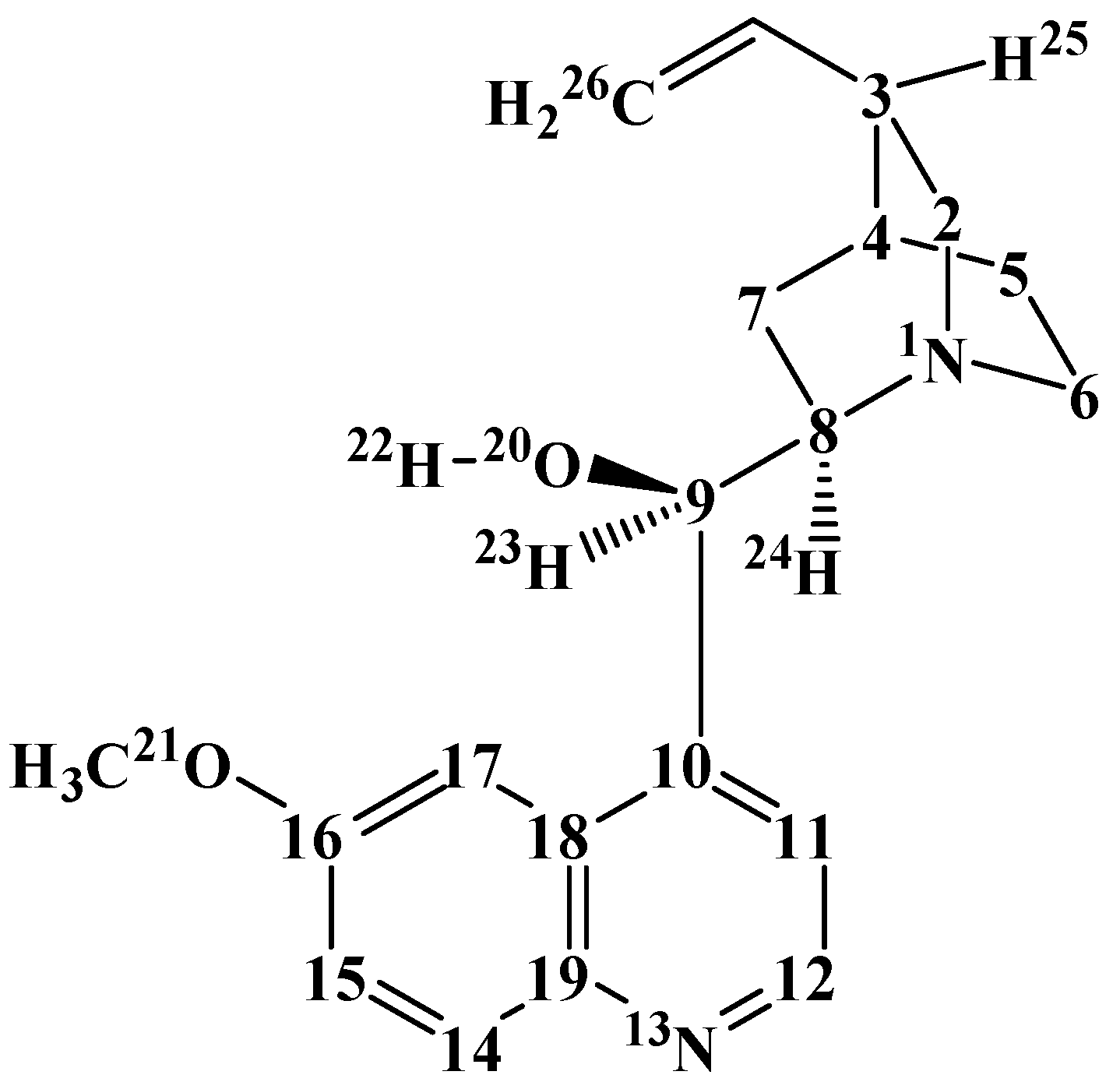
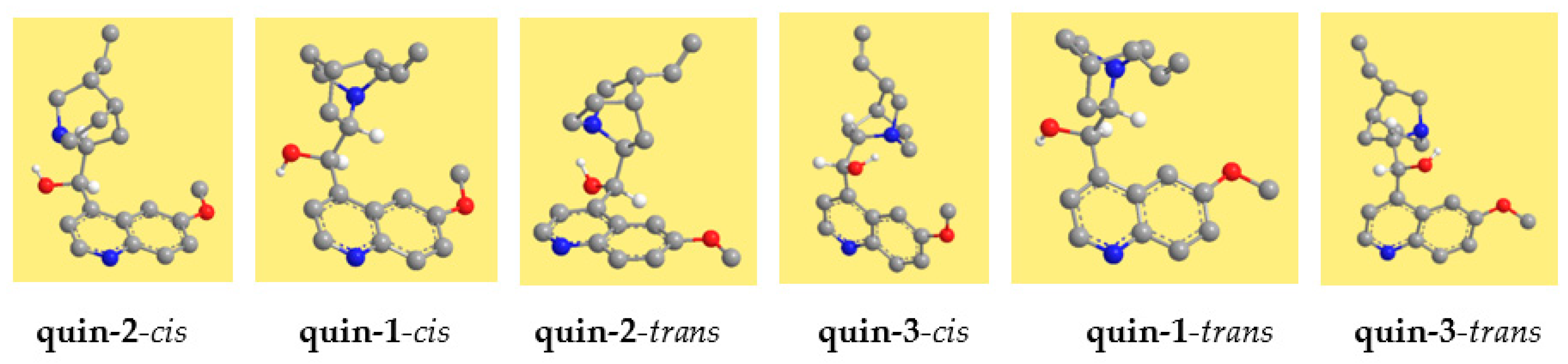
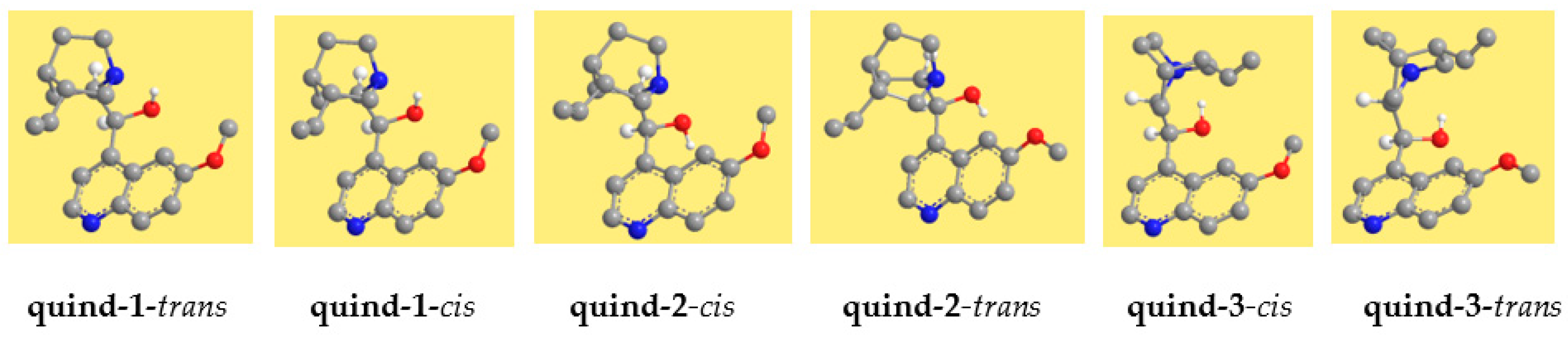
3.1. Results in Vacuo
Besides the conformers expected to have an IHB, the conformer corresponding to
cis-γ-open(3) in [
15] (the lowest energy conformer of all the previous studies, called Q1 in the earlier studies) was also calculated, as a reference to compare the energy of the conformers with the IHB. Both the
cis and
trans options, as defined in [
15], were calculated for each type of conformer to verify possible influences from the orientation of the methoxy group.
