3.2. Synthesis
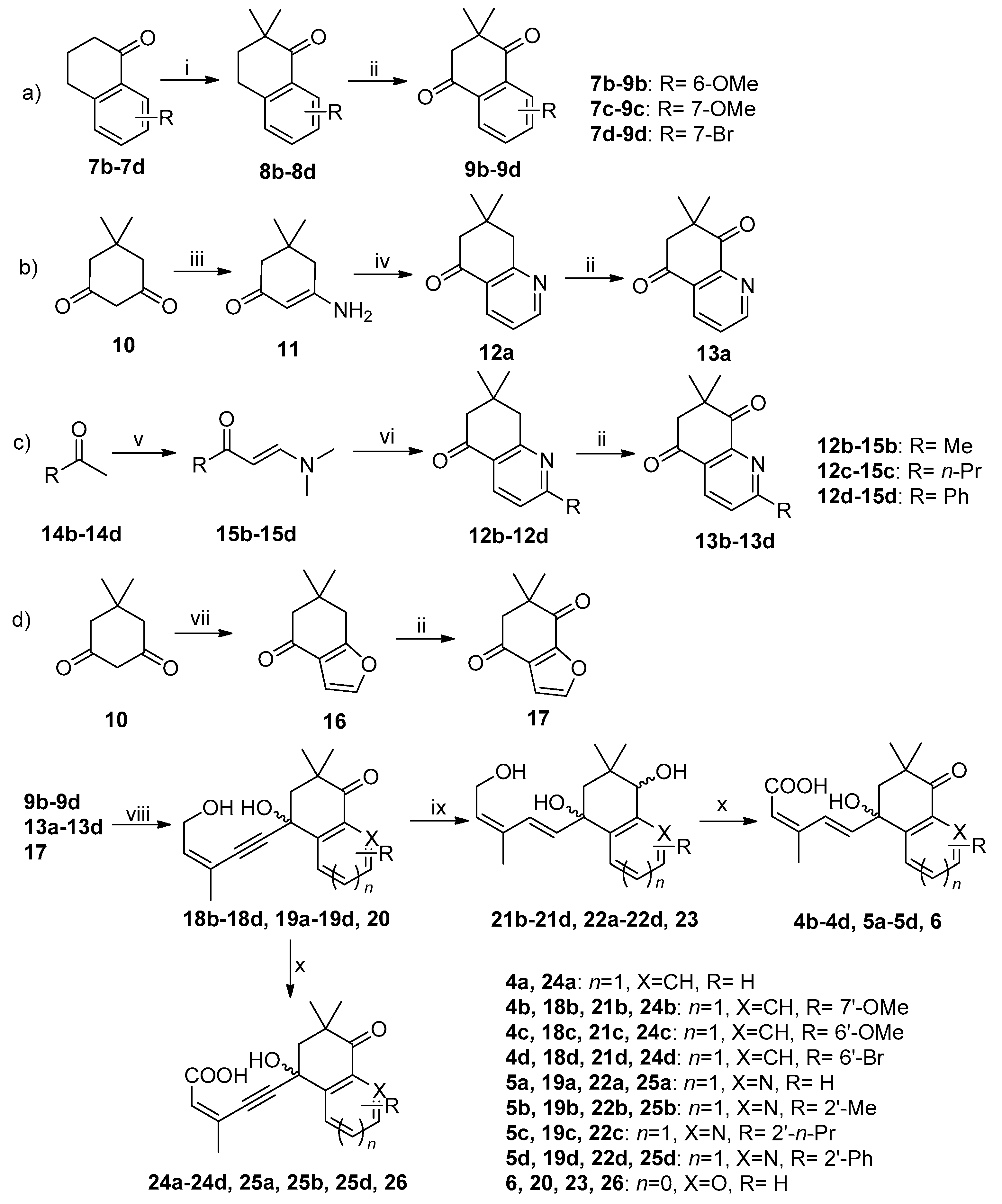
3.2.1. Synthesis of 9b–9d (Pathway a in Scheme 1, Use 9b as Example)
6-Methoxy-2,2-dimethyl-3,4-dihydronaphthalen-1(2
H)-one (
8b) [
25,
27]: To a stirred solution of 6-methoxy-3,4-dihydronaphthalen-1(2
H)-one
7b (10.0 g, 56.8 mmol) dissolved in dry tetrahydrofuran (THF, 150 mL) in a 500 mL round bottomed flask was added NaH (11.4 g, 284 mmol, 60% in oil). After stirring the mixture for 1 h under ice-water bath, methyl iodide (32.3 g, 227 mmol) in dry THF (50 mL) was added slowly. The mixture was allowed to stir at r.t. for 16 h. The reaction was quenched by addition of water (slowly and dropwise). The mixture was then extracted with ethyl acetate (3 × 100 mL), washed with water (2 × 100 mL), and dried over anhydrous Na
2SO
4. Evaporation of the solvent yielded a yellow oil. The residue was subjected to silica gel chromatography using petroleum ether (PE) and ethyl acetate (EtOAc) (10:1) as eluent to afford 6-methoxy-2,2-dimethyl-3,4-dihydronaphthalen-1(2
H)-one
8b (10.4 g, yield 90%) as a yellow oil.
