Hibiscus sabdariffa L. and Its Bioactive Constituents Exhibit Antiviral Activity against HSV-2 and Anti-enzymatic Properties against Urease by an ESI-MS Based Assay
Abstract
1. Introduction
2. Results
2.1. Determinations of Concentration of PCA and Total Polyphenols Content in Plant Material
2.2. Anti-HSV-2 Activity and Cytotoxicity
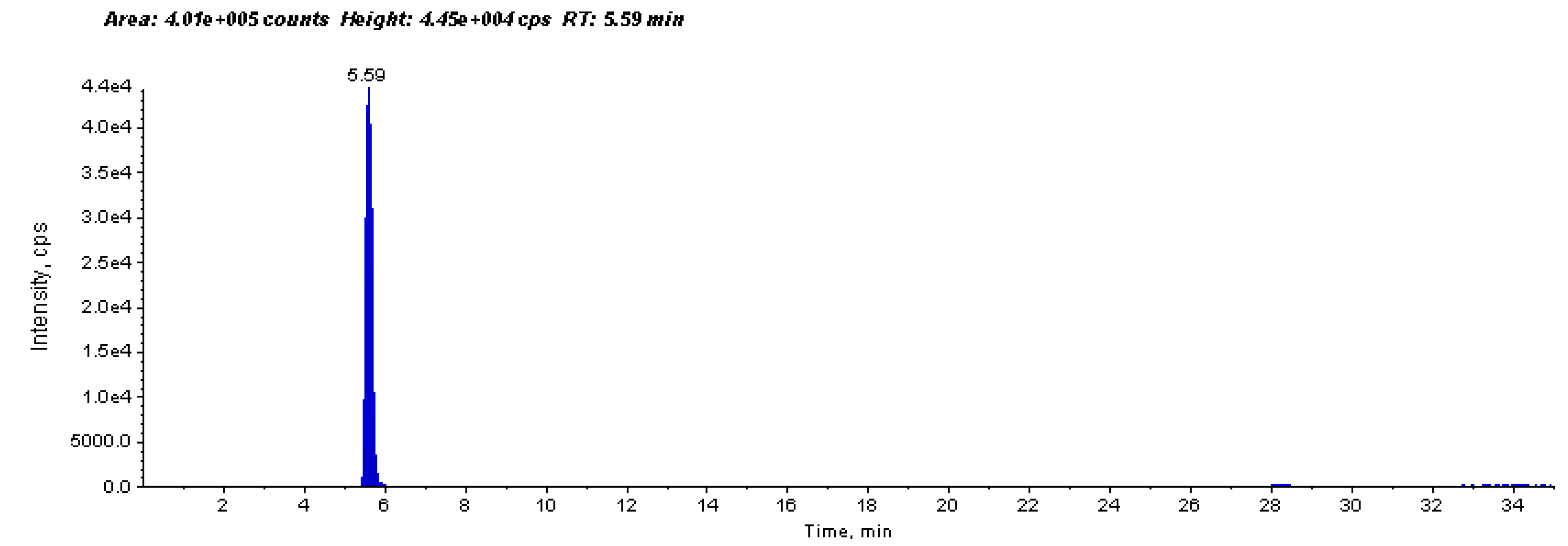
2.3. Anti-Urease Inhibitory Properties by ESI-MS-Based Assay
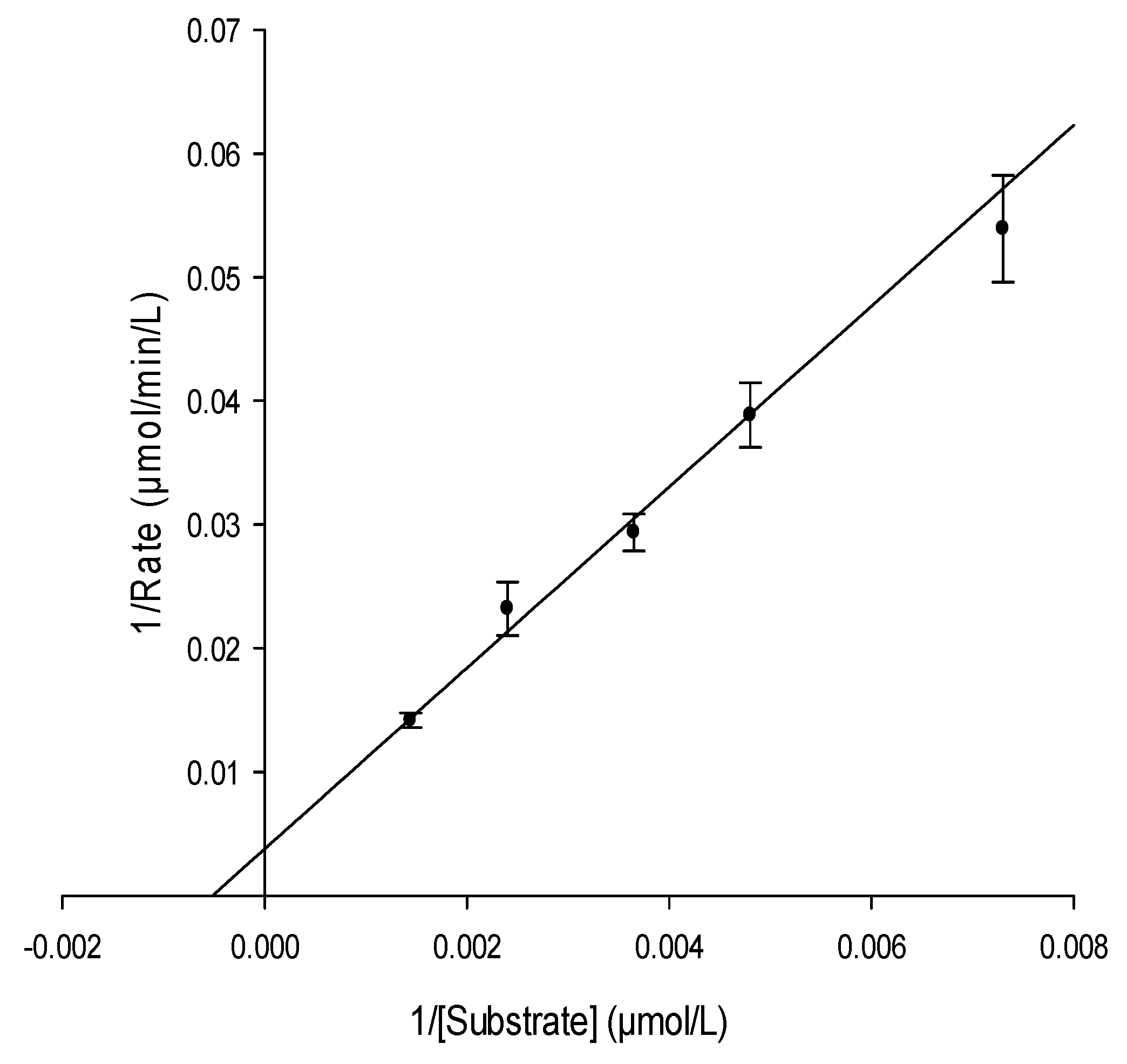
2.3.1. Determination of Urease Kinetic Parameters Km and Vmax
2.3.2. Determination of Half Maximal Inhibitory Concentration (IC50)
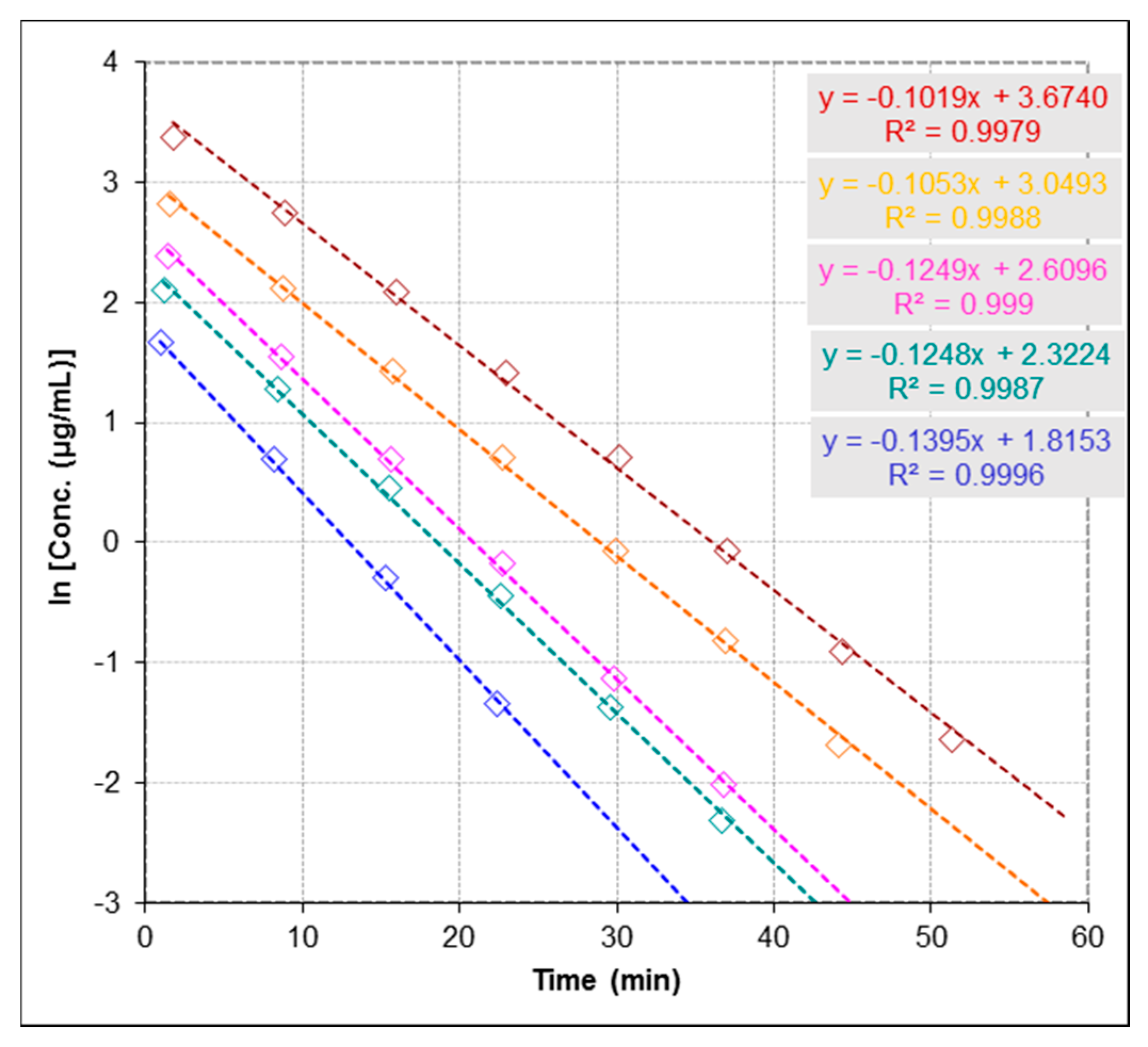
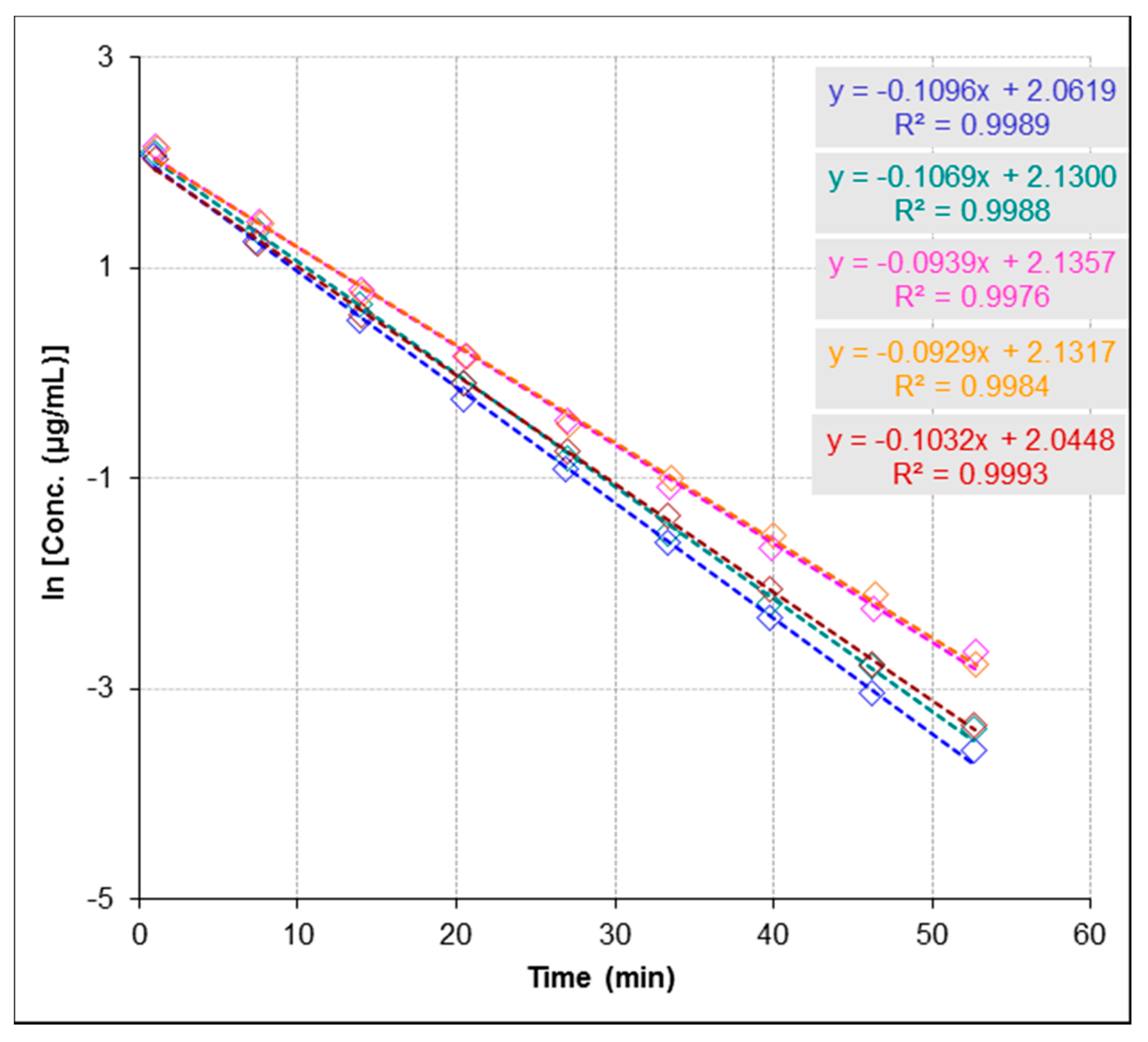
2.3.3. Repeatability and Stability Studies
3. Discussion
4. Materials and Methods
4.1. Plant Collection and Extraction Procedure
4.1.1. Determination of Concentration of PCA in Plant Material
4.1.2. Determination of Total Polyphenols by Folin-Ciocalteu Method
4.2. Antiviral Activity against HSV-2
4.2.1. Viral Strains, Cultures, Cell Lines and Reagents
4.2.2. Determination of Cytotoxicity
4.2.3. Anti-HSV-2 Activity
4.2.4. Statistical Analysis
4.3. Anti-urease Inhibitory Properties
4.3.1. Enzyme, Substrate, Inhibitors and Reagents
4.3.2. Instrumentation
4.3.3. Anti-urease Activity by ESI-MS-Based Assay
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Einsenberg, R.; Atanasiu, D.; Cairns, T.M.; Gallagher, J.R.; Krummenacher, C.; Cohen, G.H. Herpes virus fusion and entry: A story with many characters. Viruses 2012, 4, 800–832. [Google Scholar] [CrossRef] [PubMed]
