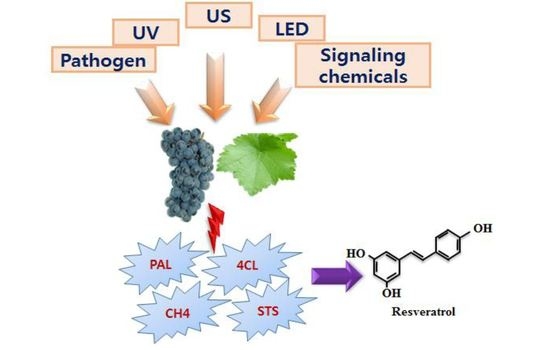
An Overview of Stress-Induced Resveratrol Synthesis in Grapes: Perspectives for Resveratrol-Enriched Grape Products
Abstract
:1. Introduction
2. Elicitation of Resveratrol Due to Pathogen Infection
3. Accumulation of Resveratrol in Response to Light
4. Induced Accumulation of Resveratrol Due to Ultrasonication
5. Accumulation of Resveratrol Due to Signaling Chemicals
6. Induction of Resveratrol by UV Irradiation
7. Elicitation of Resveratrol Due to Ozone
8. Induction of Resveratrol Due to Metal Salt
9. Induced Accumulation of Resveratrol Due to Pesticides
10. Effect of Enological Practice for the Increased Amount of Resveratrol
11. Induction of Resveratrol Due to Fertilizer
12. Conclusions and Future Prospects
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations:
| US | Ultrasonication |
| UV | Ultra violet |
| LED | Light-emitting diode |
| PAL | Phenylalanine ammonia lyase |
| STS | Stilbene synthase |
| RS | Resveratrol synthase (Syn. STS) |
| C4H | Cinnamate-4-hydroxylase |
| 4CL | Coumaroyl-CoA ligase |
| CHS | Chalcone synthase |
| TAL | Tyrosine ammonia-lyase |
| STS1 | Stilbene synthase1 |
| STS2 | Stilbene synthase2 |
| O-3-GT | 3-O-beta-glycosyltransferases |
| JA | Jasmonic acid |
| SA | Salicylic acid |
| CHI | Chitosan |
| MJ | Methyl jasmonate |
| GLU | Glucan |
| COR | Coronatine |
| OPDA | 12-oxo-phytodienoic acid |
| DAF | Days after fruiting |
| AlCl3 | Aluminum chloride |
| CaCl2 | Calcium chloride |
| CdCl2 | Cadmium chloride |
| Na2S2O5 | Sodium metabisulfite |
| FW | Fresh Weight |
References
- International Food Information Council Foundation. Sweet Weight Management Opportunities. Available online: http://www.foodinsight.org/ (accessed on 29 March 2011).
- Thomasset, S.C.; Berry, D.P.; Garcea, G.; Marczylo, T.; Steward, W.P.; Gescher, A.J. Dietary polyphenolic phytochemicals. Promising cancer chemopreventive agents in human? A review of their clinical properties. Int. J. Cancer 2006, 120, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; De Lorgeril, M. Wine, alcohol, platelets and the French Paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 10, 218–220. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Donnez, D.; Jeandet, P.; Clement, C.; Courot, E. Bioproduction of resveratrol and stilbene derivatives by plant cells and microorganisms. Trends Biotechnol. 2009, 27, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Lanz, T.; Tropf, S.; Marner, F.J.; Schroder, J.; Schroder, G. The role of cysteines in polyketide synthases. Site-directed mutagenesis of resveratrol and chalcone synthases, two key enzymes in different plant-specific pathways. J. Biol. Chem. 1991, 266, 9971–9976. [Google Scholar] [PubMed]
- Vannozzi, A.; Dry, I.B.; Fasoli, M.; Zenoni, S.; Lucchin, M. Genome-wide analysis of the grapevine stilbene synthase multigenic family: Genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol. 2012, 12, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holl, J.; Vannozzi, A.; Czemmel, S.; D’Onofrio, C.; Walker, A.R.; Rausch, T. The R2R3-MYB transcription factors MYB14 and MYB15 regulate stilbene biosynthesis in Vitis vinifera. Plant Cell 2013, 25, 4135–4149. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Wu, B.H.; Wang, L.J.; Li, S.H. Extractable amounts of trans-resveratrol in seed and berry skin in Vitis evaluated at the germplasm level. J. Agric. Food Chem. 2006, 54, 8804–8811. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tang, K.; Yang, H.R.; Wen, P.F.; Zhang, P.; Wang, H.L.; Huang, W.D. Distribution of resveratrol and stilbene synthase in young grape plants (Vitis vinifera L. cv. Cabernet Sauvignon) and the effect of UV-C on its accumulation. Plant Physiol. Biochem. 2010, 48, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Langcake, P. Disease resistance of Vitis spp. and the production of the stress metabolites resveratrol, ε-viniferin, α-viniferin, and pterostilbene. Physiol. Plant Pathol. 1981, 18, 213–226. [Google Scholar] [CrossRef]
- Dercks, W.; Creasy, L.L. The significance of stilbene phytoalexins in the Plasmopara viticola-grapevine interaction. Physiol. Mol. Plant Pathol. 1989, 34, 189–202. [Google Scholar] [CrossRef]
