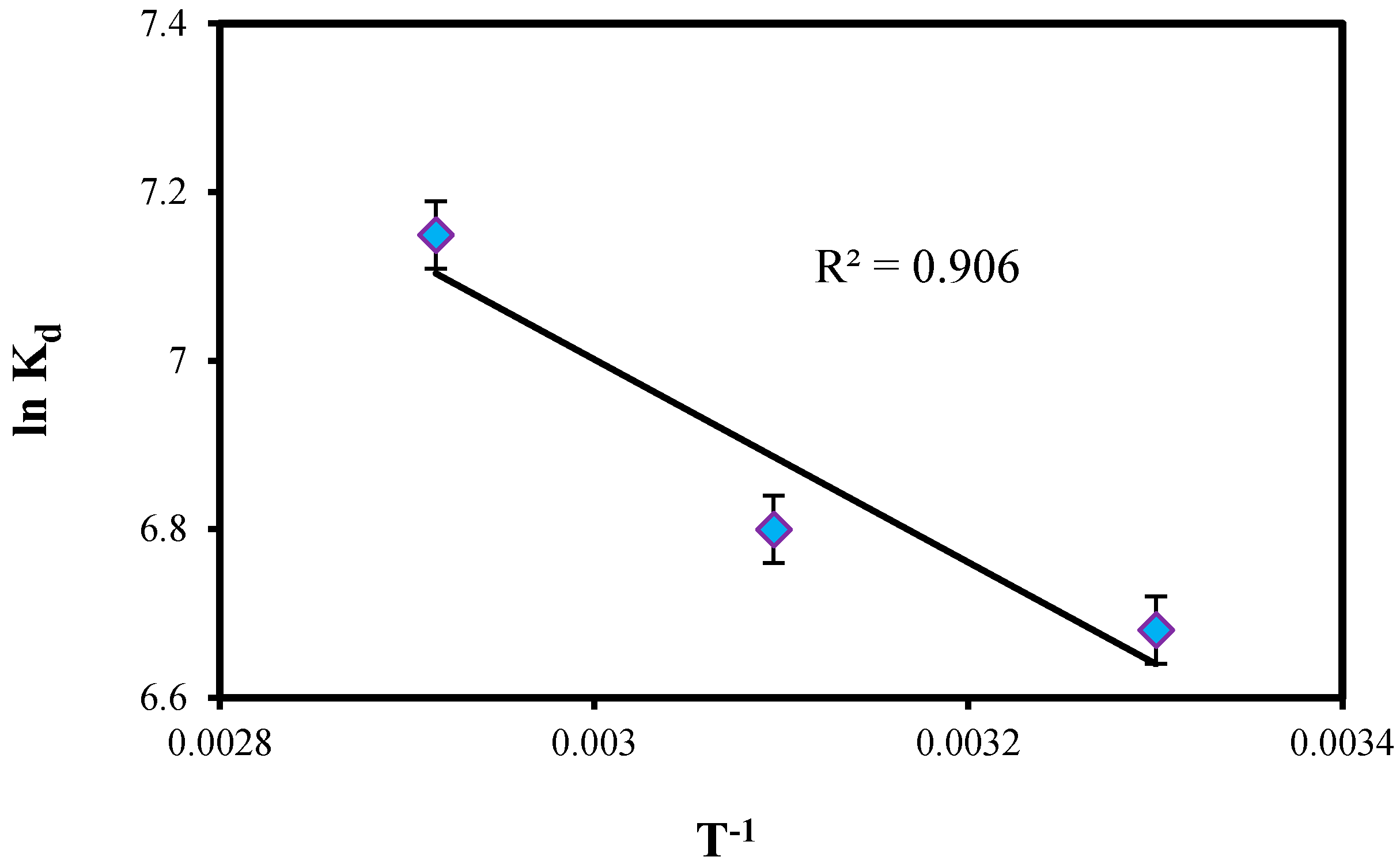
2.3.3. Thermodynamic Study
3. Materials and Methods
3.1. Materials
3.2. Extraction of Zerumbone
3.3. Synthesis of ZnO NPs
3.4. Characterization of ZnO NPs
3.5. Adsorption Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mahdavi, S.H.; Jalali, M. Removal of heavy metals from aqueous solutions using Fe3O4, ZnO, and CuO nanoparticles. J. Nanopart. Res. 2012, 14, 846. [Google Scholar] [CrossRef]
- Khan, S.H.B.; Rahman, M.M.; Marwani, H.M.; Asiri, A.M.; Alamry, K.H.A. An assessment of zinc oxide nanosheets as a selective adsorbent for cadmium. Nanoscale Res. Lett. 2013, 8, 377. [Google Scholar] [CrossRef] [PubMed]
- Ghiloufi, I.; EI Ghoul, J.; Modwi, A.; EI Mir, L. Ga-doped ZnO for adsorption of heavy metals from aqueous solution. Mater. Sci. Semicond. Process. 2016, 42, 102–106. [Google Scholar] [CrossRef]
- Agrawal, A.; Sahu, K.K. Kinetic and isotherm studies of cadmium adsorption on manganese nodule residue. J. Hazard. Mater. 2006, 137, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q.J. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.B.; Cai, W.P.; Liu, P.S.; Xu, X.X.; Zhou, H.J.; Klingshirn, C.; Kalt, H. ZnO-based hollow nanoparticles by selective etching: Elimination and reconstruction of metal-semiconductor interface.; improvement of blue emission and photocatalysis. ACS Nano 2008, 2, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.H.; Zhan, J.H. Fabrication and gas-sensing properties of porous ZnO nanoplates. Adv. Mater. 2008, 20, 547–551. [Google Scholar] [CrossRef]
- Chou, T.P.; Zhang, Q.F.; Fryxell, G.E.; Cao, G.Z. Hierarchically structured ZnO film for dye-sensitized solar cells with enhanced energy conversion efficiency. Adv. Mater. 2007, 19, 2588–2592. [Google Scholar] [CrossRef]
- Azizi, S.; Mohamad, R.; Bahadoran, A.; Bayat, S.; Abdul Rahim, R.; Ariff, A.; Zuhainis Saad, W. Effect of annealing temperature on antimicrobial and structural properties of bio-synthesized zinc oxide nanoparticles using flower extract of Anchusa italic. J. Photochem. Photobiol. B 2016, 161, 441–449. [Google Scholar] [CrossRef]
- Hassan, K.H.; Khammas, Z.A.A.; Rahman, A.M. Zinc oxide hydrogen sulfide removal catalyst/preparation, activity test and kinetic study. Al-Khwarizmi Eng. J. 2008, 4, 74–84. [Google Scholar]
- Wang, X.B.; Cai, W.P.; Lin, Y.X.; Wang, G.Z.; Liang, C.H. Mass production of micro/nanostructured porous ZnO plates and their strong structurally enhanced and selective adsorption performance for environmental remediation. J. Mater. Chem. 2010, 20, 8582–8590. [Google Scholar] [CrossRef]
- Azizi, S.; Namvar, F.; Mohamad, R.; Md Tahir, P.; Mahdavi, M. Facile biosynthesis and characterization of palm pollen stabilized ZnO nanoparticles. Mater. Lett. 2015, 148, 106–109. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Vali, H.; Orsat, V. Value-adding to grape waste: Green synthesis of gold nanoparticles. J. Food Eng. 2014, 142, 210–220. [Google Scholar] [CrossRef]