- Gescher, K.; Hensel, A.; Hafezi, W.; Derksen, A.; Kühn, J. Oligomeric proanthocyanidins from Rumex acetosa L inhibit the attachment of herpes simplex virus type-1. Antivir. Res. 2011, 89, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Alter, A.; Thompson, K.D.; Gambari, R. Extracts and molecules from medicinal plants against herpes simplex viruses. Antivir. Res. 2005, 67, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.J.; Tujama, A.; Carlucci, M.J.; Herold, B.C. Topical microbicides for prevention of genital herpes infection. J. Antimicrob. Chemother. 2005, 55, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Q.; Lu, J.; Fan, P.; Xie, W.; Qiu, W.; Wang, F.; Hu, G.; Zhang, Y. Serine/Arginine-rich splicing factor 2 modulates herpes simplex virus type 1 replication via regulating viral gene transcriptional activity and pre-mRNA splicing. J. Biol. Chem. 2016, 291, 26377–26387. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.S.; Berchová-Bímová, K.; Petráš, J.; Hassan, K.T.S. Cucurbitacin B interacts synergistically with antibiotics against Staphylococcus aureus clinical isolates and exhibits antiviral activity against HSV-1. S. Afr. J. Bot. 2017, 108, 90–94. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Masarčíková, R.; Berchová, K. Bioactive natural products with anti-herpes simplex virus properties. J. Pharm. Pharmacol. 2015, 67, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.S.; Žemlička, M. Plant-derived urease inhibitors as alternative chemotherapeutic agents. Arch. Pharm. 2016, 349, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, I.; Zarnowiec, P.; Kwinkowski, M.; Kolesinska, B.; Fraczyk, J.; Kaminski, Z.; Kaca, W. Bacterial urease and its role in long-lasting human diseases. Curr. Protein Pept. 2012, 13, 789–806. [Google Scholar] [CrossRef]