1H-NMR (300 MHz, CDCl
3) δ 7.99 (d,
J = 8.7 Hz, 1H), 6.79 (dd,
J = 8.8, 2.6 Hz, 1H), 6.65 (d,
J = 2.5 Hz, 1H), 3.81 (s, 3H), 2.92 (t,
J = 6.4 Hz, 2H), 2.00–1.85 (m, 2H), 1.19 (s, 6H).
13C-NMR (75 MHz, CDCl
3) δ 200.95, 162.86, 145.36, 129.76, 124.49, 112.84, 111.83, 54.84, 40.78, 36.30, 25.65, 24.06.
7-Methoxy-2,2-dimethyl-3,4-dihydronaphthalen-1(2H)-one 8c (yield 93%) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 7.52 (d, J = 2.8 Hz, 1H), 7.13 (d, J = 8.4 Hz, 1H), 7.04 (dd, J = 8.4, 2.8 Hz, 1H), 3.83 (s, 3H), 2.91 (t, J = 6.3 Hz, 2H), 1.96 (t, J = 6.3 Hz, 2H), 1.21 (s, 6H). 13C-NMR (75 MHz, CDCl3) δ 202.71, 158.28, 135.82, 132.10, 129.78, 121.33, 109.77, 55.35, 41.36, 36.76, 30.80, 24.82, 24.28.
7-Br-2,2-dimethyl-3,4-dihydronaphthalen-1(2H)-one 8d (yield 86%) as a red oil. 1H-NMR (300 MHz, CDCl3) δ 7.99 (d, J = 2.2 Hz, 1H), 7.37 (dd, J = 8.2, 2.2 Hz, 1H), 6.96 (d, J = 8.2 Hz, 1H), 2.78 (t, J = 6.3 Hz, 2H), 1.82 (t, J = 6.4 Hz, 2H),, 1.07 (s, 6H). 13C-NMR (75 MHz, CDCl3) δ 200.09, 140.92, 134.57, 131.85, 129.54, 129.49, 119.49, 40.32, 35.18, 24.12, 23.11.
6-Methoxy-2,2-dimethyl-2,3-dihydronaphthalene-1,4-dione (
9b) [
27,
28]: To a stirred solution of 6-methoxy-2,2-dimethyl-3,4-dihydronaphthalen-1(2
H)-one
8b (10.0 g, 49 mmol), peroxy-
t-butanol (
t-BuOOH, 62.8 g, 490 mmol, 70% aqueous) and Co(acac)
2 (1.26 g, 4.9 mmol) in 150 mL acetone at r.t. for 24 h. The mixture was then extracted with ethyl acetate (3 × 50 mL), washed with water (2 × 50 mL) and dried over anhydrous Na
2SO
4. Evaporation of the solvent yielded a brown oil. The residue was subjected to silica gel chromatography using PE and EtOAc (5:1) as eluent to afford 6-methoxy-2,2-dimethyl-2,3-dihydronaphthalene-1,4-dione
9b (7.7 g, 72%) as a colorless solid.
1H-NMR (300 MHz, CDCl
3) δ 8.04 (d,
J = 8.7 Hz, 1H), 7.42 (d,
J = 2.7 Hz, 1H), 7.23 (dd,
J = 8.7, 2.7 Hz, 1H), 3.94 (s, 3H), 2.93 (s, 2H), 1.31 (s, 6H).
13C-NMR (75 MHz, CDCl
3) δ 200.14, 196.37, 164.01, 136.94, 129.89, 127.07, 121.61, 108.31, 55.82, 52.21, 45.21, 25.90.
7-Methoxy-2,2-dimethyl-2,3-dihydronaphthalene-1,4-dione 9c (74%) as a colorless solid. 1H-NMR (300 MHz, CDCl3) δ 7.99 (d, J = 8.6 Hz, 1H), 7.48 (d, J = 2.7 Hz, 1H), 7.21 (dd, J = 8.6, 2.7 Hz, 1H), 3.94 (s, 3H), 2.89 (s, 2H), 1.31 (s, 6H). 13C-NMR (75 MHz, CDCl3) δ 201.43, 194.85, 164.42, 135.81, 128.53, 128.41, 121.05, 109.85, 55.81, 51.66, 45.60, 30.84, 25.77.
7-Br-2,2-dimethyl-2,3-dihydronaphthalene-1,4-dione 9d (69%) as a red solid. 1H-NMR (300 MHz, CDCl3) δ 8.20 (dd, J = 1.9, 0.6 Hz, 1H), 8.00–7.69 (m, 2H), 2.92 (s, 2H), 1.31 (s, 6H). 13C-NMR (75 MHz, CDCl3) δ 199.96, 195.16, 137.01, 134.84, 133.50, 130.59, 129.89, 127.96, 51.77, 45.77, 25.68.
