Discovery of Indeno[1,2-c]quinoline Derivatives as Potent Dual Antituberculosis and Anti-Inflammatory Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Results and Discussion
3. Experimental Section
3.1. General
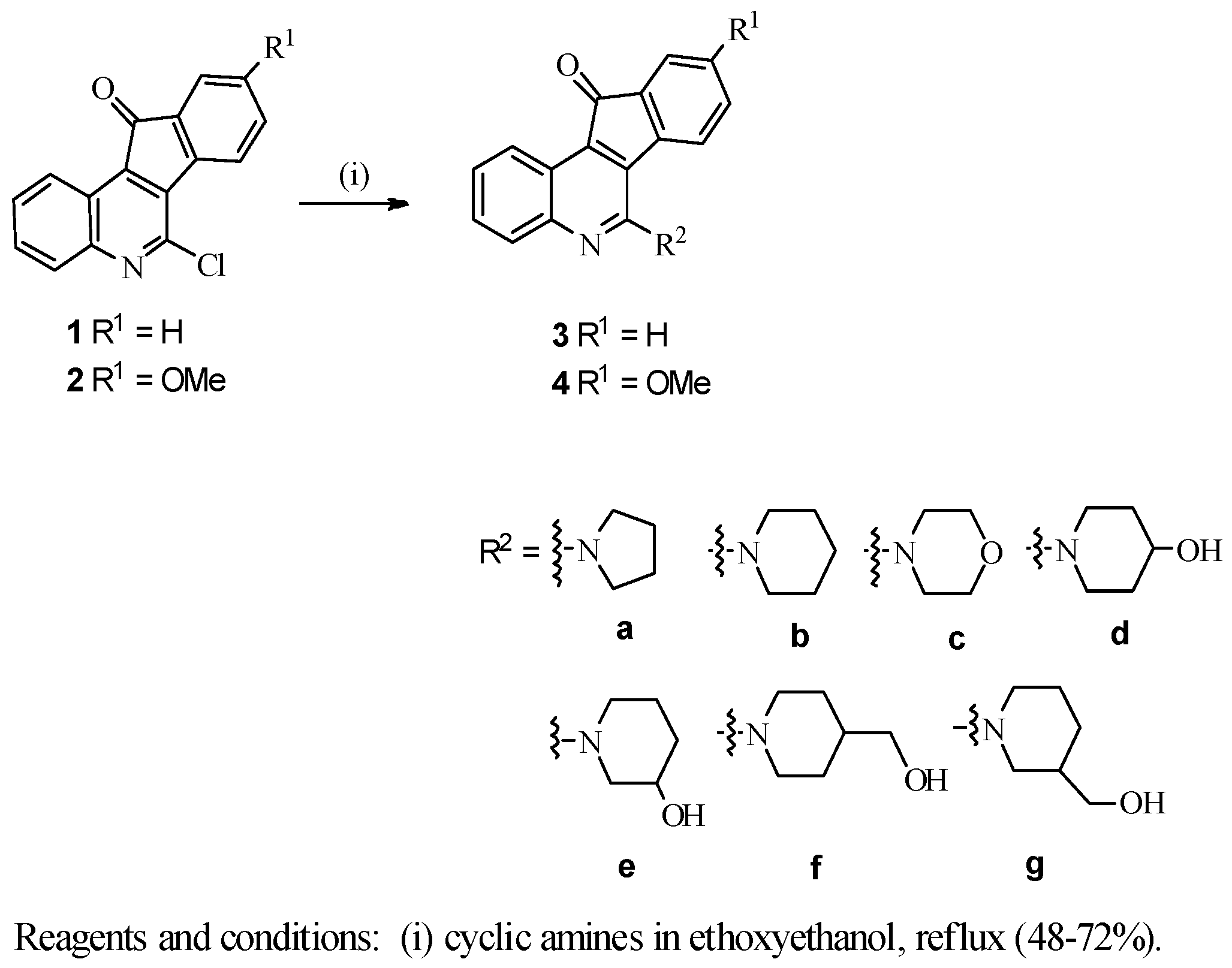
3.2. General Procedure for the Preparation of 6-Substituted-11H-indeno[1,2-c]quinolin-11-one Compounds 3a–g, 4a, 4d–g
3.2.1. 6-(Pyrrolidin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (3a)
3.2.2. 6-(Piperidin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (3b)
3.2.3. 6-(Morpholino-1-yl)-11H-indeno[1,2-c]quinolin-11-one (3c)
3.2.4. 6-(4-Hydroxypiperidin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (3d)
3.2.5. 6-(3-Hydroxypiperidin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (3e)
3.2.6. 6-(4-Piperidinemethano-1-yl)-11H-indeno[1,2-c]quinolin-11-one (3f)
3.2.7. 6-(3-Piperidinemethano-1-yl)-11H-indeno[1,2-c]quinolin-11-one (3g)
3.2.8. 9-Methoxy-6-(pyrrolidin-1-yl)-11H-indeno[1,2-c]quinolin-11-one (4a)
3.2.9. 6-(4-Hydroxypiperidin-1-yl)-9-methoxy-11H-indeno[1,2-c]quinolin-11-one (4d)
3.2.10. 6-(3-Hydroxypiperidin-1-yl)-9-methoxy-11H-indeno[1,2-c]quinolin-11-one (4e)
3.2.11. 6-[4-(Hydroxymethyl)piperidin-1-yl]-9-methoxy-11H-indeno[1,2-c]quinolin-11-one (4f)
3.2.12. 6-[3-(Hydroxymethyl)piperidin-1-yl]-9-methoxy-11H-indeno[1,2-c]quinolin-11-one (4g)
3.2.13. 1-(11H-Indeno[1,2-c]quinolin-6-yl)piperidin-4-ol (5)
3.2.14. 6-(4-Hydroxypiperidin-1-yl)-11-methyl-11H-indeno[1,2-c]quinolin-11-ol (6)
3.2.15. (E)-6-(4-Hydroxypiperidin-1-yl)-11H-indeno[1,2-c]quinolin-11-one Oxime (7)
3.2.16. (E)-6-(4-Hydroxypiperidin-1-yl)-11H-indeno[1,2-c]quinolin-11-one O-methyl Oxime (8)
3.2.17. (E)-2-[6-(4-Hydroxypiperidin-1-yl)-11H-indeno[1,2-c]quinolin-11-ylidene]hydrazine Carboxamide (9)
3.2.18. (E)-2-[6-(4-Hydroxypiperidin-1-yl)-11H-indeno[1,2-c]quinolin-11-ylidene]hydrazine Carbothioamide (10)
3.2.19. (E)-N′-[6-(4-Hydroxypiperidin-1-yl)-11H-indeno[1,2-c]quinolin-11-ylidene]benzohydrazide (11)
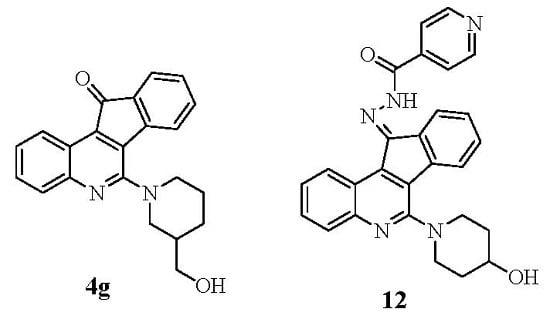
3.2.20. (E)-N′-[6-(4-Hydroxypiperidin-1-yl)-11H-indeno[1,2-c]quinolin-11-ylidene]isonicotino Hydrazide (12)
3.3. Anti-Mycobacterium Activity
3.4. Superoxide Generation and Elastase Release
3.5. Quantitative Structure–Activity Relationship (QSAR)
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scott, H.M.; Flynn, J.L. Mycobacterium tuberculosis in chemokine receptor 2-deficient mice: Influence of dose on disease progression. Infect. Immun. 2002, 70, 5946–5954. [Google Scholar] [CrossRef] [PubMed]