- Follmer, C.J. Ureases as a target for the treatment of gastric and urinary infections. Clin. Pathol. 2010, 63, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Kosikowska, P.; Berlicki, Ł. Urease inhibitors as potential drugs for gastric and urinary tract infections: A patent review. Expert Opin. Ther. Pat. 2011, 21, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, L.; Cianci, M.; Musiani, F.; Ciurli, S. Inactivation of urease by 1,4-benzoquinone: Chemistry at the protein surface. Dalton Trans. 2016, 45, 5455–5459. [Google Scholar] [CrossRef] [PubMed]
- Mobley, H.L.; Island, M.D.; Hausinger, R.P. Molecular biology of microbial ureases. Microbiol. Rev. 1995, 59, 451–480. [Google Scholar] [PubMed]
- Tanaka, T.; Kawase, M.; Tani, S. Urease inhibitory activity of simple α, β-unsaturated ketones. Life Sci. 2003, 73, 2985–2990. [Google Scholar] [CrossRef]
- Zaborska, W.; Krajewska, B.; Olech, Z. Heavy metal ions inhibition of jack bean urease: Potential for rapid contaminant probing. Enzyme Inhib. Med. Chem. 2004, 19, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Laghari, H.A.; Memona, S.; Nelofar, A.; Khan, K.M.; Yasmin, A.; Syed, M.N.; Aman, A. A new flavanenol with urease-inhibition activity isolated from roots of manna plant camelthorn (Alhagi maurorum). J. Mol. Struct. 2010, 965, 65–67. [Google Scholar] [CrossRef]
- Paulo, L.; Oleastro, M.; Gallardo, E.; Queiroz, J.A.; Domingues, F. Anti-Helicobacter pylori and urease inhibitory activities of resveratrol and red wine. Food Res. Int. 2011, 44, 964–969. [Google Scholar] [CrossRef]
- Hassan, S.T.; Berchová, K.; Šudomová, M. Antimicrobial, antiparasitic and anticancer properties of Hibiscus sabdariffa (L.) and its phytochemicals: In vitro and in vivo studies. Ceska Slov. Farm. 2016, 65, 10–14. [Google Scholar] [PubMed]