3.2.2. Synthesis of 13a (Pathway b in Scheme 1)
3-Amino-5,5-dimethylcyclohex-2-enone (
11) [
29]: 5,5-dimethylcyclohexane-1,3-dione
10 (7.0 g, 50 mmol) was added to a mixture of ammonium acetate (3.85 g, 50 mmol) in dry toluene (100 mL). The mixture was heated for 5 h under reflux using a Dean–Stark water separator. The red oily layer formed was then separated and recrystallized with ethyl acetate to afford 3-amino-5,5-dimethylcyclohex-2-enone
11 (5.9 g, yield 85%) as a yellow solid.
1H-NMR (300 MHz, DMSO-
d6) δ 6.67 (s, 2H), 4.90 (s, 1H), 2.11 (s, 2H), 1.90 (s, 2H), 0.96 (s, 6H).
7,7-Dimethyl-7,8-dihydroquinolin-5(6
H)-one (
12a) [
29]: A solution of 1,1,3,3-tetraethoxylpropane (10 mL, 40 mmol), 3-amino-5,5-dimethylcyclohex-2-enone
11 (5.6 g, 40 mmol) and a catalytical amount of
p-toluenesulfonic acid hydrate in DMF (50 mL) was heated under reflux for 18 h (monitored by TLC). The solvent was distilled in vacuo, the residue neutralized with NaHCO
3, extracted with EtOAc (3 × 100 mL) and dried (anhydrous Na
2SO
4). Column chromatography using PE and EtOAc (6:1) as eluent to afford 7,7-dimethyl-7,8-dihydroquinolin-5(6
H)-one
12a (2.1 g, yield 30%) as a yellow solid.
1H-NMR (300 MHz, CDCl
3) δ 8.71 (d,
J = 4.7 Hz, 1H), 8.25 (d,
J = 7.8 Hz, 1H), 7.30 (dd,
J = 7.8, 4.8 Hz, 1H), 3.06 (s, 2H), 2.56 (s, 2H), 1.12 (s, 6H).
13C-NMR (75 MHz, CDCl
3) δ 197.41, 161.82, 153.36, 134.25, 126.90, 121.86, 51.67, 45.94, 32.60, 27.94.
7,7-Dimethyl-6,7-dihydroquinoline-5,8-dione (13a): The synthesis of compound 13a was followed the same method as the synthesis of 9b using intermediate 12a as substrate.
7,7-Dimethyl-6,7-dihydroquinoline-5,8-dione 13a (yield 42%) as a yellow solid. 1H-NMR (300 MHz, CDCl3) δ 9.08 (dd, J = 4.6, 1.7 Hz, 1H), 8.37 (dd, J = 7.9, 1.8 Hz, 1H), 7.67 (dd, J = 7.9, 4.6 Hz, 1H), 3.02 (s, 2H), 1.37 (s, 6H). 13C-NMR (75 MHz, CDCl3) δ 199.22, 195.03, 155.53, 149.62, 134.68, 131.69, 127.66, 51.84, 45.49, 25.53.
3.2.3. Synthesis of 13b–13d (Pathway c in Scheme 1, Use 13b as Example)
4-(Dimethylamino)but-3-en-2-one (
15b) [
31]: To a mixture of acetone
14b (5.8 g, 100 mmol), in xylene (200 mL), was added dimethylformamide dimethylacetal (DMF-DMA, 11.9 g, 100 mmol) and the reaction refluxed for 15 h (monitored by TLC). The xylene was distilled off and the resulting solid residue was purified by column chromatography using PE and EtOAc (6:1) as eluent to afford 4-(dimethylamino)but-3-en-2-one
15b (4.3 g, 38%) as a yellow oil.
1H-NMR (300 MHz, Chloroform-
d) δ 7.48 (d,
J = 12.8 Hz, 1H), 5.05 (d,
J = 12.8 Hz, 1H), 2.95 (d,
J = 55.7 Hz, 6H), 2.09 (s, 3H).
1-(Dimethylamino)hex-1-en-3-one 15c (25%) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 7.19 (d, J = 12.7 Hz, 1H), 4.72 (d, J = 12.7 Hz, 1H), 2.62 (d, J = 27.6 Hz, 6H), 2.08–1.92 (m, 2H), 1.31 (h, J = 7.4 Hz, 2H), 0.61 (t, J = 7.4 Hz, 3H).
3-(Dimethylamino)-1-phenylprop-2-en-1-one 15d (81%) as a yellow solid. 1H-NMR (300 MHz, CDCl3) δ 7.90 (dd, J = 7.9, 1.7 Hz, 2H), 7.80 (d, J = 12.4 Hz, 1H), 7.54–7.35 (m, 3H), 5.72 (d, J = 12.4 Hz, 1H), 3.13 (s, 3H), 2.93 (s, 3H).