- Schmidlin, L.; Poutaraud, A.; Claudel, P.; Mestre, P.; Prado, E.; Santos-Rosa, M. A stress-inducible resveratrol O-methyltransferase involved in the biosynthesis of pterostilbene in grapevine. Plant Physiol. 2008, 148, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Tassoni, A.; Fornale, S.; Franceschetti, M.; Musiani, F.; Michael, A.J.; Perry, B. Jasmonates and Na-orthovanadate promote resveratrol production in Vitis vinifera cv. Barbera cell cultures. New Phytol. 2005, 166, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.F.; Chen, J.Y.; Kong, W.F.; Pan, Q.H.; Wan, S.B.; Huang, W.D. Salicylic acid induced the expression of phenylalanine ammonia-lyase gene in grape berry. Plant Sci. 2005, 169, 928–934. [Google Scholar] [CrossRef]
- González-Barrio, R.; Beltrán, D.; Cantos, E.; Gil, M.I.; Espín, J.C.; Tomás-Barberán, F.A. Comparison of ozone and UV-C treatments on the postharvest stilbenoid monomer, dimer, and trimer induction in var. ‘Superior’ white table grapes. J. Agric. Food Chem. 2006, 54, 4222–4228. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Ma, L.; Xi, H.F.; Duan, W.; Wang, J.F.; Li, S.H. Individual and combined effects of CaCl2 and UV-C on the biosynthesis of resveratrols in grape leaves and berry skins. J. Agric. Food Chem. 2013, 61, 7135–7141. [Google Scholar] [CrossRef] [PubMed]
- Bavaresco, L.; Pettegolli, D.; Cantu’, E.; Fregoni, M.; Chiusa, G.; Trevisan, M. Elicitation and accumulation of stilbene phytoalexins in grapevine berries infected by Botrytis cinerea. Vitis 1997, 36, 77–83. [Google Scholar]
- Langcake, P.; McCarthy, W.V. The relationship of resveratrol production to infection of grapevine leaves by Botrytis cinerea. Vitis 1979, 18, 244–253. [Google Scholar]
- Jeandet, P.; Bessis, R.; Sbaghi, M.; Meunier, P. Production of the phytoalexin resveratrol by grapes as a response to Botrytis attack under natural conditions. J. Phytopathol. 1995, 143, 135–139. [Google Scholar] [CrossRef]
- Sarig, P.; Zutkhi, Y.; Monjauze, A.; Lisker, N.; Ben-Arie, R. Phytoalexin elicitation in grape berries and their susceptibility to Rhizopus stolonifer. Physiol. Mol. Plant Pathol. 1997, 50, 337–347. [Google Scholar] [CrossRef]
- Romero-Pe´rez, A.I.; Lamuela-Ravento´s, R.M.; Andre´s-Lacueva, C.; De la Torre-Boronat, M.C. Method for the quantitative extraction of resveratrol and piceid isomers in grape berry skins. Effect of powdery mildew on the stilbene content. J. Agric. Food Chem. 2001, 49, 210–215. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- RoldaäN, A.; Palacios, V.; Caro, I.; PeäRez, L. Resveratrol content of Palomino fino grapes: Influence of vintage and fungal infection. J. Agric. Food Chem. 2003, 51, 1464–1468. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Chereyathmanjiyil, A.; Masih, I.; Chapuis, L.; Benoıˆt, A. Biological control of Botrytis cinerea causing gray mould disease of grapevine and elicitation of stilbene phytoalexin (resveratrol) by a soil bacterium. FEMS Microbiol. Lett. 1998, 165, 65–70. [Google Scholar] [CrossRef]
- Montero, C.; Cristescu, S.M.; Jiménez, J.B.; Orea, J.M.; Lintel Hekkert, S.T.; Harren, F.J.M.; González Ureń, A. Trans-resveratrol and grape disease resistance. A dynamical study by high-resolution laser-based techniques. Plant Physiol. 2003, 131, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Nikfardjam, M.S.P.; László, G.; Dietrich, H. Resveratrol-derivatives and antioxidative capacity in wines made from botrytized grapes. Food Chem. 2006, 96, 74–79. [Google Scholar] [CrossRef]
- Timperio, A.M.; D’Alessandro, A.; Fagioni, M.; Magro, P.; Zolla, L. Production of the phytoalexins trans-resveratrol and delta-viniferin in two economy-relevant grape cultivars upon infection with Botrytis cinerea in field conditions. Plant Physiol. Biochem. 2012, 50, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Mihai, R.; Cristina, S.; Helepciuc, F.; Brezeanu, A.; Stoian, G. Biotic and abiotic elicitors induce biosynthesis and accumulation of resveratrol with antitumoral activity in the long-term Vitis vinifera L. callus cultures. Rom. Biotechnol. Lett. 2011, 16, 6683–6689. [Google Scholar]
- Feys, B.J.; Parker, J.E. Interplay of signaling pathways in plant disease resistance. Trends Genet. 2000, 16, 449–455. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Kim, S.A.; Cho, K.S.; Yun, H.K. Expression of genes related to flavonoid and stilbene synthesis as affected by signaling chemicals and Botrytis cinerea in grapevines. Biol. Plant. 2014, 58, 758–767. [Google Scholar] [CrossRef]