- De Arruda, A.; Cardoso, C.A.; Vieira Mdo, C.; Arena, A.C. Safety assessment of Hibiscus sabdariffa after maternal exposure on male reproductive parameters in rats. Drug Chem. Toxicol. 2016, 39, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Ademiluyi, A.O.; Oboh, G. Aqueous extracts of Roselle (Hibiscus sabdariffa Linn.) varieties inhibit α-amylase and α-glucosidase activities in vitro. J. Med. Food. 2013, 16, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, X.; Jiang, H.; Qi, Y.; Chin, K. L.; Yue, Y. Antioxidant activity of leaf extracts from different Hibiscus sabdariffa accessions and simultaneous determination five major antioxidant compounds by LC-Q-TOF-MS. Molecules 2014, 19, 21226–21238. [Google Scholar] [CrossRef] [PubMed]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.-Z.; Wang, X.-F.; Zhang, X.; Su, J.-Y.; Chen, H.-M.; Liu, Y.-H.; Zhang, Z.-B.; Xie, J.-H.; Su, Z.-R. Andrographolide sodium bisulphite-induced inactivation of urease: Inhibitory potency, kinetics and mechanism. BMC Complement. Altern. Med. 2015, 15, 238. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Du, N.; Chen, M.; Liu, Z.; Sheng, L.; Xu, H.; Chen, S. Kinetics and mechanism of jack bean urease inhibition by Hg2+. Chem. Cent. J. 2012, 6, 154. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, B. Mono- (Ag, Hg) and di- (Cu, Hg) valent metal ions effects on the activity of jack bean urease. Probing the modes of metal binding to the enzyme. J. Enzyme Inhib. Med. Chem. 2008, 23, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Yuan, Y.; Hodge, C.N. Determining appropriate substrate conversion for enzymatic assays in high-throughput screening. J. Biomol. Screen. 