2,7,7-Trimethyl-7,8-dihydroquinolin-5(6
H)-one (
12b) [
31]: To a mixture of 4-(dimethylamino)but-3-en-2-one
15b (2.5 g, 22 mmol), 5,5-dimethylcyclohexane-1,3-dione (3.1 g, 22 mmol),ammonium acetate (NH
4Oac, 3.4 g, 44 mmol) in 50 mL of AcOH refluxed for 15 h (monitored by TLC). The resulting mixture was cooled to r.t., the solution was concentrated on rotary evaporator and then purified by column chromatography using PE and EtOAc (4:1) as eluent to afford 2,7,7-trimethyl-7,8-dihydroquinolin-5(6
H)-one
12b (3.4 g, 81%) as a yellow oil.
1H-NMR (300 MHz, CDCl
3) δ 8.15 (d,
J = 8.0 Hz, 1H), 7.14 (d,
J = 8.0 Hz, 1H), 3.00 (s, 2H), 2.60 (s, 3H), 2.52 (s, 2H), 1.11 (s, 6H).
13C-NMR (75 MHz, CDCl
3) δ 197.57, 163.46, 161.66, 134.66, 124.71, 121.77, 51.84, 46.26, 32.79, 28.13, 24.80.
7,7-Dimethyl-2-propyl-7,8-dihydroquinolin-5(6H)-one 12c (63%) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 7.79 (d, J = 8.0 Hz, 1H), 6.78 (d, J = 8.0 Hz, 1H), 2.66 (s, 2H), 2.52–2.37 (m, 2H), 2.16 (s, 2H), 1.43 (h, J = 7.4 Hz, 2H), 0.75 (s, 6H), 0.63 (t, J = 7.4 Hz, 3H). 13C-NMR (75 MHz, CDCl3) δ 196.59, 166.65, 161.07, 133.91, 124.30, 120.52, 51.29, 45.89, 40.12, 32.19, 27.63, 22.17, 13.25.
7,7-Dimethyl-2-phenyl-7,8-dihydroquinolin-5(6H)-one 12d (92%) as a yellow solid. 1H-NMR (300 MHz, CDCl3) δ 8.31 (d, J = 8.2 Hz, 1H), 8.06 (dd, J = 7.8, 1.7 Hz, 2H), 7.71 (d, J = 8.2 Hz, 1H), 7.51–7.47 (m, 3H), 3.11 (s, 2H), 2.57 (s, 2H), 1.14 (s, 6H).
2,7,7-Trimethyl-6,7-dihydroquinoline-5,8-dione (13b): The synthesis of compound 13b–13d was followed the same method as the synthesis of 9b using intermediate 12b–12d as substrate.
2,7,7-Trimethyl-6,7-dihydroquinoline-5,8-dione 13b (yield 57%) as a yellow solid. 1H-NMR (300 MHz, CDCl3) δ 8.24 (d, J = 8.1 Hz, 1H), 7.53 (d, J = 8.1 Hz, 1H), 2.99 (s, 2H), 2.77 (s, 3H), 1.36 (s, 6H). 13C-NMR (75 MHz, CDCl3) δ 199.54, 194.92, 165.77, 149.03, 134.68, 129.39, 127.65, 51.64, 45.37, 25.45, 25.21.
7,7-Dimethyl-2-propyl-6,7-dihydroquinoline-5,8-dione 13c (yield 61%) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 8.04 (d, J = 8.1 Hz, 1H), 7.36 (d, J = 8.1 Hz, 1H), 2.90–2.67 (m, 4H), 1.62 (h, J = 7.4 Hz, 2H), 1.15 (s, 6H), 0.79 (t, J = 7.3 Hz, 3H). 13C-NMR (75 MHz, CDCl3) δ 199.01, 194.49, 169.05, 148.73, 134.24, 129.20, 126.56, 51.24, 44.93, 40.34, 25.06, 22.37, 13.34.
7,7-Dimethyl-2-phenyl-6,7-dihydroquinoline-5,8-dione 13d (yield 51%) as a yellow solid. 1H-NMR (300 MHz, CDCl3) δ 8.40 (d, J = 8.3 Hz, 1H), 8.22–8.15 (m, 2H), 8.08 (d, J = 8.3 Hz, 1H), 7.52 (dd, J = 5.1, 1.9 Hz, 3H), 3.02 (s, 2H), 1.39 (s, 6H). 13C-NMR (75 MHz, CDCl3) δ 199.27, 194.78, 162.64, 149.54, 137.25, 135.39, 130.57, 129.81, 129.76, 128.82, 128.41, 127.62, 123.89, 51.71, 45.43, 25.40.
3.2.4. Synthesis of 17 (Pathway d in Scheme 1)
6,6-Dimethyl-6,7-dihydrobenzofuran-4(5
H)-one (
16) [
32,
33]: To a stirred ice-cooled suspension of 5,5-dimethylcyclohexane-1,3-dione
10 (14 g, 100 mmol) in water (100 mL) was added dropwise a solution of KOH (7 g, 125 mmol) in water (100 mL). Then, KI (0.3 g, 20 mmol) was added to the resulting clear solution followed by the dropwise addition of 40% aqueous chloroacetaldehyde (ClCH
2CHO, 20 mL) over 25 min. The reaction mixture was allowed to warm to reflux and stir overnight. The reaction was quenched by the dropwise addition of 2 M HCl until acid to pH paper. The solution was extracted by EtOAc (3 × 100 mL). The combined organic layers were dried (anhydrous Na
2SO
4), concentrated, and vacuum distilled to afford 6,6-dimethyl-6,7-dihydrobenzofuran-4(5
H)-one
16 (12.5 g, 76%) as a yellow oil.