- Ghorbani, P.; Soltani, M.; Homayouni-Tabrizi, M.; Namvar, F.; Azizi, S.; Mohammad, R.; Boroumand Moghaddam, A. Sumac silver novel biodegradable nano composite for bio-medical application: Antibacterial activity. Molecules 2015, 20, 12946–12958. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Takahashita, D.; Kinoshita, T.; Koshimizu, K.; Kim, H.W.; Yoshihiro, A.; Nakamura, Y.; Jiwajinda, S.; Tereo, J.; Ohigashi, H. Zerumbone, a Southeast Asian ginger sesquiterpene, markedly suppress free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis. Carcinogenesis 2002, 23, 1961–1963. [Google Scholar] [CrossRef]
- Takada, Y.; Murakami, A.; Aggarwal, B.B. Zerumbone abolishes NF-kappaB and IkappaBalpha kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis and down regulation of invasion. Oncogene 2005, 17, 122–130. [Google Scholar]
- Khorsand Zak, A.; Majid, W.H.A.; Mahmoudian, M.R.; Darroudi, M.; Yousefi, R. Starch-stabilized synthesis of ZnO nanopowders at low temperature and optical properties study. Adv. Powder Technol. 2013, 24, 618–624. [Google Scholar] [CrossRef]
- Azizi, S.; Mohamad, R.; Mahdavi Shahri, M. Green Microwave-assisted combustion synthesis of zinc oxide nanoparticles with citrullus colocynthis (l.) schrad: Characterization and biomedical applications. Molecules 2017, 22, 301. [Google Scholar] [CrossRef] [PubMed]
- Eid Eltayeb, E.M.; Bustamam Abdul, A.; Suliman Fakhr Eldin, O.; Mohd Sukari, A.; Rasedee, A.; Fatah Safa, S. Characterization of the inclusion complex of zerumbone with hydroxypropyl—Cyclodextrin. Carbohydr. Polym. 2011, 83, 1707–1714. [Google Scholar]
- Senthil Kumar, P.; Vincent, C.; Kirthika, K.; Sathish Kumar, K. Kinetics and equilibrium studies of Pb2+ in removal from aqueous solutions by use of nano-silversol-coated activated carbon. Braz. J. Chem. Eng. 2010, 27, 339–346. [Google Scholar] [CrossRef]
- Kumar Meena, A.; Kadirvelu, K.; Mishra, G.K.; Rajagopal, C.H.; Nagar, P.N. Adsorptive removal of heavy metals from aqueous solution by treated sawdust (Acacia arabica). J. Hazard. Mater. 2008, 150, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Tobin, J.M.; Cooper, D.G.; Neufeld, R.J. Uptake of Metal Ions by rhizopus arrhizus biomass microbiol. Appl. Environ. 1984, 47, 821–824. [Google Scholar]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Gharayebi, Y.; Shameli, K.; Nadi, B. Fabrication and characterization of SiO2/(3-aminopropyl) triethoxysilane-coated magnetite nanoparticles for lead (II) removal from aqueous Solution. J. Inorg. Organomet. Polym. Mater. 2013, 23, 599–607. [Google Scholar] [CrossRef]
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Ab Rahman, M.Z.; Azizi, S. Rapid removal of Cu(II) ion by chemically modified rubber wood fibre. Environ. Eng. Sci. 2012, 29, 101–107. [Google Scholar] [CrossRef]
- Sheela, T.; Arthoba Nayaka, Y.; Viswanatha, R.; Basavanna, S.; Venkatesha, T.G. Kinetics and thermodynamics studies on the adsorption of Zn(II).; Cd(II) and Hg(II) from aqueous solution using zinc oxide nanoparticles. Powder Technol. 2012, 217, 163–170. [Google Scholar] [CrossRef]