2003, 8, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kawase, M.; Tani, S. Alpha-hydroxyketones as inhibitors of urease. Bioorg. Med. Chem. 2004, 12, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Firdous, S.; Ansari, N.H.; Fatima, I.; Malik, A.; Afza, N.; Iqbal, L.; Lateef, M. Ophiamides A-B, new potent urease inhibitory sphingolipids from Heliotropium ophioglossum. Arch. Pharm. Res. 2012, 35, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Cheng, H.Y.; Lin, T.C.; Chiang, L.C.; Lin, C.C. The in vitro activity of geraniin and 1,3,4,6-tetra-O-galloyl-β-d-glucose isolated from Phyllanthus urinaria against herpes simplex virus type 1 and type 2 infection. J. Ethnopharmacol. 2007, 110, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.H.; Hsu, J.D.; Lo, M.H.; Chu, C.Y.; Chou, F.P.; Huang, C.L.; Wang, C.J. Inhibitory effect of Hibiscus protocatechuic acid on tumor promotion in mouse skin. Cancer Lett. 1998, 126, 199–207. [Google Scholar] [CrossRef]
- Tseng, T.H.; Kao, T.W.; Chu, C.Y.; Chou, F.P.; Lin, W.L.; Wang, C.J. Induction of apoptosis by hibiscus protocatechuic acid in human leukemia cells via reduction of retinoblastoma (RB) phosphorylation and Bcl-2 expression. Biochem. Pharmacol. 2000, 60, 307–315. [Google Scholar] [CrossRef]
- Charlton, A.J.; Baxter, N.J.; Khan, M.L.; Moir, A.J.; Haslam, E.; Davies, A.P.; Williamson, M.P. Polyphenol/peptide binding and precipitation. J. Agric. Food Chem. 2002, 50, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Friend, D.R. Pharmaceutical development of microbicide drug products. Pharm. Dev. Technol. 2010, 15, 562–581. [Google Scholar] [CrossRef] [PubMed]
- Benini, S.; Rypniewski, W.R.; Wilson, K.S.; Mangani, S.; Ciurli, S. Molecular details of urease inhibition by boric acid: Insights into the catalytic mechanism. J. Am. Chem. Soc. 2004, 126, 3714–3715. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.; Adams, S.D.; Lee, L.H.; Murray, S.R.; Hsu, S.D.; Hammond, J.R.; Dickinson, D.; Chen, P.; Chu, T.C. Inhibition of herpes simplex virus 1 with the modified green tea polyphenol palmitoyl-epigallocatechin gallate. Food Chem. Toxicol. 