- Peters, W.; Scott, H.M.; Chambers, H.F.; Flynn, J.L.; Charo, I.F.; Ernst, J.D. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2001, 98, 7958–7963. [Google Scholar] [CrossRef] [PubMed]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P.R. The immune response in tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef] [PubMed]
- Dooley, D.P.; Carpenter, J.L.; Rademacher, S. Adjunctive corticosteroid therapy for tuberculosis: A critical reappraisal of the literature. Clin. Infect. Dis. 1997, 25, 872–887. [Google Scholar] [CrossRef] [PubMed]
- Worthington, R.J.; Melander, C. Combination approaches to combat multi-drug-resistant bacteria. Trends Biotechnol. 2013, 31, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Wallis, R.S.; Maeurer, M.; Mwaba, P.; Chakaya, J.; Rustomjee, R.; Migliori, G.B.; Marais, B.; Schito, M.; Churchyard, G.; Swaminathan, S.; et al. Tuberculosis—Advances in development of new drugs, treatment regimens, host-directed therapies, and biomarkers. Lancet Infect. Dis. 2016, 16, e34–e46. [Google Scholar] [CrossRef]
- Rayasam, G.V.; Balganesh, T.S. Exploring the potential of adjunct therapy in tuberculosis. Trends Pharmacol. Sci. 2015, 36, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Cholo, M.C.; Steel, H.C.; Fourie, P.B.; Germishuizen, W.A.; Anderson, R. Clofazimine: Current status and future prospects. J. Antimicrob. Chemother. 2012, 67, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Van Heeswijk, R.P.G.; Dannemann, B.; Hoetelmans, R.M.W. Bedaquiline: A review of human pharmacokinetics and drug–drug interactions. J. Antimicrob. Chemother. 2014, 69, 2310–2318. [Google Scholar] [CrossRef] [PubMed]
- Upadhayaya, R.S.; Shinde, P.D.; Sayyed, A.Y.; Kadam, S.A.; Bawane, A.N.; Poddar, A.; Plashkevych, O.; Földesi, A.; Chattopadhyaya, J. Synthesis and structure of azole-fused indeno[2,1-c]quinolines and their anti-mycobacterial properties. Org. Biomol. Chem. 2010, 8, 5661–5673. [Google Scholar] [CrossRef] [PubMed]
- Upadhayaya, R.S.; Lahore, S.V.; Sayyed, A.Y.; Dixit, S.S.; Shinde, P.D.; Chattopadhyaya, J. Conformationally-constrained indeno[2,1-c]quinolones—A new class of anti-mycobacterial agents. Org. Biomol. Chem. 2010, 8, 2180–2197. [Google Scholar] [CrossRef] [PubMed]
- Upadhayaya, R.S.; Shinde, P.D.; Kadam, S.A.; Bawane, A.N.; Sayyed, A.Y.; Kardile, R.A.; Gitay, P.N.; Lahore, S.V.; Dixit, S.S.; Földesi, A.; et al. Synthesis and antimycobacterial activity of prodrugs of indeno[2,1-c]quinoline derivatives. Eur. J. Med. Chem. 2011, 46, 1306–1324. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.Y.; Chen, Y.L.; Fang, K.C.; Wang, T.C.; Tzeng, C.C.; Peng, C.F. Synthesis and antibacterial activity of 1-(substituted-benzyl)-6-fluoro-1,4-dihydro-4-oxoquinoline-3-carboxylic acids and their 6,8-difluoro analogs. J. Heterocycl. Chem. 1998, 35, 955–964. [Google Scholar] [CrossRef]
