Thermal and Antioxidant Properties of Polysaccharides Sequentially Extracted from Mulberry Leaves (Morus alba L.)
Abstract
:1. Introduction
2. Results
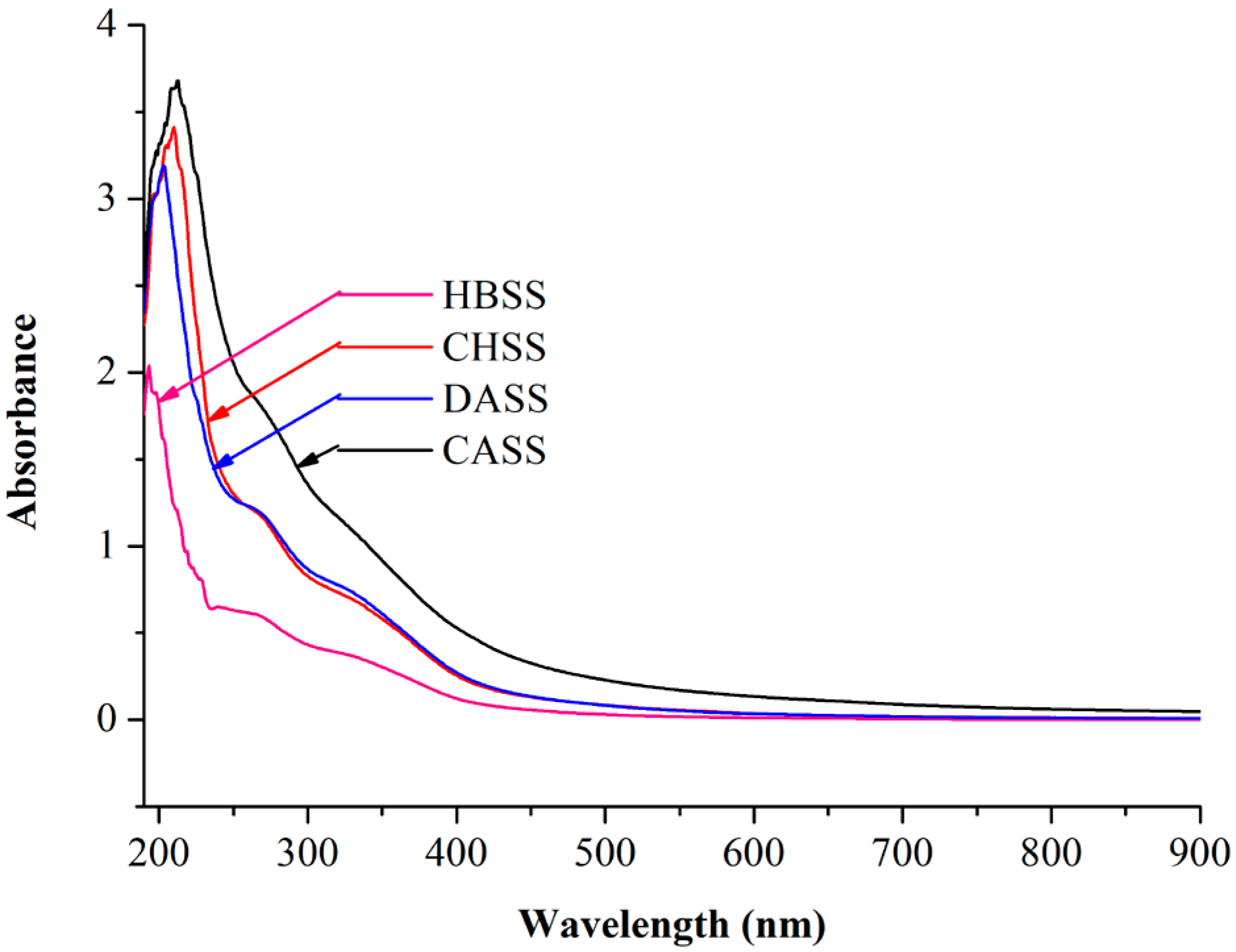
2.1. UV Absorption Peak Detection
2.2. Chemical Composition Analysis of MLPs
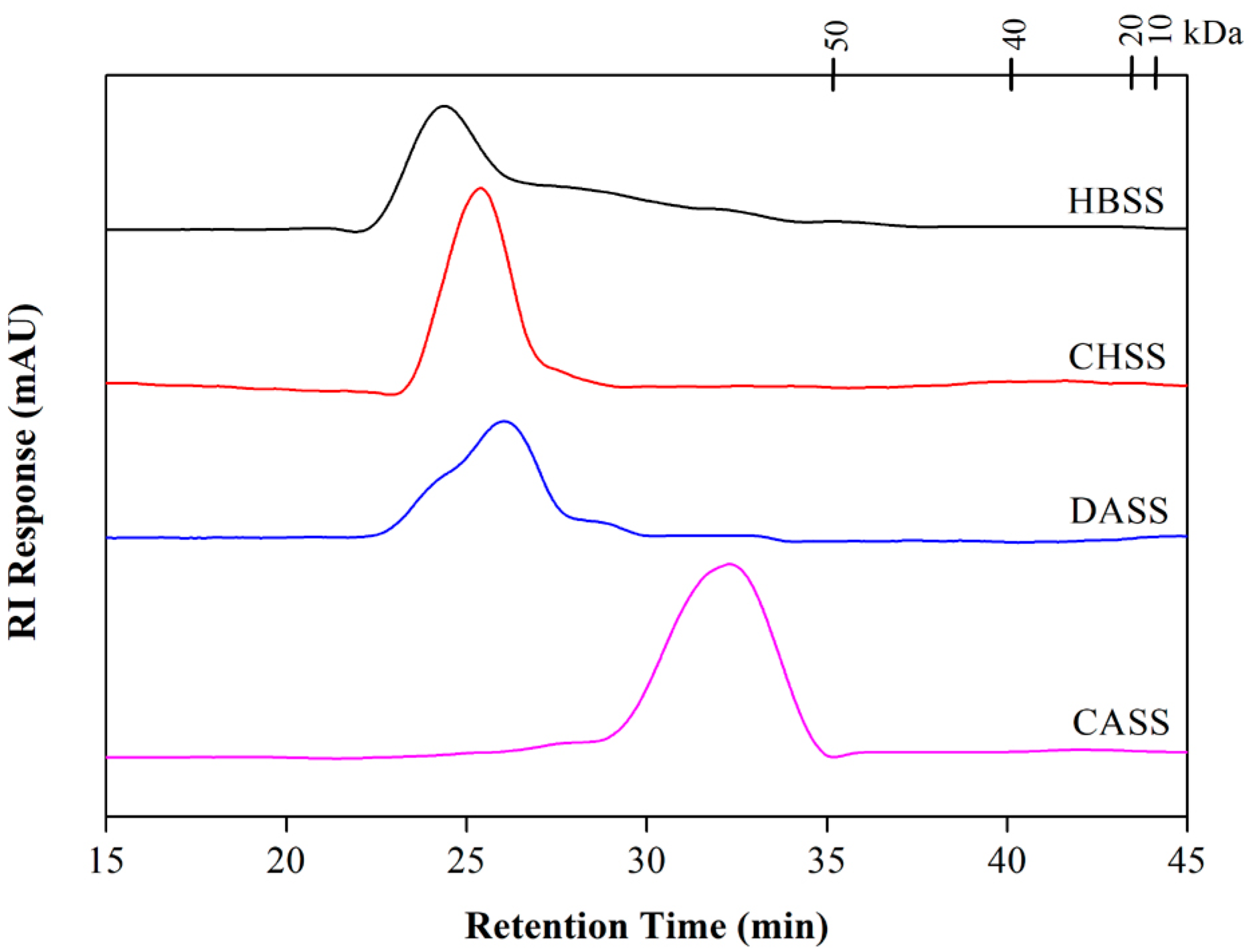
2.3. Determination of Molecular Weight and Monosaccharide Composition
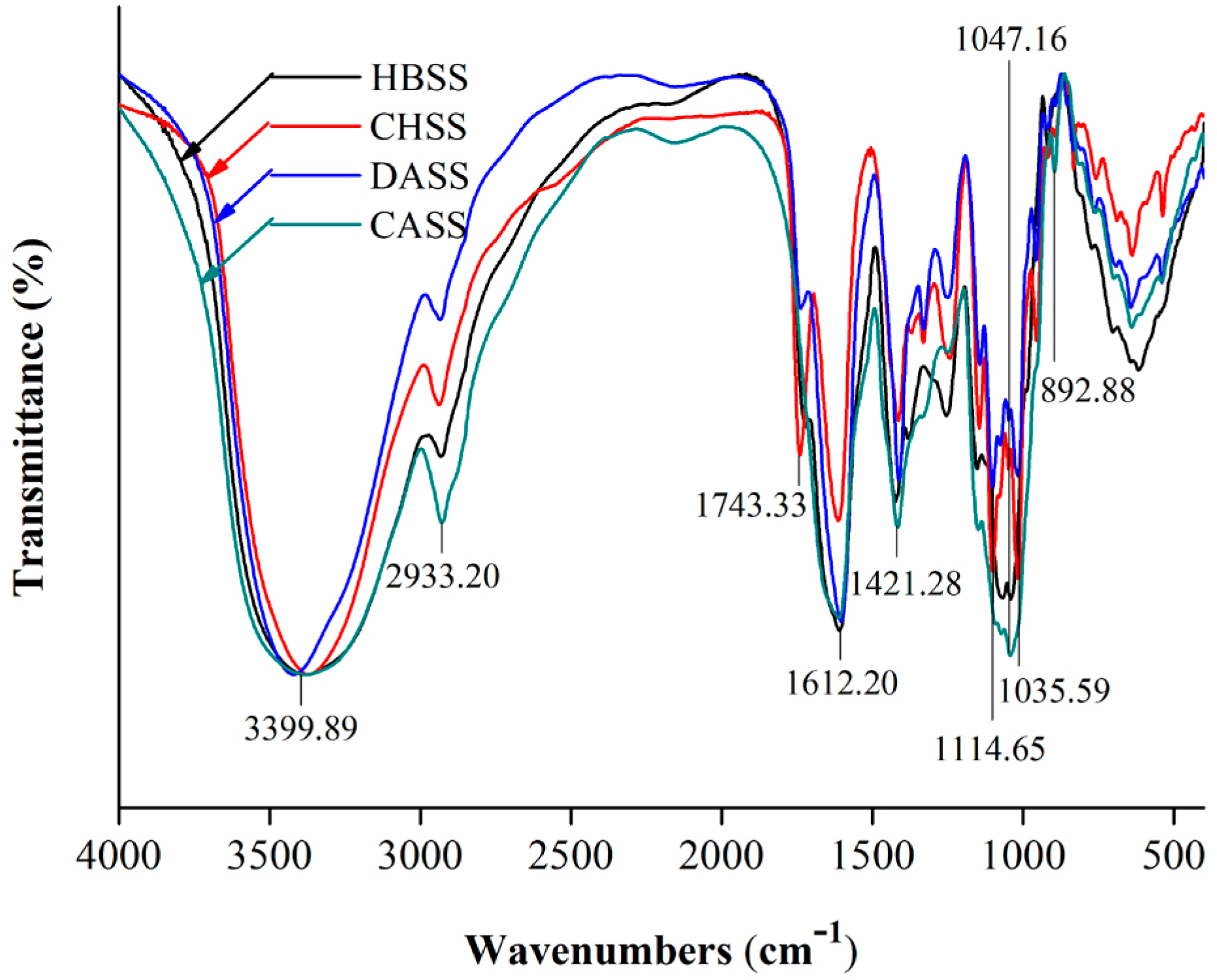
2.4. FTIR Spectrum Analysis of MLPs
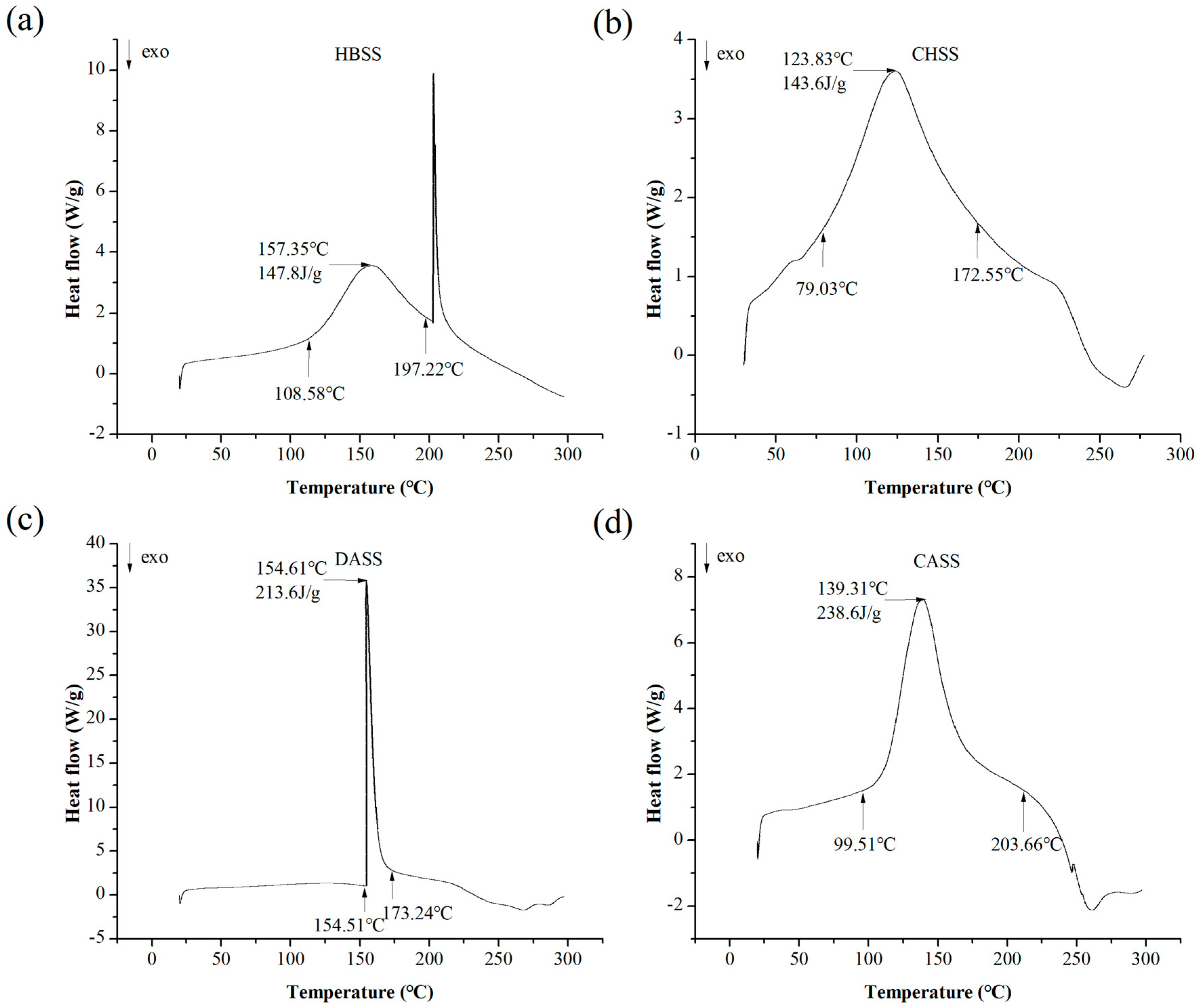
2.5. Differential Scanning Calorimetric (DSC) Analysis
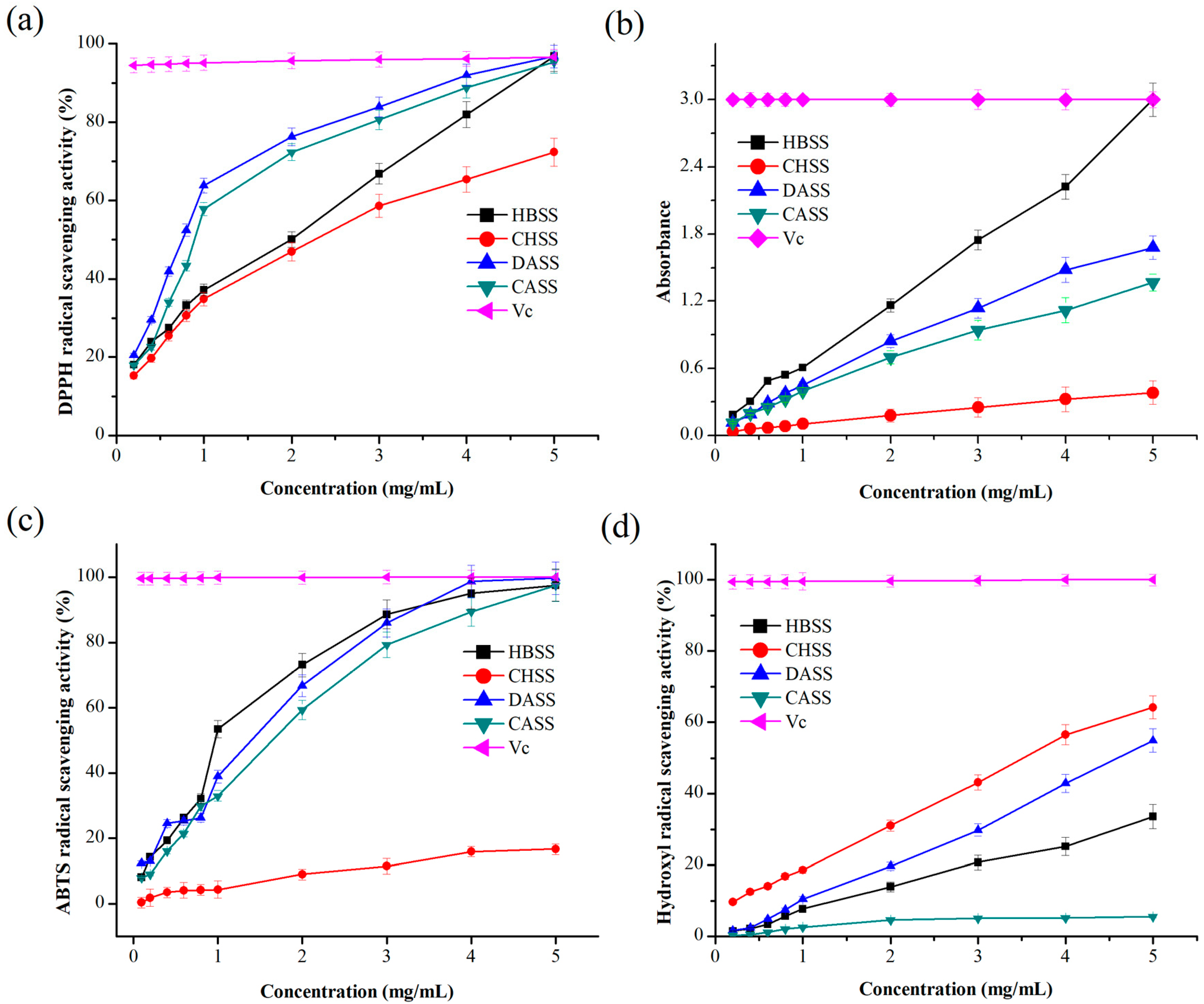
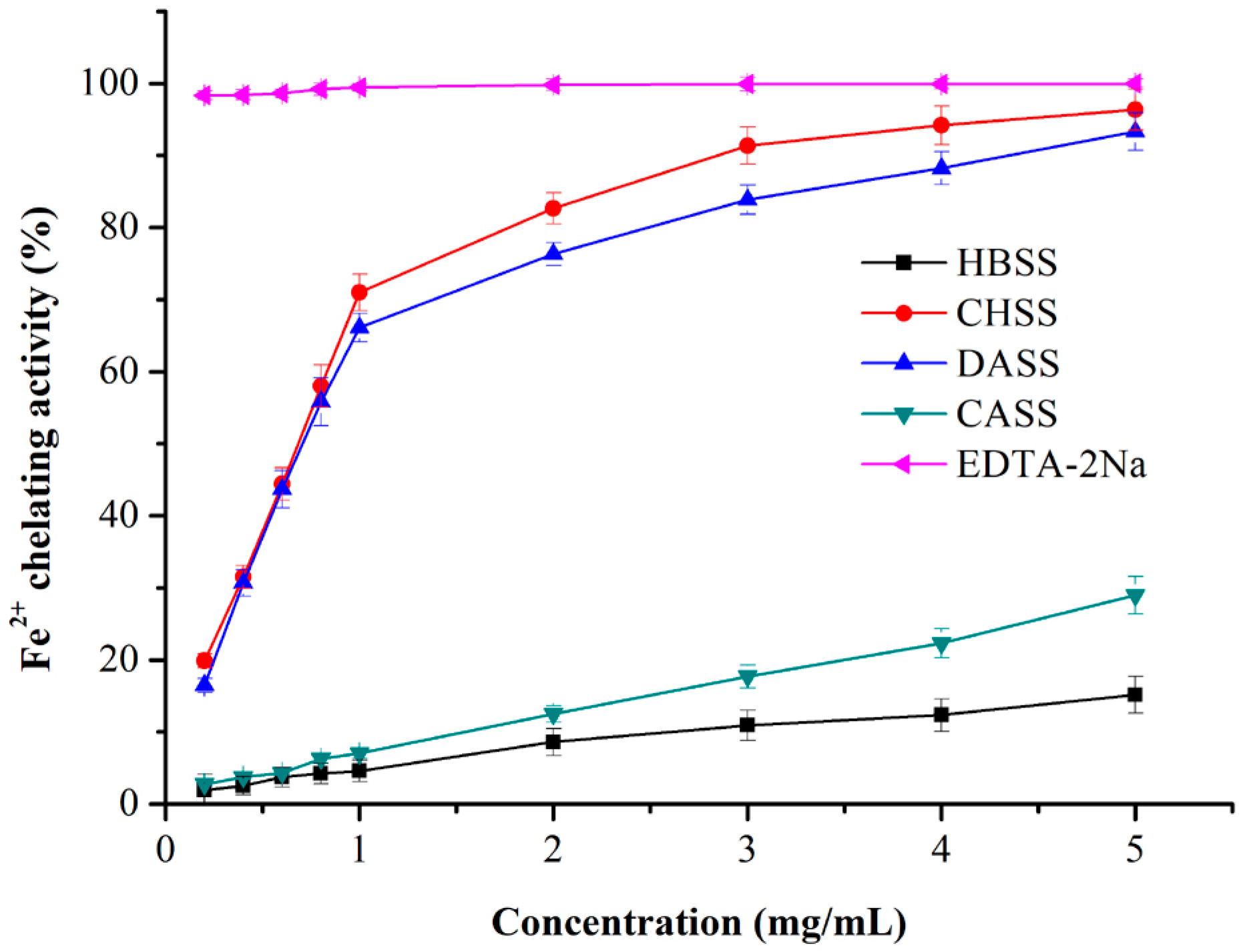
2.6. Antioxidant Activities of MLPs
2.6.1. DPPH Radical Scavenging Activity
2.6.2. Determination of Reducing Power
2.6.3. ABTS Radical Scavenging Activity
2.6.4. Hydroxyl Radical Scavenging Activity
2.6.5. Iron (Fe2+) Chelating Activity
3. Materials and Methods
3.1. Materials and Chemicals
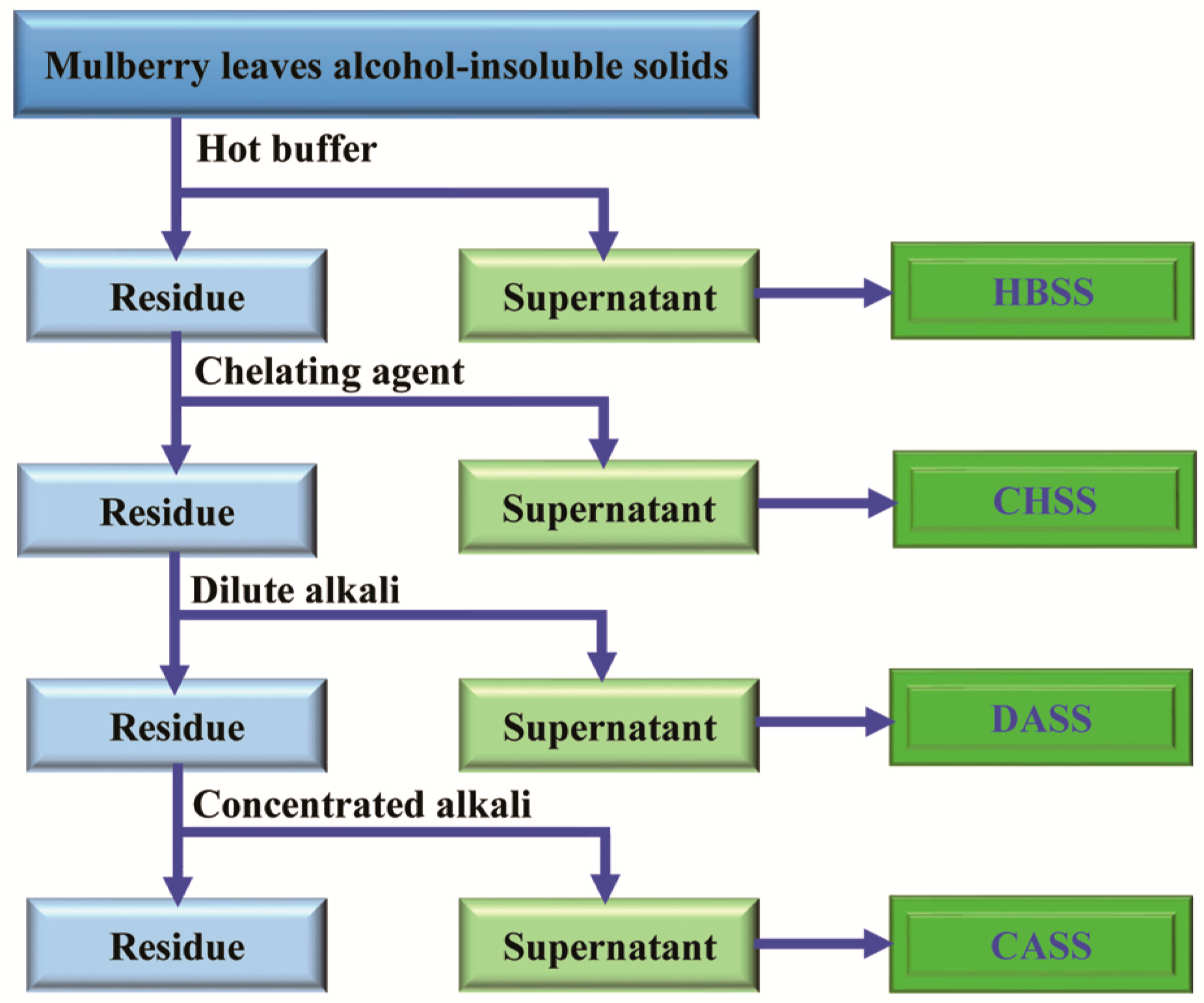
3.2. Sequential Extraction of MLPs
3.3. Purification of Crude Mulberry Leaf Polysaccharides
3.4. UV Absorption Peak Detection
3.5. Chemical Composition Analysis of MLPs
3.6. Homogeneity and Molecular Weight Determination
3.7. Monosaccharide Composition of MLPs
3.8. Infrared Spectrum Analysis of MLPs
3.9. Differential Scanning Calorimetric (DSC) Analysis
3.10. Antioxidant Activities of MLPs
3.10.1. DPPH Radical Scavenging Activity
3.10.2. Determination of Reducing Power
3.10.3. ABTS Radical Scavenging Activity
3.10.4. Hydroxyl Radical Scavenging Activity
3.10.5. Iron (Fe2+)-Chelating Activity