- Shohael, A.M.; Ali, M.B.; Yu, K.W.; Hahn, E.J.; Islam, R.; Paek, K.Y. Effect of light on oxidative stress, secondary metabolites and induction of antioxidant enzymes in Eleutherococcus senticosus somatic embryos in bioreactor. Process Biochem. 2006, 41, 1179–1185. [Google Scholar] [CrossRef]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. The effect of bunch shading on berry development and flavonoid accumulation in Shiraz grapes. Aust. J. Grape Wine Res. 2004, 10, 55–73. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. Hortic. Sci. 2010, 45, 1809–1814. [Google Scholar]
- Potrebko, I.; Resurreccion, A.V.A. Effect of ultraviolet doses in combined ultraviolet-ultrasound treatments on trans-resveratrol and trans-piceid contents in sliced peanut kernels. J. Agric. Food Chem. 2009, 57, 7750–7756. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Zhang, L.; Kato, M.; Yamawaki, K.; Kiriiwa, Y.; Yahata, M.; Ikoma, Y.; Matsumoto, H. Effect of blue and red LED light irradiation on β-cryptoxanthin accumulation in the flavedo of citrus fruits. J. Agric. Food Chem. 2012, 60, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Tuan, P.A.; Thwe, A.A.; Kim, Y.B.; Kim, J.K.; Kim, S.J.; Lee, S.; Chung, S.O.; Park, S.U. Effects of white, blue, and red lightemitting diodes on carotenoid biosynthetic gene expression levels and carotenoid accumulation in sprouts of tartary buckwheat (Fagopyrum tataricum Gaertn.). J. Agric. Food Chem. 2013, 61, 12356–12361. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.W.; Lee, C.W.; Chakrabarty, D.; Paek, K.Y. Growth responses of marigold and salvia bedding plants as affected by monochromic or mixture radiation provided by a light-emitting diode (LED). Plant Growth Regul. 2002, 38, 225–230. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Kim, S.A.; Choi, S.J.; Yun, H.K. Comparison of accumulation of stilbene compounds and stilbene related gene expression in two grape berries irradiated with different light sources. Hortic. Environ. Biotechnol. 2015, 56, 36–43. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, Y.; Zhan, Y.; Li, Y.; Kawabata, S. Chalcone synthase family genes have redundant roles in anthocyanin biosynthesis and in response to blue/UV-A light in turnip (Brassica rapa; Brassicaceae). Am. J. Bot. 2013, 100, 2458–2467. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.; Erel, D. Dynamic Ultrasonic Cleaning and Disinfecting Device and Method. U.S. Patent 5,113,881, 19 May 1992. [Google Scholar]
- Hasan, M.M.; Baek, K.H. Induction of resveratrol biosynthesis in grape skin and leaves by ultrasonication treatment. Korean J. Hortic. Sci. Technol. 2013, 31, 496–502. [Google Scholar] [CrossRef]
- Rudolf, J.R.; Resurreccion, A.V.A. Elicitation of resveratrol in peanut kernels by application of abiotic stresses. J. Agric. Food Chem. 2005, 53, 10186–10192. [Google Scholar] [CrossRef] [PubMed]
- Sales, J.M.; Resurreccion, A.V.A. Maximising resveratrol and piceid contents in UV and ultrasound treated peanuts. Food Chem. 2009, 11, 7674–7680. [Google Scholar] [CrossRef]
- Hasan, M.M.; Yun, H.K.; Kwak, E.J.; Baek, K.H. Preparation of resveratrol-enriched grape juice from ultrasonication treated grape fruits. Ultrason. Sonochem. 2014, 21, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wu, J.; Ho, K.P.; Qi, S. Ultrasound-induced physiological effects and secondary metabolite (saponin) production in Panax ginseng cell cultures. Ultrasound Med. Biol. 2001, 27, 1147–1152. [Google Scholar] [CrossRef]
- Xiao, Y.M.; Wu, Q.; Cai, Y.; Lin, X.F. Ultrasound accelerated enzymatic synthesis of sugar esters in nonaqueous solvents. Carbohydr. Res. 2005, 340, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, L. Ultrasound-induced stress responses of Panax ginseng cells: Enzymatic browning and phenolics production. Biotechnol. Prog. 2002, 18, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Resveratrol: A molecule whose time has come? And gone? Clin. Biochem. 1997, 30, 91–113. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Kim, S.A.; Han, J.H.; Choi, S.J.; Yun, H.K. Induction of defense-related responses and suppression of grey mold in grapevines treated with defense response signaling molecules. J. Am. Pomol. Soc. 2013, 67, 104–116. [Google Scholar]
- Jeandet, P.; Breuil, A.C.D.; Bessis, R.; Debord, S.; Sbaghi, M.; Adrian, M. Phytoalexins from the Vitaceae: Biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J. Agric. Food Chem. 2002, 50, 2731–2741. [Google Scholar] [CrossRef]