- Sheu, J.Y.; Chen, Y.L.; Tzeng, C.C.; Hsu, S.L.; Fang, K.C.; Wang, T.C. Synthesis, and antimycobacterial and cytotoxic evaluation of certain fluoroquinolone derivatives. Helv. Chim. Acta 2003, 86, 2481–2489. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Chen, Y.L.; Sheu, J.Y.; Chen, I.L.; Wang, T.C.; Tzeng, C.C. Synthesis and antimycobacterial evaluation of certain fluoroquinolone derivatives. Bioorg. Med. Chem. 2005, 13, 3921–3926. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Huang, H.Y.; Chen, Y.W.; Huang, Z.Y.; Tzeng, C.C.; Liu, C.L.; Yao, C.W. Synthesis and antimycobacterial evaluation of metal-chelator bearing fluoroquinolones. Chin. Pharm. J. 2005, 57, 57–70. [Google Scholar]
- Yang, C.L.; Tseng, C.H.; Chen, Y.L.; Lu, C.M.; Kao, C.L.; Tseng, H.Y.; Wu, M.H.; Tzeng, C.C. Identification of benzofuro[2,3-b]quinoline derivatives as a new class of antituberculosis agents. Eur. J. Med. Chem. 2010, 45, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Lu, C.M.; Chen, I.L.; Tsao, L.T.; Wang, J.P. Synthesis and antiinflammatory evaluation of 9-anilinoacridine and 9-phenoxyacridine derivatives. J. Med. Chem. 2002, 45, 4689–4694. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Chen, I.L.; Lu, C.M.; Tzeng, C.C.; Tsao, L.T.; Wang, J.P. Synthesis and anti-inflammatory evaluation of 9-phenoxyacridine and 4-phenoxyfuro[2,3-b]quinoline derivatives. Part 2. Bioorg. Med. Chem. 2003, 11, 3921–3927. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chen, I.L.; Lu, C.M.; Tzeng, C.C.; Tsao, L.T.; Wang, J.P. Synthesis and anti-inflammatory evaluation of 4-anilinofuro[2,3-b]quinoline and 4-phenoxyfuro[2,3-b]quinoline derivatives. Part 3. Bioorg. Med. Chem. 2004, 12, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Kuan, Y.H.; Lin, R.H.; Chen, Y.L.; Tsao, L.T.; Tzeng, C.C.; Wang, J.P. Effective attenuation of acute lung injury in vivo and the formyl peptide-induced neutrophil activation in vitro by CYL-26z through the phosphoinositide 3-kinase gamma pathway. Biochem. Pharmacol. 2006, 72, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Zhao, Y.L.; Lu, C.M.; Tzeng, C.C.; Wang, J.P. Synthesis, cytotoxicity, and anti-inflammatory evaluation of 2-(furan-2-yl)-4-(phenoxy)quinoline derivatives, Part 4. Bioorg. Med. Chem. 2006, 14, 4373–4378. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.W.; Tsao, L.T.; Chang, L.C.; Chen, Y.L.; Huang, L.J.; Kuo, S.C.; Tzeng, C.C.; Lee, M.R.; Wang, J.P. Inhibition of lipopolysaccharide-stimulated NO production by a novel synthetic compound CYL-4d in RAW 264.7 macrophages involving the blockade of MEK4/JNK/AP-1 pathway. Biochem. Pharmacol. 