2013, 52, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.S.; Šudomová, M. The development of urease inhibitors: What opportunities exist for better treatment of Helicobacter pylori infection in children? Children 2017, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Nyam, K.L.; Leao, S.Y.; Tan, C.P.; Long, K. 2014. Functional properties of roselle (Hibiscus sabdariffa L.) seed and its application as bakery product. J. Food Sci. Technol. 2014, 51, 3830–3837. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.O.; Curto, A.F.; Guido, L.F. Determination of phenolic content in different barley varieties and corresponding malts by liquid chromatography-diode array detection-electrospray ionization tandem mass spectrometry. Antioxidants 2015, 4, 563–576. [Google Scholar] [CrossRef] [PubMed]
- ISO 14502–1: Determination of Substances Characteristic of Green and Black Tea—Part 1: Content of Total Polyphenols in Tea—Colorimetric Method Using Folin-Ciocalteu Reagent; International Organization for Standardization: Geneva, Switzerland, 2005.
- Markoulatos, P.; Georgopoulou, A.; Siafakas, N.; Plakokefalos, E.; Tzanakaki, G.; Kourea-Kremastinou, J. Laboratory diagnosis of common herpesvirus infections of the central nervous system by a multiplex PCR assay. J. Clin. Microbiol. 2001, 39, 4426–4432. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Walker, W.E.; Waisbren, B.A.; Martins, R.R.; Batayias, G.E. A method for determining sensitivities of antiviral drugs in vitro for possible use as clinical consultation. Am. J. Clin. Pathol. 1971, 56, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Nishimura, T.; Toku, H.; Fukuyasu, H. Antiviral compounds. XII. Antiviral activity of amidinohydrazones of alkoxyphenyl-substituted carbonyl compounds against influenza virus in eggs and in mice. Kitasato Arch. Exp. Med. 1977, 50, 39–46. [Google Scholar] [PubMed]