- Krisa, S.; Larronde, F.; Budzinski, H.; Decendit, A.; Deffieux, G.; Michel, M.J. Stilbene production by Vitis vinifera cell suspension cultures: methyl jasmonate induction and 13C biolabeling. J. Nat. Prod. 1999, 62, 1688–1690. [Google Scholar] [CrossRef]
- Belhadj, A.; Telef, N.; Saigne, C.; Cluzet, S.; Barrieu, F.; Hamdi, S.; Mérillon, J.M. Effect of methyl jasmonate in combination with carbohydrates on gene expression of PR proteins, stilbene and anthocyanin accumulation in grapevine cell cultures. Plant Physiol. Biochem. 2008, 46, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Lijavetzky, D.; Almagro, L.; Belchi-Navarro, S.; Martínez-Zapater, J.M.; Bru, R.; Pedreño, M.A. Synergistic effect of methyljasmonate and cyclodextrin on stilbene biosynthesis pathway gene expression and resveratrol production in Monastrell grapevine cell cultures. BMC Res. Notes 2008, 1, 132. [Google Scholar] [CrossRef] [PubMed]
- Belchí-Navarro, S.; Almagro, L.; Lijavetzky, D.; Bru, R.; Pedreño, M.A. Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and methyljasmonate. Plant Cell Rep. 2012, 31, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Vuonga, T.V.; Francoa, C.; Zhang, W. Treatment strategies for high resveratrol induction in Vitis vinifera L. cell suspension culture. Biotechnol. Rep. 2014, 1–2, 15–21. [Google Scholar] [CrossRef]
- Martinez, C.; Blanc, F.; Le Claire, E.; Besnard, O.; Nicole, M.; Baccou, J.C. Salicylic acid and ethylene pathways are differentially activated in melon cotyledons by active or heat-denatured cellulase from Trichoderma longibrachiatum. Plant Physiol. 2001, 127, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Taurino, M.; Ingrosso, I.; D’amico, L.; Domenico, S.D.; Nicoletti, I.; Corradini, D.; Santino, A.; Giovinazzo, G. Jasmonates elicit different sets of stilbenes in Vitis vinifera cv. Negramaro cell cultures. SpringerPlus 2015, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Almagro, L.; Belchí-Navarro, S.; Martínez-Marquez, A.; Bru, R.; Pedrēno, M.A. Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and coronatine. Plant Physiol. Biochem. 2015, 97, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Dipalo, S.C.F.; Bagni, N.; Tassoni, A. Chitosan elicits mono-glucosylated stilbene production and release in fed-batch bioreactor cultures of grape cells. Food Chem. 2011, 124, 1473–1479. [Google Scholar] [CrossRef]
- Caia, Z.; Knorra, D.; Smetanskaa, I. Enhanced anthocyanins and resveratrol production in Vitis vinifera cell suspension culture by indanoyl-isoleucine, N-linolenoyl-l-glutamine and insect saliva. Enzym. Microb. Technol. 2012, 50, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Righetti, L.; Franceschetti, M.; Ferri, M.; Tassoni, A.; Bagni, N. Resveratrol production in Vitis vinifera cell suspensions treated with several elicitors. Carpologia 2007, 60, 169–171. [Google Scholar]
- Santamaria, A.R.; Mulinacci, N.; Valletta, A.; Innocenti, M.; Pasqua, G. Effects of elicitors on the production of resveratrol and viniferins in cell cultures of Vitis vinifera L. cv Italia. J. Agric. Food Chem. 2011, 59, 9094–9101. [Google Scholar] [CrossRef] [PubMed]
- Portu, J.; López, R.; Baroja, E.; Santamaría, P.; Garde-Cerdán, T. Improvement of grape and wine phenolic content by foliar application to grapevine of three different elicitors: Methyl jasmonate, chitosan, and yeast extract. Food Chem. 2016, 201, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Keptayen, J.C.; Van Den Ende, H.; Klis, F.M. The contribution of cell wall proteins to the organization of the yeast cell wall. Biochim. Biophys. Acta 1999, 1426, 373–383. [Google Scholar]
- Peltonen, S.; Mannonen, L.; Karjalainen, R. Elicitor-induced changes of phenylalanine ammonia-lyase activity in barley cell suspension cultures. Plant Cell Tissue Org. 1997, 50, 185–193. [Google Scholar] [CrossRef]
- Ferri, M.; Tassoni, A.; Franceschetti, M.; Righetti, L.; Naldrett, M.J.; Bagni, N. Chitosan treatment induces changes of protein expression profile and stilbene distribution in Vitis vinifera cell suspensions. Proteomics 2009, 9, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Trotel-Aziz, P.; Dhuicq, L.; Jeandet, P.; Couderchet, M.; Fernet, G. Chitosan oligomers and copper sulfate induce grapevine defense reactions and resistance to gray mold and downy mildew. Phytopathology 2006, 96, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Ghaouth, E.A.; Wilson, C.L.; Callahan, A.M. Induction of chitinase, β-1, 3 glucanase, and phenylalanine ammonia-lyase in peach fruit by UV-C treatment. Phytopathology 2003, 93, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Romanazzi, G.; Gabler, F.M.; Smilanick, J.L. Preharvest chitosan and postharvest UV irradiation treatments suppress gray mold of table grapes. Plant Dis. 2006, 90, 445–450. [Google Scholar] [CrossRef]