2007, 73, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Lin, C.S.; Shih, P.K.; Tsao, L.T.; Wang, J.P.; Cheng, C.M.; Tzeng, C.C.; Chen, Y.L. Furo[3, 2:3,4]naphtho[1,2-d]imidazole derivatives as potential inhibitors of inflammatory factors in sepsis. Bioorg. Med. Chem. 2009, 17, 6773–6779. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Tzeng, C.C.; Shih, P.K.; Yang, C.N.; Chuang, Y.C.; Peng, S.I.; Lin, C.S.; Wang, J.P.; Cheng, C.M.; Chen, Y.L. Identification of furo[3′,2′:3,4]naphtho[1,2-d]imidazole derivatives as orally active and selective inhibitors of microsomal prostaglandin E(2) synthase-1 (mPGES-1). Mol. Divers. 2012, 16, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.R.; Huang, L.J.; Lee, M.R.; Chen, Y.L.; Kuo, S.C.; Tzeng, C.C.; Hsu, M.F.; Wang, J.P. The signaling mechanisms mediating the inhibitory effect of TCH-1116 on formyl peptide-stimulated superoxide anion generation in neutrophils. Eur. J. Pharm. 2012, 682, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Cheng, C.M.; Tzeng, C.C.; Peng, S.I.; Yang, C.L.; Chen, Y.L. Synthesis and anti-inflammatory evaluations of â-lapachone derivatives. Bioorg. Med. Chem. 2013, 21, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Tseng, C.H.; Chen, Y.L.; Hwang, T.L.; Tzeng, C.C. Discovery of benzo[f]indole-4,9-dione derivatives as new type of anti-inflammatory agents. Int. J. Mol. Sci. 2015, 16, 6532–6544. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Chen, Y.L.; Lu, P.J.; Yang, C.N.; Tzeng, C.C. Synthesis and antiproliferative evaluation of certain indeno[1,2-c]quinoline derivatives. Bioorg. Med. Chem. 2008, 16, 3153–3162. [Google Scholar] [CrossRef] [PubMed]
- Tung, C.W. Prediction of pupylation sites using the composition of k-spaced amino acid pairs. J. Theor. Biol. 2013, 336, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Tung, C.W.; Wu, M.T.; Chen, Y.K.; Wu, C.C.; Chen, W.C.; Li, H.P.; Chou, S.H.; Wu, D.C.; Wu, I.C. Identification of biomarkers for esophageal squamous cell carcinoma using feature selection and decision tree methods. Sci. World J. 2013, 2013, 782031. [Google Scholar] [CrossRef] [PubMed]
- Kier, L.B.; Hall, L.H. Molecular Connectivity in Chemistry and Drug Research; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Wildman, S.A.; Crippen, G.M. Prediction of physicochemical parameters by atomic contributions. J. Chem. Inf. Comput. Sci. 1999, 39, 868–873. [Google Scholar] [CrossRef]