- Andal, V.; Buvaneswari, G. Removal of lead ions by NiFe2O4 nanoparticles. IJRET 2014, 3, 475–483. [Google Scholar]
- Verma, M.; Tyagi, I.; Chandra, R.; Kumar Gupt, V. Adsorptive removal of Pb(II) ions from aqueous solution using CuO nanoparticles synthesized by sputtering method. J. Mol. Liq. 2017, 225, 936–944. [Google Scholar] [CrossRef]
- Beheshti, H.; Irani, M. Removal of lead(II) ions from aqueous solutions using diatomite nanoparticles. Desalination Water Treat. 2016, 57, 18799–18805. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Tay, A.S.H.; Tan, P.Y.; Hidajat, K.; Uddin, M.S. Carboxymethyl—Cyclodextrin conjugated magnetic nanoparticles as nano-adsorbents for removal of copper ions: Synthesis and adsorption studies. J. Hazard. Mater. 2011, 185, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Salleh, N.; Jais, U.S.; Sarijo, S.H. Gelatin-coated zeolite y for controlled release of anticancer drug (zerumbone). In Proceedings of the IEEE Symposium on Business, Engineering and Industrial Applications, Bandung, Indonesia, 23–26 September 2012; pp. 124–129. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

| Pseudo-First Order | Pseudo-Second Order | ||||||
|---|---|---|---|---|---|---|---|
| Conc (mg L−1) | qe exp (mg g−1) | k1 (min−1) | qe (cal) | R2 | k2 (g mg−1min−1) | qe (cal) | R2 |
| 10 | 2.1 | 4.8 × 10−2 | 1.3 | 0.868 | 22.7 × 10−2 | 2.2 | 0.999 |
| 20 | 3.7 | 2.3 × 10−2 | 4.2 | 0.817 | 10.7 × 10−2 | 3.8 | 0.989 |
| 30 | 4.8 | 1.4 × 10−2 | 5.8 | 0.959 | 4.8 × 10−2 | 4.5 | 0.997 |
| Langmuir Isotherm | Freundlich Isotherm | |||||
|---|---|---|---|---|---|---|
| Temp (°C) | Qm (mg g−1) | B (L mg−1) | R2 | KF (mg g−1) | n | R2 |
| 30 | 15.65 | 5.93 × 10−2 | 0.988 | 1.98 | 2.34 | 0.929 |
| 50 | 17.84 | 7.37 × 10−2 | 0.999 | 2.94 | 2.54 | 0.961 |
| 70 | 19.65 | 8.39 × 10−2 | 0.999 | 3.16 | 2.81 | 0.914 |
| Temp (K) | ΔH0 (kJ mol−1) | ΔG0 (kJ mol−1) | ΔS0 (J mol−1 K−1) |
|---|---|---|---|
| 30 | 11.13 | –16.19 | 0.7 |
| 50 | −18.40 | ||
| 70 | –19.91 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azizi, S.; Mahdavi Shahri, M.; Mohamad, R. Green Synthesis of Zinc Oxide Nanoparticles for Enhanced Adsorption of Lead Ions from Aqueous Solutions: Equilibrium, Kinetic and Thermodynamic Studies. Molecules 2017, 22, 831. https://doi.org/10.3390/molecules22060831
Azizi S, Mahdavi Shahri M, Mohamad R. Green Synthesis of Zinc Oxide Nanoparticles for Enhanced Adsorption of Lead Ions from Aqueous Solutions: Equilibrium, Kinetic and Thermodynamic Studies. Molecules. 2017; 22(6):831. https://doi.org/10.3390/molecules22060831
Chicago/Turabian StyleAzizi, Susan, Mahnaz Mahdavi Shahri, and Rosfarizan Mohamad. 2017. "Green Synthesis of Zinc Oxide Nanoparticles for Enhanced Adsorption of Lead Ions from Aqueous Solutions: Equilibrium, Kinetic and Thermodynamic Studies" Molecules 22, no. 6: 831. https://doi.org/10.3390/molecules22060831