3.11. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ji, T.; Li, J.; Su, S.-L.; Zhu, Z.-H.; Guo, S.; Qian, D.-W.; Duan, J.-A. Identification and Determination of the Polyhydroxylated Alkaloids Compounds with α-Glucosidase Inhibitor Activity in Mulberry Leaves of Different Origins. Molecules 2016, 21, 206. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, C.-Q.; Zhang, H.; Li, J.-W. Response Surface Optimized Extraction of Deoxynojirimycin from Mulberry Leaves (Morus alba L.) and Preparative Separation with Resins. Molecules 2014, 19, 7040–7056. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Q.; Thakur, K.; Chen, G.-H.; Hu, F.; Zhang, J.-G.; Zhang, H.-B.; Wei, Z.-J. Metabolic Effect of 1-Deoxynojirimycin from Mulberry Leaves on db/db Diabetic Mice Using Liquid Chromatography-Mass Spectrometry Based Metabolomics. J. Agric. Food Chem. 2017, 65, 4658–4667. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, V.K.; Negi, N.; Khurana, P. Auxin Response Factor Genes Repertoire in Mulberry: Identification, and Structural, Functional and Evolutionary Analyses. Genes 2017, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Yang, L.; Zheng, H.Y. Hypolipidemic and antioxidant effects of mulberry (Morus alba L.) fruit in hyperlipidaemia rats. Food Chem. Toxicol. 2010, 48, 2374–2379. [Google Scholar] [CrossRef] [PubMed]
- El-Beshbishy, H.A.; Singab, A.N.; Sinkkonen, J.; Pihlaja, K. Hypolipidemic and antioxidant effects of Morus alba L. (Egyptian mulberry) root bark fractions supplementation in cholesterol-fed rats. Life Sci. 2006, 78, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.-C.; Jhou, K.-Y.; Tseng, C.-Y. Antihypertensive effect of mulberry leaf aqueous extract containing γ-aminobutyric acid in spontaneously hypertensive rats. Food Chem. 2012, 132, 1796–1801. [Google Scholar] [CrossRef]