1H-NMR (300 MHz, CDCl
3) δ 7.25 (d,
J = 2.0 Hz, 1H), 6.57 (d,
J = 2.0 Hz, 1H), 2.66 (s, 2H), 2.29 (s, 2H), 1.05 (s, 6H).
13C-NMR (75 MHz, CDCl
3) δ 193.53, 166.03, 142.70, 119.66, 106.07, 51.86, 37.13, 35.03, 28.31.
6,6-Dimethyl-5,6-dihydrobenzofuran-4,7-dione (17): The synthesis of compound 17 was followed the same method as the synthesis of 9b using intermediate 16 as substrate.
6,6-Dimethyl-5,6-dihydrobenzofuran-4,7-dione 17 (yield 52%) as a white solid. 1H-NMR (300 MHz, CDCl3) δ 7.72 (d, J = 1.9 Hz, 1H), 6.80 (d, J = 1.9 Hz, 1H), 2.90 (s, 2H), 1.35 (s, 6H). 13C-NMR (75 MHz, CDCl3) δ 192.11, 190.88, 153.06, 148.64, 132.08, 107.55, 53.99, 46.59, 26.23.
3.2.5. General Procedure for the Preparation of Intermediate 18b–18d, 19a–19d and 20 (Use 18b as Example) [34]
To a stirred solution of (Z)-3-methylpent-2-en-4-yn-1-ol (0.88 g, 9.2 mmol) in dry THF (20 mL) was cooled to −78 °C under an atmosphere of argon. n-Butyl lithium (7.7 mL, 18.4 mmol, 2.4 M in hexane) was then added slowly, via syringe. The mixture was allowed to stir at −78 °C for 1 h, after which, 6-methoxy-2,2-dimethyl-2,3-dihydronaphthalene-1,4-dione 9b (2 g, 9.2 mmol), dissolved in dry THF (10 mL) was added. The mixture was stirred for a further 1 h at −78 °C, and then the cold bath was removed. The reaction mixture was stirred at r.t. for a further 16 h. The reaction was quenched by addition of a saturated aqueous solution of NH4Cl. The mixture was stirred for 10 min and extracted with ethyl acetate (3 × 50 mL), washed with water (2 × 30 mL) and dried over anhydrous Na2SO4. Evaporation of the solvent yielded the desired alcohol as a yellowish oil. The residue was subjected to silica gel chromatography using PE and EtOAc (4:1) as eluent to afford (Z)-(1′-hydroxy-3′,3′-dimethyl-4′-oxo-7′-methoxy-tetrahydronaphthalene-one-yl)-3-methyl-pentyl-2-em-4-yn-1-ol 18b (2.37 g, 82%) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 7.80 (d, J = 8.6 Hz, 1H), 7.46 (d, J = 2.8 Hz, 1H), 7.13 (dd, J = 8.6, 2.9 Hz, 1H), 5.88 (t, J = 7.5 Hz, 1H), 4.27 (d, J = 6.7 Hz, 2H), 3.83 (s, 3H), 2.92 (d, J = 16.9 Hz, 1H), 2.73 (s, 1H), 2.58 (d, J = 17.4 Hz, 1H), 1.89 (s, 3H), 1.21 (s, 3H), 1.13 (s, 3H). 13C-NMR (75 MHz, CDCl3) δ 197.49, 159.63, 136.82, 136.36, 131.11, 129.04, 121.66, 120.16, 109.42, 94.54, 85.58, 74.29, 61.03, 55.54, 48.38, 41.60, 25.02, 23.31, 23.02.
(Z)-(1′-Hydroxy-3′,3′-dimethyl-4′-oxo-6′-methoxy-tetrahydronaphthalene-one-yl)-3-methyl-pentyl-2-em-4-yn-1-ol 18c (75%) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 7.96 (d, J = 8.7 Hz, 1H), 7.39 (d, J = 2.4 Hz, 1H), 6.90 (d, J = 8.7 Hz, 1H), 5.89 (t, J = 6.4 Hz, 1H), 4.27 (d, J = 6.4 Hz, 2H), 3.87 (s, 3H), 3.01–2.41 (m, 3H), 1.88 (s, 3H), 1.16 (s, 6H). 13C-NMR (75 MHz, CDCl3) δ 196.35, 164.42, 147.09, 136.57, 129.46, 123.57, 120.04, 114.19, 111.84, 94.29, 85.76, 74.60, 60.98, 55.52, 48.48, 41.51, 30.86, 24.94, 22.97.