- Bintsis, T.; Litopoulou-Tzanetaki, E.; Robinson, R.K. Existing and potential applications of ultraviolet light in food industry-A critical review. J. Sci. Food Agric. 2000, 80, 637–645. [Google Scholar] [CrossRef]
- Yaun, B.R.; Summer, S.S.; Eifert, J.D.; Marcy, J.E. Inhibition of pathogens on fresh produce by ultraviolet energy. Int. J. Food Microbiol. 2004, 90, 1–8. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. The production of resveratrol and the viniferins by grapevines in response to ultraviolet irradiation. Phytochemistry 1977, 16, 1193–1196. [Google Scholar] [CrossRef]
- Jeandet, P.; Bessis, R.; Gautheron, B. The production of resveratrol (3,5,4′-trihydroxystilbene) by grape berries in different developmental stages. Am. J. Enol. Vitic. 1991, 42, 41–46. [Google Scholar]
- Douillet-Breuil, A.C.; Jeandet, P.; Adrian, M.; Bessis, R. Changes in the phytoalexin content of various Vitis spp. in response to UV-C elicitation. J. Agric. Food Chem. 1999, 47, 4456–4461. [Google Scholar] [CrossRef] [PubMed]
- Adrian, M.; Jeandet, P.; Douillet-Breuil, A.C.; Tesson, L.; Bessis, R. Stilbene content of mature Vitis vinifera berries in response to UV-C elicitation. J. Agric. Food Chem. 2000, 48, 6103–6105. [Google Scholar] [CrossRef] [PubMed]
- Versari, A.; Parpinello, G.P.; Tornielli, G.B.; Ferrarini, R.; Giulivo, C. Stilbene compounds and stilbene synthase expression during ripening, wilting, and UV treatment in grape cv. Corvina. J. Agric. Food Chem. 2001, 49, 5531–5536. [Google Scholar] [CrossRef] [PubMed]
- Cantos, E.; Garcia-Viguera, C.; De Pascual-Teresa, S.; Tomas-Barberan, F.A. Effect of postharvest ultraviolet irradiation on resveratrol and other phenolics of cv. Napoleon table grapes. J. Agric. Food Chem. 2000, 48, 4606–4612. [Google Scholar] [CrossRef] [PubMed]
- Moriartry, J.M.; Harmon, R.; Weston, L.A.; Bessis, R.; Breuil, A.C.; Adrian, M.; Jeandet, P. Resveratrol content of two Californian table grape cultivars. Vitis 2001, 40, 43–44. [Google Scholar]
- Petit, A.N.; Baillieul, F.; Vaillant-Gaveau, N.; Jacquens, L.; Conreux, A.; Jeandet, P.; Clement, C.; Fontaine, F. Low responsiveness of grapevine flowers and berries at fruit set to UV-C irradiation. J. Exp. Bot. 2009, 60, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Cantos, E.; Espin, J.C.; Tomas-Barberan, F.A. Postharvest stilbene-enrichment of red and white table grape varieties using UV-C irradiation pulses. J. Agric. Food Chem. 2002, 50, 6322–6329. [Google Scholar] [CrossRef] [PubMed]
- Cantos, E.; Tomas-Barberan, F.A.; Martinez, A.; Espin, J.C. Differential stilbene induction susceptibility of seven red wine grape varieties upon post-harvest UV-C irradiation. Eur. Food Res. Technol. 2003, 217, 253–258. [Google Scholar] [CrossRef]
- Cantos, E.; Espin, J.C.; Fernandez, M.J.; Oliva, J.; Tomas-Barberan, F.A. Postharvest UV-C-irradiated grapes as a potential source for producing stilbene-enriched red wines. J. Agric. Food Chem. 2003, 51, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.; Espín, J.C.; Cantos, V.E. Postharvest Treatment of Fruits and Vegetables Using Ultra Violet Irradiation Pulses. Patent WO/2002/085137, 31 October 2002. [Google Scholar]
- González-Barrio, R.; Salmenkallio-Marttila, M.; Tomás-Barberán, F.A.; Cantos, E.; Espín, J.C. Etiology of UV-C-induced browning in var. Superior white table grapes. J. Agric. Food Chem. 2005, 53, 5990–5996. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R.F.; Puertas, B.; Fernández, M.I.; Palma, M.; Cantos-Villar, E. Induction of stilbenes in grapes by UV-C: Comparison of different subspecies of Vitis. Innov. Food Sci. Emerg. Technol. 2010, 11, 231–238. [Google Scholar] [CrossRef]
- Cho, Y.J.; Kim, N.; Kim, C.T.; Maeng, J.S.; Pyee, J. Quantitative evaluation of resveratrol enrichment induced by UV stimulus in harvested grapes. Food Sci. Biotechnol. 2012, 21, 597–601. [Google Scholar] [CrossRef]