- Chan, K.C.; Yang, M.Y.; Lin, M.C.; Lee, Y.J.; Chang, W.C.; Wang, C.J. Mulberry leaf extract inhibits the development of atherosclerosis in cholesterol-fed rabbits and in cultured aortic vascular smooth muscle cells. J. Agric. Food Chem. 2013, 61, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Dua, K.; Kazmi, I.; Anwar, F. Anticonvulsant activity of Morusin isolated from Morus alba: Modulation of GABA receptor. Biomed. Aging Pathol. 2014, 4, 29–32. [Google Scholar] [CrossRef]
- Kurniati, N.F.; Suryani, G.P.; Sigit, J.I. Vasodilator Effect of Ethanolic Extract of Mulberry Leaves (Morus alba L.) in Rat and Rabbit. Procedia Chem. 2014, 13, 142–146. [Google Scholar] [CrossRef]
- Katsube, T.; Imawaka, N.; Kawano, Y.; Yamazaki, Y.; Shiwaku, K.; Yamane, Y. Antioxidant flavonol glycosides in mulberry (Morus alba L.) leaves isolated based on LDL antioxidant activity. Food Chem. 2006, 97, 25–31. [Google Scholar] [CrossRef]
- Katsube, T.; Tsurunaga, Y.; Sugiyama, M.; Furuno, T.; Yamasaki, Y. Effect of air-drying temperature on antioxidant capacity and stability of polyphenolic compounds in mulberry (Morus alba L.) leaves. Food Chem. 2009, 113, 964–969. [Google Scholar] [CrossRef]
- Wang, J.H.; Zuo, S.R.; Luo, J.P. Structural Analysis and Immuno-Stimulating Activity of an Acidic Polysaccharide from the Stems of Dendrobium nobile Lindl. Molecules 2017, 22, 611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shi, J.J.; Thakur, K.; Hu, F.; Zhang, J.G.; Wei, Z.J. Anti-Cancerous Potential of Polysaccharide Fractions Extracted from Peony Seed Dreg on Various Human Cancer Cell Lines Via Cell Cycle Arrest and Apoptosis. Front. Pharmacol. 2017, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Duan, W.; Liu, Y.; Cen, Y. Anti-inflammatory effect of the polysaccharides of Golden needle mushroom in burned rats. Int. J. Biol. Macromol. 