(Z)-(1′-Hydroxy-3′,3′-dimethyl-4′-oxo-6′-Br-tetrahydronaphthalene-one-yl)-3-methyl-pentyl-2-em-4-yn-1-ol 18d (70%) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 8.06 (d, J = 1.9 Hz, 1H), 7.85 (d, J = 8.3 Hz, 1H), 7.56 (dd, J = 8.3, 1.9 Hz, 1H), 5.92 (t, J = 6.8 Hz, 1H), 4.27 (d, J = 6.8 Hz, 2H), 4.03 (s, 1H), 2.85 (d, J = 18.7 Hz, 1H), 2.64 (d, J = 23.1 Hz, 2H), 1.89 (s, 3H), 1.17 (s, 6H). 13C-NMR (75 MHz, CDCl3) δ 196.68, 146.17, 136.66, 131.84, 130.46, 129.55, 128.80, 128.58, 120.18, 93.72, 86.38, 74.11, 60.94, 48.57, 41.62, 24.92, 22.95.
(Z)-(5′-Hydroxy-7′,7′-dimethyl-8′-oxo-tetrahydroquinolin-one-yl)-3-methyl-pentyl-2-em-4-yn-1-ol 19a (yield 79%) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 8.77 (dd, J = 4.8, 1.8 Hz, 1H), 8.30 (dd, J = 7.8, 1.8 Hz, 1H), 7.45 (dd, J = 7.8, 4.8 Hz, 1H), 5.85 (t, J = 6.6 Hz, 1H), 5.48 (s, 1H), 4.14 (d, J = 6.5 Hz, 2H), 3.00 (d, J = 17.4 Hz, 2H), 2.68 (d, J = 17.4 Hz, 1H), 1.79 (s, 3H), 1.39 (s, 3H), 1.02 (s, 3H). 13C-NMR (75 MHz, CDCl3) δ 196.02, 161.46, 153.11, 136.96, 135.23, 124.98, 123.67, 119.37, 93.94, 85.72, 73.93, 60.68, 50.01, 40.70, 24.67, 22.63, 21.14.
(Z)-(5′-Hydroxy-2′,7′,7′-trimethyl-8′-oxo-tetrahydroquinolin-one-yl)-3-methyl-pentyl-2-em-4-yn-1-ol 19b (yield 85%) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 8.17 (d, J = 7.9 Hz, 1H), 7.28 (d, J = 8.0 Hz, 1H), 5.84 (t, J = 6.6 Hz, 1H), 5.35 (s, 1H), 4.16 (d, J = 6.6 Hz, 2H), 3.00 (d, J = 17.5 Hz, 1H), 2.66 (s, 3H), 2.59 (d, J = 17.5 Hz, 2H), 1.80 (s, 3H), 1.40 (s, 3H), 0.97 (s, 3H). 13C-NMR (75 MHz, CDCl3) δ 195.87, 163.50, 160.80, 136.79, 135.44, 123.43, 122.57, 119.43, 94.30, 85.23, 73.63, 60.86, 50.24, 40.79, 24.67, 24.66, 22.68, 20.93.
(Z)-(5′-Hydroxy-7′,7′-dimethyl-2′-propyl-8′-oxo-tetrahydroquinolin-one-yl)-3-methyl-pentyl-2-em-4-yn-1-ol 19c (yield 73%) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 8.10 (d, J = 7.9 Hz, 1H), 7.19 (d, J = 7.9 Hz, 1H), 5.75 (t, J = 6.1 Hz, 1H), 5.31 (s, 1H), 4.09 (d, J = 6.3 Hz, 2H), 2.93 (d, J = 17.4 Hz, 1H), 2.80 (d, J = 7.3 Hz, 2H), 2.50 (d, J = 17.5 Hz, 1H), 1.81–1.65 (m, 5H), 1.32 (s, 3H), 0.92–0.86 (m, 6H). 13C-NMR (75 MHz, CDCl3) δ 195.60, 166.92, 160.52, 136.72, 135.12, 122.56, 122.38, 118.79, 94.04, 84.93, 73.34, 60.55, 50.03, 40.45, 39.82, 24.42, 22.39, 21.98, 20.59, 13.30.
(Z)-(5′-Hydroxy-7′,7′-dimethyl-2′-phenyl-8′-oxo-tetrahydroquinolin-one-yl)-3-methyl-pentyl-2-em-4-yn-1-ol 19d (yield 79%) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 8.35 (d, J = 8.2 Hz, 1H), 8.20–8.02 (m, 2H), 7.87 (d, J = 8.2 Hz, 1H), 7.53 (qd, J = 4.6, 1.6 Hz, 3H), 5.82 (t, J = 7.4 Hz, 1H), 5.34 (s, 1H), 4.17 (s, 2H), 3.08 (d, J = 17.7 Hz, 1H), 2.62 (d, J = 17.6 Hz, 1H), 1.80 (s, 3H), 1.45 (s, 3H), 1.00 (s, 3H). 13C-NMR (75 MHz, CDCl3) δ 195.74, 161.32, 160.49, 137.29, 136.59, 136.36, 130.62, 128.99, 127.50, 123.46, 120.24, 119.88, 94.60, 85.30, 73.89, 61.28, 50.61, 40.97, 24.81, 22.87, 21.00.