The resulting conformers are shown in
Figure 2 for quinine and in
Figure 3 for quinidine, and their relative energies are reported in
Table 2. The conformers are denoted with acronyms starting with ‘quin’ for quinine and ‘quind’ for quinidine. This is followed by a number, increasing according to the relative energy in the DFT results. The relative energy sequence for quinine is considerably different in the results of the different methods utilised. However, the spectroscopic experimental information reported in [
15] shows that the lowest energy conformer does not have an IHB. Therefore, it is opted here to choose a relative energy sequence consistent with this, in assigning numbers to conformers. DFT results are chosen with preference to HF results because DFT takes into account part of the electronic correlation. Furthermore, they are closer to MP2 results, which take into account both electron correlation and dispersion energy. The conformers with the IHB (quin-2-c, quin-2-t, quin-3-c and quin 3-t for quinine and quind-1-t, quind-1-c, quind-3-c and quind-3-t for quinidine) have relative energy only marginally different from those without the IHB in the MP2 results, whereas the difference is not negligible in the DFT results, but also not so large as to exclude them from being potentially responsible for the biological activity. This consistency in the DFT and MP2 results in terms of yielding a low enough relative energy for the conformers with the IHB to include them as potentially responsible for the biological activity can be considered as a clear indication that such conformers need to be included.
In the acronym denoting the conformers, the number is followed by ‘c’ for
cis or ‘t’ for
trans. For quinine,
cis-γ-open(3) corresponds to Q1 in [
15]; for quinidine, quind-2-t corresponds with the conformer with the lowest relative free energy in [
15]. For quinine,
cis conformers have lower energy than corresponding
trans conformers, whereas for quinidine,
trans conformers have lower energy. For both molecules, the difference between corresponding
cis and
trans is considerable. For quinine, this energy difference (kcal/mol) is ≈2/HF, 1.6–3/MP2 and ≈1/DFT; for quinidine, it is 2–3/HF, 1–5/MP2 and 1–3/DFT.
Table 3 reports the relative free energies (∆G, sums of electronic and thermal free energies) of the calculated conformers in the HF and DFT results in vacuo. For quinine, the conformer with the lowest relative energy (quin-1-c, not having an IHB) also has the lowest ∆G. For quinidine, the quind-1-c conformer (having the IHB) has the lowest ∆G. Comparisons with the ∆G values reported in [
15] is not easy because only the lowest energy conformer of [
15] was calculated in this work, and, therefore, the comparison can refer only to this conformer. For quinine, the conformer with the lowest ∆G coincides in the current results and in [
15]; for quinidine, a different conformer (quin-3-c, not present in [
15] and with an H···N distance too long to be considered an IHB but still suggesting a weak interaction between the two atoms) has the lowest ∆G in the current results.
Table 4 reports the relevant torsion angles, i.e., the torsion angles indicating the mutual orientations of the ring systems (C11–C10–C9–C8 and C10–C9–C8–N1) and the orientation of the OH group (H22–O20–C9–C8 and O20–C9–C8–N1). The orientation of the methoxy group does not influence the rest of the geometry significantly, and corresponding pairs of
cis and
trans conformers have close values for these torsion angles. Conformers 1 and 2 differ for the C10–C9–C8–N1 torsion angle, and conformers 2 and 3 differ for the C11–C10–C9–C8 torsion angle; both angles are associated with the orientation of the two moieties.
Table 5 reports the parameters of the IHBs. The H···N bond length would suggest a moderate strength for the IHB [
38]. However, the directionality, as shown by the NĤO angle, is not optimal, which may contribute to decreasing the strength. The parameters indicate a stronger IHB for quinine than for quinidine, and the directionality is also more favourable for quinine. Comparison of the three methods shows that MP2 optimises for geometries with better IHB parameters.
Frequency calculations confirm that the identified stationary points correspond to true minima.