2010, 46, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Sheng, K.; Yan, E.; Qiao, J.; Lv, F. Extraction, purification and antibacterial activities of a polysaccharide from spent mushroom substrate. Int. J. Biol. Macromol. 2012, 50, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhai, X.; Li, L.; Wu, X.; Li, B. Response surface optimization of ultrasound-assisted polysaccharides extraction from pomegranate peel. Food Chem. 2015, 177, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, W.; Wang, Q.; Wang, D.; Dong, D.; Mu, H.; Ye, X.; Duan, J. Purification, antioxidant and immunological activities of polysaccharides from Actinidia Chinensis roots. Int. J. Biol. Macromol. 2015, 72, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-M.; Wang, F.-Y.; Liu, Y.-L. Hot-compressed water extraction of polysaccharides from soy hulls. Food Chem. 2016, 202, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Khemakhem, I.; Abdelhedi, O.; Trigui, I.; Ayadi, M.A.; Bouaziz, M. Structural, antioxidant and antibacterial activities of polysaccharides extracted from olive leaves. Int. J. Biol. Macromol. 2018, 106, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Willför, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Shi, J.-J.; Zhang, J.-G.; Sun, Y.-H.; Qu, J.; Li, L.; Prasad, C.; Wei, Z.-J. Physicochemical properties and antioxidant activities of polysaccharides sequentially extracted from peony seed dreg. Int. J. Biol. Macromol. 2016, 91, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.X.; Shi, J.J.; Zhang, J.G.; Li, L.; Jiang, L.; Wei, Z.J. Thermal, emulsifying and rheological properties of polysaccharides sequentially extracted from Vaccinium bracteatum Thunb. leaves. Int. J. Biol. Macromol 2016, 93, 1240–1252. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.-Y.; Ma, Y.-L.; Wang, C.-H.; Wang, H.; Ren, Y.-F.; Zhang, J.-G.; Thakura, K.; Wei, Z.-J. Insights into physicochemical and functional properties of polysaccharides sequentially extracted from onion (Allium cepa L.). Int. J. Biol. Macromol. 2017, 105, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liao, B.-Y.; Thakur, K.; Zhang, J.-G.; Wei, Z.-J. The rheological behavior of polysaccharides sequential extracted from Polygonatum cyrtonema Hua. Int. J. Biol. Macromol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Han, X.; Li, J. Ultrasound-assisted extraction of polysaccharides from mulberry leaves. Food Chem. 2011, 127, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Thirugnanasambandham, K.; Sivakumar, V.; Maran, J.P. Microwave-assisted extraction of polysaccharides from mulberry leaves. Int. J. Biol. Macromol. 2015, 72, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Romdhane, M.B.; Haddar, A.; Ghazala, I.; Jeddou, K.B.; Helbert, C.B.; Ellouz-Chaabouni, S. Optimization of polysaccharides extraction from watermelon rinds: Structure, functional and biological activities. Food Chem. 2017, 216, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.-Y.; Abdul Manaf, N.H.; Latiff, A.A. Physico-chemical properties of alcohol precipitate pectin-like polysaccharides from Parkia speciose pod. Food Hydrocoll. 2010, 24, 471–478. [Google Scholar] [CrossRef]
- Cui, C.; Lu, J.; Sun-Waterhouse, D.; Mu, L.; Sun, W.; Zhao, M.; Zhao, H. Polysaccharides from Laminaria japonica: Structural characteristics and antioxidant activity. LWT - Food Sci. Technol. 2016, 73, 602–608. [Google Scholar] [CrossRef]