(Z)-(4′-Hydroxy-6′,6′-dimethyl-7′-oxo-tetrahydrobenzofuran-one-yl)-3-methyl-pentyl-2-em-4-yn-1-ol 20 (yield 90%) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 7.42 (d, J = 2.0 Hz, 1H), 6.68 (d, J = 2.0 Hz, 1H), 5.95 (t, J = 6.8 Hz, 1H), 4.31 (d, J = 6.7 Hz, 2H), 2.80 (d, J = 16.4 Hz, 1H), 2.45 (d, J = 16.5 Hz, 1H), 1.88 (s, 3H), 1.21 (d, J = 12.0 Hz, 6H). 13C-NMR (75 MHz, CDCl3) δ 193.65, 163.80, 143.91, 137.11, 119.66, 119.21, 106.44, 90.38, 85.67, 70.16, 60.77, 49.40, 43.91, 24.79, 22.67, 22.55.
3.2.6. General Procedure for the Preparation of Intermediate 21b−21d, 22a−22d and 23 (Use 21b as Example)
To a stirred solution of (Z)-(1′-hydroxy-3′,3′-dimethyl-4′-oxo-7′-methoxy-tetrahydronaphthalene-one-yl)-3-methyl-pentyl-2-em-4-yn-1-ol 18b (1 g, 3.2 mmol) in dry THF (20 mL) was cooled to 0 °C and Red-Al reagent (2.9 mL, 9.6 mmol, 3.3 M in toluene) added dropwise via syringe. After 4 h stirring at r.t., the reaction was quenched by slow addition of saturate brine (10 mL) and extracted with diethyl ether (3 × 30 mL). The organic phase was washed with water (2 × 20 mL) and dried over anhydrous Na2SO4 and filtered, filtrate concentrated under reduced pressure. The residue was subjected to silica gel chromatography using CH2Cl2 and methanol (25:1) as eluent to afford (1E,3Z)-(1′,4′-dihydroxy-3′,3′-dimethyl-7′-methoxy-tetraloneyl)-3-methyl-pentyl-2-ene-4-yn-1-ol 21b (0.57 g, 56%) as a white solid. 1H-NMR (300 MHz, MeOD) δ 7.32 (d, J = 8.7 Hz, 1H), 7.06 (d, J = 2.5 Hz, 1H), 6.81 (dd, J = 8.7, 2.7 Hz, 1H), 6.31 (d, J = 15.6 Hz, 1H), 6.05 (d, J = 15.7 Hz, 1H), 5.44 (t, J = 6.9 Hz, 1H), 4.76 (dd, J = 9.9, 6.8 Hz, 1H), 4.09 (d, J = 7.1 Hz, 2H), 3.79 (s, 3H), 1.91 (dd, J = 13.3, 6.7 Hz, 1H), 1.82 (d, J = 13.3 Hz, 4H), 0.99 (d, J = 5.8 Hz, 6H). 13C-NMR (75 MHz, MeOD) δ 160.23, 141.14, 138.07, 134.97, 134.39, 130.26, 129.55, 126.56, 115.18, 111.87, 79.59, 67.32, 58.48, 55.63, 44.95, 39.99, 25.70, 23.24, 20.82.
(1E,3Z)-(1′,4′-Dihydroxy-3′,3′-dimethyl-6′-methoxy-tetraloneyl)-3-methyl-pentyl-2-ene-4-yn-1-ol 21c (49%) as a white solid. 1H-NMR (300 MHz, MeOD) δ 7.42 (d, J = 8.1 Hz, 1H), 6.98 (d, J = 2.7 Hz, 1H), 6.83 (dd, J = 8.6, 2.7 Hz, 1H), 6.37 (d, J = 15.7 Hz, 1H), 6.06 (d, J = 15.5 Hz, 1H), 5.46 (t, J = 6.8 Hz, 1H), 4.75 (dd, J = 9.7, 7.0 Hz, 1H), 4.10 (dd, J = 6.5, 2.8 Hz, 2H), 3.75 (s, 3H), 1.91 (dd, J = 13.4, 6.9 Hz, 1H), 1.87–1.72 (m, 4H), 0.99 (d, J = 10.5 Hz, 6H). 13C-NMR (75 MHz, MeOD) δ 160.48, 143.82, 137.72, 134.99, 132.00, 129.69, 129.51, 126.76, 114.75, 112.80, 79.89, 67.00, 58.47, 55.61, 45.06, 40.26, 25.65, 23.10, 20.79.