Table 6 reports the vibrational frequencies of the OH bond (harmonic approximation) in the HF and DFT results. With reference to the 3650 cm
−1 experimental frequency value for the ground state reported in [
15], the HF results obtained here are considerably higher, whereas the DFT result obtained here (3766 cm
−1), although being 116 cm
−1 higher than the experimental value, is closer to the experimental value than the calculated result reported in [
15].
It is interesting to consider the decrease in the vibrational frequency caused by the formation of the IHB (red shift). The red shift is evaluated with reference to the vibration when the OH is not engaged in the IHB. Although the frequency of quin-1-c and quin-1-t, or quind-2-c and quind-2-t, are very close, it was opted to evaluate the red shifts of the
cis conformers with respect to the frequency of the OH in quin-1-c and quind-2-c, and the red shifts of the
trans conformers with respect to the frequency in quin-1-t and quind-2-t. The red shifts are reported in
Table 7. For quinine, the value of the red shift is 67–98 cm
−1/HF and about 250 cm
−1/DFT; for the quind-1-c and quind-1-t conformers of quinidine, it is 63 and 68 cm
−1/HF and 210 and 248 cm
−1/DFT, respectively. The quind-3 conformers do not show any red shift with respect to quind-2, and the HF and DFT N···H values are longer than what was normally accepted for an H-bond, although they suggest the presence of a weak interaction between the two atoms (only the MP2 values suggest an IHB).
Table 8 reports the dipole moment of the calculated conformers. The dipole moment of the conformers with the IHB is significantly greater than for that of conformers without the IHB for both quinine and quinidine and in the results of all the methods.
3.2. Results in Solution
Table 2 also shows the relative energies in chloroform and water solutions. For quinine, the increasing relative-energy sequence remains the same in vacuo and in solution. For the lower energy conformers of quinine having the IHB, the relative energy is lower in chloroform than in vacuo; in water, it is higher than in chloroform and, for some conformers, it becomes close or slightly higher than in vacuo. For quinidine, the increasing relative-energy sequence changes in solution. The lowest energy conformer is a conformer with the IHB both in vacuo and in chloroform (quind-1-t and quind-1-c respectively), whereas quind-3-c (with a very weak IHB-type interaction) is the lowest energy conformer in water. The relative energy increases in solution with respect to in vacuo for
trans conformers and decreases for
cis conformers, up to the point that, differently from in vacuo,
trans conformers have higher relative energy than the corresponding
cis conformers in solution. For conformers quind-1-t, quind-1-c and quind-2-c, the relative energy is higher in water than in chloroform; for the other three conformers, it is lower in water than in chloroform.
The dipole moment (
Table 8) increases as the medium polarity increases (which is the most common trend). Since calculations in solution were SP calculations, the increase is due only to the polarization of the solute molecule by the solvent (not to a change in the molecular geometry). The dipole moment of the conformers with the IHB remains higher than that of conformers without the IHB in all the media and with all the calculation methods, both for quinine and for quinidine. The
cis conformers have higher dipole moment than the
trans conformers in all the media and with all the levels of theory, for both quinine and quinidine.
Table 9 shows the free energy of solvation (ΔG
solv) of the calculated conformers. The presence or absence of the IHB does not appear to influence ΔG
solv. The magnitude of ΔG
solv is much greater for water than for chloroform. For quinine, ΔG
solv is negative for all the conformers and with all the calculation methods. For quinidine, ΔG
solv is negative in water for all the conformers; in chloroform, it is negative for most conformers but positive for quind-2-c in the HF and DFT results. For quinine, ΔG
solv is smaller for
cis conformers than for
trans conformers in all the solvents and with all the calculation methods. For quinidine, ΔG
solv is greater for quind-3-c and quind-1-c than for their corresponding
trans conformers, whereas it is smaller for quind-2-c than for quind-2-t in both solvents and with all the calculation methods.
Table 10 reports the electrostatic component (G
el) of the free energy of solvation (ΔG
solv) of the calculated conformers. G
el has negative values in both solvents for all the conformers, and its magnitude is considerably greater in water than in chloroform. The non-electrostatic component of ΔG
solv can be easily calculated as G
non-el = ΔG
solv − G
el.