- Yin, X.; Singer, S.D.; Qiao, H.; Liu, Y.; Jiao, C.; Wang, H.; Li, Z.; Fei, Z.; Wang, Y.; Fan, C.; et al. Insights into the mechanisms underlying ultraviolet-C induced resveratrol metabolism in grapevine (V. amurensis Rupr.) cv. “Tonghua-3”. Front. Plant Sci. 2016, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nigro, F.; Ippolito, A.; Lima, G. Use of UV-C light to reduce Botrytis storage rot of table grapes. Postharvest Biol. Technol. 1998, 13, 171–181. [Google Scholar] [CrossRef]
- González-Barrio, R.; Vidal-Guevara, M.L.; Tomás-Barberán, F.A.; Espín, J.C. Preparation of a resveratrol-enriched grape juice based on ultraviolet C-treated berries. Innov. Food Sci. Emerg. Technol. 2009, 10, 374–382. [Google Scholar] [CrossRef]
- Jiménez Sánchez, J.B.; Corral, C.E.; Orea, J.M.; Delgado, S.M.J.; Ureña, G.A. Elicitation of trans-resveratrol by laser resonant irradiation of table grapes. Appl. Phys. B 2007, 87, 559–563. [Google Scholar] [CrossRef]
- Borie, B.; Jeandet, P.; Parize, A.; Bessis, R.; Adrian, M. Resveratrol and stilbene synthase mRNA production in grapevine leaves treated with biotic and abiotic phytoalexin elicitors. Am. J. Enol. Vitic. 2004, 55, 60–64. [Google Scholar]
- Gonzálvez, A.G.; Jiménez, J.B.; Ureña, Á.G. Fruit-enhanced resistance to microbial infection induced by selective laser excitation. J. Spectrosc. 2013, 2013, 789159. [Google Scholar] [CrossRef]
- Takayanagi, T.; Okuda, T.; Mine, Y.; Yokotsuka, K. Induction of resveratrol biosynthesis in skins of three grape cultivars by ultraviolet irradiation. J. Jpn. Soc. Hortic. Sci. 2004, 73, 193–199. [Google Scholar] [CrossRef]
- Pan, Q.H.; Wang, L.; Li, J.M. Amounts and subcellular localization of stilbene synthase in response of grape berries to UV irradiation. Plant Sci. 2009, 176, 360–366. [Google Scholar] [CrossRef]
- Wang, J.F.; Maa, L.; Xi, H.F.; Wang, L.J.; Li, S.H. Resveratrol synthesis under natural conditions and after UV-C irradiation in berry skin is associated with berry development stages in ‘Beihong’ (V. vinifera × V. amurensis). Food Chem. 2015, 168, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Ma, L.; Liu, G.; Wang, N.; Wang, J.; Wang, L. Transcriptomic analysis of grape (Vitis vinifera L.) leaves after exposure to ultraviolet C irradiation. PLoS ONE 2014, 9, e113772. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Nakabayashi, R.; Ogata, Y.; Sakurai, N.; Tokimatsu, T.; Goto, S. Multi omics in grape berry skin revealed specific induction of stilbene synthetic pathway by UV-C irradiation. Plant Physiol. 2015, 168, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Palou, L.; Crisosto, C.H.; Smilanick, J.L.; Adaskaveg, J.E.; Zoffoli, J.P. Effects of continuous 0.3 ppm ozone exposure on decay development and physiological responses of peaches and table grapes in cold storage. Posthavest Biol. Technol. 2002, 24, 39–48. [Google Scholar] [CrossRef]
- Sarig, P.; Zahavi, T.; Zutkhi, Y.; Yannai, S.; Lisker, N.; Ben-Arie, R. Ozone for control of post-harvest decay of table grapes caused by Rhizopus stolonifer. Physiol. Mol. Plant Pathol. 1996, 48, 403–415. [Google Scholar] [CrossRef]
- Artés-Herna´ndez, F.; Artés, F.; Toma´s-Barbera´n, F.A. Quality and enhancement of bioactive phenolics in cv. Napoleon table grapes exposed to different postharvest gaseous treatments. J. Agric. Food Chem. 2003, 51, 5290–5295. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, J.A.; Vázquez, A.; Pérez, A.G.; García, J.M. Control of table grapes postharvest decay by ozone treatment and resveratrol induction. Food Sci. Technol. Int. 2009, 15, 495–502. [Google Scholar] [CrossRef]
- Sgarbi, E.; Baroni Fornasicro, R.; Lins, A.A.P. Phenol metabolism is differentially affected by ozone in two cell lines from grape (Vitis vinifera L.) leaf. Plant Sci. 2003, 165, 951–957. [Google Scholar] [CrossRef]
- Grimmig, B.; Schubert, R.; Fischer, R.; Hain, R.; Schreie, P.H.; Betz, C.; Langebartels, C.; Ernst, D.; Sandermann, J.H. Ozone-and ethylene-induced regulation of a grapevine resveratrol synthase promoter in transgenic tobacco. Acta Physiol. Plant. 1997, 19, 467–474. [Google Scholar] [CrossRef]
- Valletta, A.; Salvatori, E.; Santamaria, A.R.; Nicoletti, M.; Toniolo, C.; Caboni, E.; Bernardini, A.; Pasqua, G.; Manes, F. Ecophysiological and phytochemical response to ozone of wine grape cultivars of Vitis vinifera L. Nat. Prod. Res. 2016, 30, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Beltŕan, D.; Selma, M.V.; Tudela, J.A.; Gil, M.I. Effect of different sanitizers on microbial and sensory quality of fresh-cut potato strips stored under modified atmosphere or vacuum packaging. Postharvest Biol. Technol. 2005, 37, 37–46. [Google Scholar] [CrossRef]