- Li, S.; Shah, N.P. Characterization, antioxidative and bifidogenic effects of polysaccharides from Pleurotus eryngii after heat treatments. Food Chem. 2016, 197, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Winkler, H.; Vorwerg, W.; Rihm, R. Thermal and mechanical properties of fatty acid starch esters. Carbohydr. Polym. 2014, 102, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Lin, L.; Xie, J.; Wang, Z.; Wang, H.; Dong, Y.; Shen, M.; Xie, M. Effect of ultrasonic treatment on the physicochemical properties and antioxidant activities of polysaccharide from Cyclocarya paliurus. Carbohydr. Polym. 2016, 151, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Jeddou, K.B.; Chaari, F.; Maktouf, S.; Nouri-Ellouz, O.; Helbert, C.B.; Ghorbel, R.E. Structural, functional, and antioxidant properties of water-soluble polysaccharides from potatoes peels. Food Chem. 2016, 205, 97–105. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Zhao, Y.; Zhao, R.; Sun, P. Antioxidant and antitumor activities in vitro of polysaccharides from E. sipunculoides. Int. J. Biol. Macromol. 2015, 78, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ai, L.; Hang, F.; Ding, S.; Liu, Y. Composition and antioxidant activity of polysaccharides from jujuba by classical and ultrasound extraction. Int. J. Biol. Macromol. 2014, 63, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, L.; Zhang, B.; Niu, T. Structural characterisation and antioxidant properties of polysaccharides from the fruiting bodies of Russula virescens. Food Chem. 2010, 118, 675–680. [Google Scholar] [CrossRef]