- Gramatica, P.; Corradi, M.; Consonni, V. Modelling and prediction of soil sorption coefficients of non-ionic organic pesticides by molecular descriptors. Chemosphere 2000, 41, 763–777. [Google Scholar] [CrossRef]
- Hall, L.H.; Kier, L.B. Electrotopological state indices for atom types: A novel combination of electronic, topological, and valence state information. J. Chem. Inf. Comput. Sci. 1995, 35, 1039–1045. [Google Scholar] [CrossRef]
- Liu, R.; Sun, H.; So, S.S. Development of quantitative structure—Property relationship models for early ADME evaluation in drug discovery. 2. Blood-brain barrier penetration. J. Chem. Inf. Comput. Sci. 2001, 41, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.; Franzblau, S.G. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 1997, 41, 1004–1009. [Google Scholar] [PubMed]
- Hwang, T.L.; Li, G.L.; Lan, Y.H.; Chia, Y.C.; Hsieh, P.W.; Wu, Y.H.; Wu, Y.C. Potent inhibition of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the chinese medicinal herb Fissistigma oldhamii. Free Radic. Biol. Med. 2009, 46, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.W. PaDEL-descriptor: An open source software to calculate molecular descriptors and fingerprints. J. Comput. Chem. 2011, 32, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, C.; Han, Y.; Kuhn, S.; Horlacher, O.; Luttmann, E.; Willighage, E. The Chemistry Development Kit (CDK): An open-source Java library for Chemo- and Bioinformatics. J. Chem. Inf. Comput. Sci. 2003, 43, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Tung, C.W.; Jheng, J.L. Interpretable prediction of non-genotoxic hepatocarcinogenic chemicals. Neurocomputing 2014, 145, 68–74. [Google Scholar] [CrossRef]
- Chauhan, J.S.; Dhanda, S.K.; Singla, D.; The Open Source Drug Discovery; Agarwal, S.M.; Raghava, G.P.S. QSAR-based models for designing quinazoline/imidazothiazoles/pyrazolopyrimidines based inhibitors against wild and mutant EGFR. PLoS ONE 2014, 9, e101079. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds reported herein are available from the authors. |

| Compounds | R1 | R2 | MIC (μg/ mL) | Survival Rate of Vero Cells @ 20 μg/mL (%) |
|---|---|---|---|---|
| 3a | H |  | 4.56 | 88.61 |
| 3b | H |  | 4.93 | 96.18 |
| 3c | H |  | 3.12 | 91.19 |
| 3d | H |  | 2.09 | 91.23 |
| 3e | H |  | 4.97 | 87.78 |
| 3f | H |  | 3.98 | 91.11 |
| 3g | H |  | 3.07 | 92.25 |
| 4a | OMe |  | 17 | 89.62 |
| 4b | OMe |  | >20 | 99.55 |
| 4c | OMe |  | >20 | 94.47 |
| 4d | OMe |  | 7.74 | 84.72 |
| 4e | OMe |  | 19.10 | 87.59 |
| 4f | OMe |  | >20 | 90.26 |
| 4g | OMe |  | >20 | 87.60 |
| 5 | 18.0 | 85.41 | ||
| 6 | >20 | 89.83 | ||