- Hanawa, F.; Tahara, S.; Mizutani, J. Antifungal stress compounds from Veratrum grandiflorum leaves treated with cupric chloride. Phytochemistry 1992, 31, 3005–3007. [Google Scholar] [CrossRef]
- Pool, R.M.; Creasy, L.L.; Frackelton, A.S. Resveratrol and the viniferins, their application to screening for disease resistance in grape breeding programs. Vitis 1981, 20, 136–145. [Google Scholar]
- Adrian, M.; Jeandet, P.; Bessis, R.; Joubert, J.M. Induction of phytoalexin (resveratrol) synthesis in grapevine leaves treated with aluminum chloride (AlCl3). J. Agric. Food Chem. 1996, 44, 1979–1981. [Google Scholar] [CrossRef]
- Loschke, D.C.; Hadwiger, L.A.; Wagoner, W. Comparison of mRNA populations coding for phenylalanine ammonia lyase and other peptides from pea tissue treated with biotic and abiotic inducers. Physiol. Plant Pathol. 1983, 23, 163–173. [Google Scholar] [CrossRef]
- Dugo, G.; Saitta, M.; Giuffrida, D.; Vilasi, F.; LA Torre, G.L. Determination of resveratrol and other phenolic compounds in experimental wines from grapes subjected to different pesticide treatments. Ital. J. Food Sci. 2004, 3, 16–20. [Google Scholar]
- Dugo, G.; Bambara, G.; Salvo, F.; Saitta, M.; Lo Curto, S. Contenuto di resveratrolo in vini rossi da uve alloctone e autoctone prodotte in Sicilia. Inst. Res. Inf. Syst. 2000, 12, 79. [Google Scholar]
- Pezet, R.; Cuenat, P. Resveratrol in wine: Extraction from skin during fermentation and post-fermentation standing of must from Gamay grapes. Am. J. Enol. Vitic. 1996, 47, 287–290. [Google Scholar]
- Vrhovsek, U.; Wendelin, S.; Eder, R. Effects of various vinification techniques on the concentration of cis- and trans-resveratrol and resveratrol glucoside isomers in wine. Am. J. Enol. Vitic. 1997, 48, 214–219. [Google Scholar]
- Kostadinović, S.; Wilkens, A.; Stefova, M.; Ivanova, V.; Vojnoski, B.; Mirhosseini, H.; Winterhalter, P. Stilbene levels and antioxidant activity of Vranec and Merlot wines from Macedonia: Effect of variety and enological practices. Food Chem. 2012, 135, 3003–3009. [Google Scholar] [CrossRef] [PubMed]
- Sakuta, M.; Takagi, T.; Komamine, A. Effects of nitrogen source on betacyanin accumulation and growth in suspension cultures of Phytolacca americana. Physiol. Plant. 1987, 71, 459–463. [Google Scholar] [CrossRef]
- Bavaresco, L.; Eibach, R. Investigations of the influence of N fertilizer on resistance to powdery mildew (Oidium tuckeri), downy mildew (Plasmopara viticola) and on phytoalexins synthesis in different grapevine varieties. Vitis 1987, 26, 192–200. [Google Scholar]
- Bavaresco, L.; Fregoni, M.; Petegolli, D. Effect of nitrogen and potassium fertilizer on induced resveratrol synthesis in two grapevine genotypes. Vitis 1994, 33, 175–176. [Google Scholar]
- Graham, R.D. Effects of nutrient stress on susceptibility of plants to disease with particular reference to the trace elements. Adv. Bot. Res. 1983, 10, 221–276. [Google Scholar]
- Bavaresco, L.; Pezzutto, S.; Ragga, A.; Ferrari, F.; Trevisan, M. Effect of nitrogen supply on trans-resveratrol concentration in berries of Vitis Vinifera L. cv. Cabernet Sauvignon. Vitis 2001, 40, 229–230. [Google Scholar]
- Jiménez, J.B.; Orea, J.M.; González-Ureña, A.; Escribano, P.; López de la Osa, P.; Guadarrama, A. Short anoxic treatments to enhance trans-resveratrol content in grapes and wine. Eur. Food Res. Technol. 2007, 224, 373–378. [Google Scholar] [CrossRef]
- Sae-Lee, N.; Kerdchoechuen, O.; Laohakunjit, N. Enhancement of phenolics, resveratrol and antioxidant activity by nitrogen enrichment in cell suspension culture of Vitis vinifera. Molecules 2014, 19, 7901–7912. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, K.V. Perspectives for production and application of resveratrol. Appl. Microbiol. Biotechnol. 2011, 90, 417–425. [Google Scholar] [CrossRef] [PubMed]
 the induction of the corresponding enzymes by the mentioned elicitors or inducer). PAL, Phenylalanine ammonia lyase; C4H, Cinnamate-4-hydroxylase; 4CL, Coumaroyl-CoA ligase; TAL, Tyrosine ammonia-lyase; CHS, Chalcone synthase; STS, Stilbene synthase; UV, Ultra violet; US, Ultrasonication; LED, Light-emitting diode; O3, Ozone; MJ = Methyl jasmonate.