- Sengkhamparn, N.; Verhoef, R.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G. Characterisation of cell wall polysaccharides from okra (Abelmoschus esculentus (L.) Moench). Carbohydr. Res. 2009, 344, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Liu, M.; Fang, Z.; Wu, J.; Zhang, Q. Composition and cytotoxicity of a novel polysaccharide from brown alga (Laminaria japonica). Carbohydr. Polym. 2012, 89, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-J.; Zhang, J.-G.; Sun, Y.-H.; Xu, Q.-X.; Li, L.; Prasad, C.; Wei, Z.-J. The rheological properties of polysaccharides sequentially extracted from peony seed dreg. Int. J. Biol. Macromol. 2016, 91, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, L.; Li, J.; Liu, H. Characterization and antioxidant activities of degraded polysaccharides from two marine Chrysophyta. Food Chem. 2014, 160, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.C.; Zhang, Z.; Jia, L.R. Antioxidant activity and characterization of antioxidant polysaccharides from pine needle (Cedrus deodara). Carbohydr. Polym. 2014, 108, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Li, X.; Jiao, Y.; Jiang, D.; Zhang, L.; Fan, B.; Zhang, Q. Optimization for ultrasound-assisted extraction of polysaccharides with antioxidant activity in vitro from the aerial root of Ficus microcarpa. Carbohydr. Polym. 2014, 110, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, J.; Ye, H.; Sun, Y.; Lu, Z.; Zeng, X. In vitro and in vivo antioxidant activity of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr. Polym. 2010, 82, 1278–1283. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Samples | Yield (g/100 g AIS) | Total Sugar Content (%) | Uronic Acid (%) |
|---|---|---|---|
| HBSS | 9.4 ± 0.4 b | 82.3 ± 3.5 a,b | 12.4 ± 0.5 d |