| 7 | >20 | 84.14 | ||
| 8 | >20 | 91.48 | ||
| 9 | >20 | 81.20 | ||
| 10 | >20 | 80.28 | ||
| 11 | 1.98 | 89.55 | ||
| 12 | 0.96 | 94.25 | ||
| INH | 0.8–1.2 | 84.80 | ||
| Compounds | Superoxide Anion | Elastase Release |
|---|---|---|
| 3a | >10 | >10 |
| 3b | >10 | >10 |
| 3c | >10 | >10 |
| 3d | 1.78 ± 0.44 | 2.20 ± 0.69 |
| 3e | 2.90 ± 0.11 | 2.80 ± 0.09 |
| 3f | 3.37 ± 0.17 | 4.50 ± 1.32 |
| 3g | 2.77 ± 0.47 | 2.12 ± 1.19 |
| 4a | >10 | >10 |
| 4b | >10 | >10 |
| 4c | >10 | >10 |
| 4d | 2.82 ± 0.30 | 2.05 ± 0.24 |
| 4e | 1.89 ± 0.65 | 2.93 ± 0.70 |
| 4f | 1.93 ± 0.92 | 0.88 ± 0.30 |
| 4g | 0.68 ± 0.14 | 0.46 ± 0.08 |
| 5 | 4.02 ± 1.08 | 5.29 ± 0.48 |
| 6 | >10 | >10 |
| 7 | 0.83 ± 0.47 | 1.39 ± 0.64 |
| 8 | 2.68 ± 0.95 | 2.13 ± 0.92 |
| 9 | 3.64 ± 1.43 | >10 |
| 10 | 2.41 ± 0.36 | >10 |
| 11 | 1.67 ± 0.87 | >10 |
| 12 | 1.72 ± 0.23 | 1.76 ± 0.49 |
| LY294002 b | 1.36 ± 0.33 | 2.21 ± 0.45 |
| Coefficient | Estimate Std. Error | t-Value | Pr (>|t|) | ||
|---|---|---|---|---|---|
| (Intercept) | 0.5035 | 1.1585 | 0.435 | 0.681929 | |
| SCH-6 | 11.463 | 1.0533 | 10.883 | 0.000114 | *** |
| CrippenLogP | −0.5711 | 0.1296 | −4.407 | 0.006977 | ** |
| hmax | −3.8978 | 1.4043 | −2.776 | 0.039106 | * |
| Compound | Observed pMIC | Predicted pMIC | Error |
|---|---|---|---|
| 12 | 2.67 | 2.64 | 0.03 |
| 11 | 2.35 | 2.37 | −0.02 |
| 3g | 2.05 | 1.86 | 0.19 |
| 3c | 2.01 | 2.08 | −0.08 |
| 3e | 1.82 | 1.80 | 0.02 |
| 3a | 1.82 | 1.88 | −0.06 |
| 4d | 1.67 | 1.73 | −0.06 |
| 4a | 1.29 | 1.38 | −0.10 |
| 5 | 1.24 | 1.18 | 0.07 |
| Compound | Observed pMIC | Predicted pMIC | Error |
|---|---|---|---|
| 3d | 2.20 | 2.00 | 0.20 |
| 3f | 1.94 | 1.95 | −0.01 |
| 3b | 1.80 | 1.87 | −0.07 |
| 4e | 1.28 | 1.58 | −0.30 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, C.-H.; Tung, C.-W.; Wu, C.-H.; Tzeng, C.-C.; Chen, Y.-H.; Hwang, T.-L.; Chen, Y.-L. Discovery of Indeno[1,2-c]quinoline Derivatives as Potent Dual Antituberculosis and Anti-Inflammatory Agents. Molecules 2017, 22, 1001. https://doi.org/10.3390/molecules22061001
Tseng C-H, Tung C-W, Wu C-H, Tzeng C-C, Chen Y-H, Hwang T-L, Chen Y-L. Discovery of Indeno[1,2-c]quinoline Derivatives as Potent Dual Antituberculosis and Anti-Inflammatory Agents. Molecules. 2017; 22(6):1001. https://doi.org/10.3390/molecules22061001
Chicago/Turabian StyleTseng, Chih-Hua, Chun-Wei Tung, Chen-Hsin Wu, Cherng-Chyi Tzeng, Yen-Hsu Chen, Tsong-Long Hwang, and Yeh-Long Chen. 2017. "Discovery of Indeno[1,2-c]quinoline Derivatives as Potent Dual Antituberculosis and Anti-Inflammatory Agents" Molecules 22, no. 6: 1001. https://doi.org/10.3390/molecules22061001