the induction of the corresponding enzymes by the mentioned elicitors or inducer). PAL, Phenylalanine ammonia lyase; C4H, Cinnamate-4-hydroxylase; 4CL, Coumaroyl-CoA ligase; TAL, Tyrosine ammonia-lyase; CHS, Chalcone synthase; STS, Stilbene synthase; UV, Ultra violet; US, Ultrasonication; LED, Light-emitting diode; O3, Ozone; MJ = Methyl jasmonate.
 the induction of the corresponding enzymes by the mentioned elicitors or inducer). PAL, Phenylalanine ammonia lyase; C4H, Cinnamate-4-hydroxylase; 4CL, Coumaroyl-CoA ligase; TAL, Tyrosine ammonia-lyase; CHS, Chalcone synthase; STS, Stilbene synthase; UV, Ultra violet; US, Ultrasonication; LED, Light-emitting diode; O3, Ozone; MJ = Methyl jasmonate.
the induction of the corresponding enzymes by the mentioned elicitors or inducer). PAL, Phenylalanine ammonia lyase; C4H, Cinnamate-4-hydroxylase; 4CL, Coumaroyl-CoA ligase; TAL, Tyrosine ammonia-lyase; CHS, Chalcone synthase; STS, Stilbene synthase; UV, Ultra violet; US, Ultrasonication; LED, Light-emitting diode; O3, Ozone; MJ = Methyl jasmonate.
| Eliciting Agent | Induction of Resveratrol up to (by Fold/Amount) | References | |||||
|---|---|---|---|---|---|---|---|
| Grape | Leaf | Juice | Wine | Cell/Callus Cultures | |||
| Biotic agents | B. cinerea | 3.2 | 5 | - | little | - | [20,21,22,26,27,28,29,30] |
| P. viticola | 5 | - | - | - | - | [13] | |
| U. necator | 12 | - | - | - | - | [14] | |
| R. stolonifer | 8.5 | - | - | - | - | [23] | |
| F. oxysporum + mannitol | - | - | - | - | 6 | [31] | |
| Bacillus +Botrytis | - | 16 | - | - | - | [27] | |
| Abiotic agent/Physical force | LED | 8.4 | - | - | - | - | [41] |
| US | 7.7 | 1.8 | 1.53 | - | - | [44,47] | |
| UV | 2315 | 750 | 35 | 2 | 2 | [18,23,25,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,92,93,94,95,96] | |
| UV + CHI | 23.2 | - | - | - | - | [72] | |
| UV + CaCl2 | 16.6 | 37.2 | - | - | - | [19] | |
| Ozonization | 3.14 | - | - | - | 1.5 | [18,101,102,103,104,105] | |
| Elicitors/signaling molecules | JA/MJ | - | - | - | - | 20 | [54,55,57,58,64,65,66] |
| JA + Mannitol | - | - | - | - | 10.5 | [31] | |
| MJ + Sucrose | - | - | - | - | 6 | [55] | |
| MJ + GLU | - | - | - | - | 10 | [58,66] | |
| SA | - | - | - | - | 2 | [58,59,64,66] | |
| Manduca sexta larvae | - | - | - | - | 7 | [63] | |
| Cyclodextrins | - | - | - | - | 3 | [57] | |
| Cyclodextrins + MJ | - | - | - | - | >20 | [56] | |
| Amberlite XAD-7 + JA + GLU | - | - | - | - | 2400 µg/g dw | [58] | |
| COR | - | - | - | - | 1900 µg/g dw | [60] | |
| OPDA | - | - | - | - | 2200 µg/g dw | [60] | |
| CHI | - | - | - | - | 63% | [58,62,64,66,69] | |
| AlCl3 (Fosetylaluminum) | - | 350 | - | - | - | [110] | |
| CaCl2 | 1.5 | 5.2 | - | - | - | [19] | |
| Fertilizer | Nitrogen | - | 5.6 | - | - | - | [118,121,123] |
| Potassium + Nitrogen | - | >2 | - | - | - | [119] | |
| Fungicides | Wettable sulpher | - | - | - | 1.6 | - | [112,113] |
| Quinoxyfen, Fenarimol, Penconazole, Dinocap | - | - | - | 2.25 | - | [112] | |
| Others | Enological practice | - | - | 13 | - | - | [22,114,115] |
| Maceration | - | - | - | 10 | - | [22,114,115,116] | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.; Bae, H. An Overview of Stress-Induced Resveratrol Synthesis in Grapes: Perspectives for Resveratrol-Enriched Grape Products. Molecules 2017, 22, 294. https://doi.org/10.3390/molecules22020294
Hasan M, Bae H. An Overview of Stress-Induced Resveratrol Synthesis in Grapes: Perspectives for Resveratrol-Enriched Grape Products. Molecules. 2017; 22(2):294. https://doi.org/10.3390/molecules22020294
Chicago/Turabian StyleHasan, Mohidul, and Hanhong Bae. 2017. "An Overview of Stress-Induced Resveratrol Synthesis in Grapes: Perspectives for Resveratrol-Enriched Grape Products" Molecules 22, no. 2: 294. https://doi.org/10.3390/molecules22020294