| CHSS | 5.2 ± 0.2 c | 78.4 ± 3.1 b | 22.9 ± 0.8 a |
| DASS | 10.5 ± 0.5 a | 80.3 ± 3.2 a,b | 14.7 ± 0.6 c |
| CASS | 5.7 ± 0.3 c | 85.5 ± 3.5 a | 20.8 ± 0.9 b |
| Samples | Molecular Weight (kDa) | Sugar Components (%) | |||||
|---|---|---|---|---|---|---|---|
| Rha | Ara | Xyl | Man | Glu | Gal | ||
| HBSS | 7.812 × 103 | 21.18 | 25.99 | 2.22 | 4.96 | 18.58 | 27.07 |
| CHSS | 3.279 × 103 | 26.11 | 30.55 | 2.76 | 2.11 | 17.96 | 20.51 |
| DASS | 6.912 × 103 | 11.96 | 20.42 | 17.89 | 2.70 | 24.96 | 22.07 |
| CASS | 1.408 × 103 | 13.69 | 18.92 | 14.18 | 2.94 | 27.51 | 22.76 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, B.-Y.; Zhu, D.-Y.; Thakur, K.; Li, L.; Zhang, J.-G.; Wei, Z.-J. Thermal and Antioxidant Properties of Polysaccharides Sequentially Extracted from Mulberry Leaves (Morus alba L.). Molecules 2017, 22, 2271. https://doi.org/10.3390/molecules22122271
Liao B-Y, Zhu D-Y, Thakur K, Li L, Zhang J-G, Wei Z-J. Thermal and Antioxidant Properties of Polysaccharides Sequentially Extracted from Mulberry Leaves (Morus alba L.). Molecules. 2017; 22(12):2271. https://doi.org/10.3390/molecules22122271
Chicago/Turabian StyleLiao, Bu-Yan, Dan-Ye Zhu, Kiran Thakur, Ling Li, Jian-Guo Zhang, and Zhao-Jun Wei. 2017. "Thermal and Antioxidant Properties of Polysaccharides Sequentially Extracted from Mulberry Leaves (Morus alba L.)" Molecules 22, no. 12: 2271. https://doi.org/10.3390/molecules22122271
APA StyleLiao, B.-Y., Zhu, D.-Y., Thakur, K., Li, L., Zhang, J.-G., & Wei, Z.-J. (2017). Thermal and Antioxidant Properties of Polysaccharides Sequentially Extracted from Mulberry Leaves (Morus alba L.). Molecules, 22(12), 2271. https://doi.org/10.3390/molecules22122271