Sample Availability: Samples of all compounds used in the study are available from the authors. |

| Compound | EC50 (µg∙mL−1) | CC50 (µg∙mL−1) | SI |
|---|---|---|---|
| AEHS | n.d | n.d | n.d |
| PCA | 0.92 ± 0.21 | >200 | >217 |
| Acyclovir | 1.43 ± 0.43 | >200 | >140 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, S.T.S.; Švajdlenka, E.; Berchová-Bímová, K. Hibiscus sabdariffa L. and Its Bioactive Constituents Exhibit Antiviral Activity against HSV-2 and Anti-enzymatic Properties against Urease by an ESI-MS Based Assay. Molecules 2017, 22, 722. https://doi.org/10.3390/molecules22050722
Hassan STS, Švajdlenka E, Berchová-Bímová K. Hibiscus sabdariffa L. and Its Bioactive Constituents Exhibit Antiviral Activity against HSV-2 and Anti-enzymatic Properties against Urease by an ESI-MS Based Assay. Molecules. 2017; 22(5):722. https://doi.org/10.3390/molecules22050722
Chicago/Turabian StyleHassan, Sherif T. S., Emil Švajdlenka, and Kateřina Berchová-Bímová. 2017. "Hibiscus sabdariffa L. and Its Bioactive Constituents Exhibit Antiviral Activity against HSV-2 and Anti-enzymatic Properties against Urease by an ESI-MS Based Assay" Molecules 22, no. 5: 722. https://doi.org/10.3390/molecules22050722
APA StyleHassan, S. T. S., Švajdlenka, E., & Berchová-Bímová, K. (2017). Hibiscus sabdariffa L. and Its Bioactive Constituents Exhibit Antiviral Activity against HSV-2 and Anti-enzymatic Properties against Urease by an ESI-MS Based Assay. Molecules, 22(5), 722. https://doi.org/10.3390/molecules22050722