(1E,3Z)-(1′,4′-Dihydroxy-3′,3′-dimethyl-6′-Br-tetraloneyl)-3-methyl-pentyl-2-ene-4-yn-1-ol 21d (45%) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 7.59–7.50 (m, 1H), 7.50–7.39 (m, 1H), 7.36–7.26 (m, 2H), 6.29 (d, J = 15.7 Hz, 1H), 6.03 (d, J = 15.7 Hz, 1H), 5.51 (t, J = 7.1 Hz, 1H), 4.95–4.77 (m, 1H), 4.06 (d, J = 7.8 Hz, 2H), 2.17 (s, 2H), 2.03 (q, J = 6.9 Hz, 2H), 1.81 (s, 3H), 1.01 (d, J = 15.2 Hz, 6H). 13C-NMR (75 MHz, CDCl3) δ 140.59, 138.16, 135.73, 135.22, 128.17, 128.03, 127.62, 127.31, 127.24, 126.71, 78.87, 66.64, 57.97, 43.85, 39.11, 25.05, 22.51, 20.48.
(1E,3Z)-(5′,8′-Dihydroxy-7′,7′-dimethyl-tetrahyroquinolinyl)-3-methyl-pentyl-2-ene-4-yn-1-ol 22a (yield 12%) as a white solid. 1H-NMR (300 MHz, CDCl3) δ 8.54–8.45 (m, 1H), 7.95 (dd, J = 7.8, 1.4 Hz, 1H), 7.33–7.23 (m, 1H), 6.06 (m, 2H), 5.51 (t, J = 7.7 Hz, 1H), 4.99–4.67 (m, 1H), 4.02 (dd, J = 7.2, 3.9 Hz, 2H), 3.00 (d, J = 16.9 Hz, 1H), 2.65 (d, J = 17.5 Hz, 1H), 1.80 (s, 3H), 1.10 (s, 3H), 0.97 (s, 3H). 13C-NMR (75 MHz, CDCl3) δ 157.52, 152.85, 148.02, 135.90, 135.14, 132.74, 127.71, 126.82, 122.41, 78.03, 65.54, 57.50, 43.22, 38.94, 24.60, 21.88, 20.01.
(1E,3Z)-(5′,8′-Dihydroxy-2′,7′,7′-trimethyl-tetrahyroquinolin-yl)-3-methyl-pentyl-2-ene-4-yn-1-ol 22b (yield 43%) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 7.77 (d, J = 7.9 Hz, 1H), 7.11 (d, J = 7.9 Hz, 1H), 6.45 (d, J = 15.6 Hz, 1H), 5.87 (d, J = 15.6 Hz, 1H), 5.51 (t, J = 7.0 Hz, 1H), 4.86 (dd, J = 6.9, 3.2 Hz, 1H), 4.18 (dd, J = 6.6, 3.2 Hz, 2H), 2.51 (s, 3H), 2.20 (dd, J = 14.9, 6.9 Hz, 1H), 1.88–1.82 (m, 1H), 1.79 (s, 3H), 1.02 (d, J = 8.3 Hz, 6H). 13C-NMR (75 MHz, CDCl3) δ 157.03, 156.50, 136.95, 134.22, 134.16, 128.87, 128.01, 125.70, 122.30, 77.53, 64.78, 57.87, 50.20, 42.65, 36.27, 24.22, 23.74, 20.32.
(1E,3Z)-(5′,8′-Dihydroxy-7′,7′-dimethyl-2′-propyl-tetrahyroquinolin-yl)-3-methyl-pentyl-2-ene-4-yn-1-ol 22c (yield 26%) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 7.82 (d, J = 7.9 Hz, 1H), 7.10 (d, J = 7.9 Hz, 1H), 6.15–5.91 (m, 2H), 5.51 (t, J = 7.3 Hz, 1H), 4.89 (s, 1H), 4.86–4.75 (m, 1H), 4.01 (d, J = 8.8 Hz, 2H), 2.77–2.72 (m, 2H), 2.03–1.84 (m, 2H), 1.81 (s, 3H), 1.77–1.69 (m, 2H), 1.11 (s, 3H), 0.94 (d, J = 7.3 Hz, 6H). 13C-NMR (75 MHz, CDCl3) δ 160.78, 156.37, 136.29, 135.98, 135.36, 129.53, 127.44, 126.81, 121.49, 77.78, 65.59, 57.52, 43.41, 39.39, 39.04, 24.60, 22.35, 21.85, 20.09, 13.38.
(1E,3Z)-(5′,8′-Dihydroxy-7′,7′-dimethyl-2′-phenyl-tetrahyroquinolin-yl)-3-methyl-pentyl-2-ene-4-yn-1-ol 22d (yield 65%) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 7.98 (d, J = 7.9 Hz, 3H), 7.69 (d, J = 8.1 Hz, 1H), 7.43 (m, 3H), 6.08 (s, 2H), 5.48 (t, J = 7.1 Hz, 1H), 4.93 (s, 1H), 4.90–4.77 (m, 1H), 3.99 (dd, J = 6.9, 3.6 Hz, 2H), 2.29 (t, J = 6.0 Hz, 1H), 1.97 (m, 3H), 1.79 (s, 3H), 1.13 (s, 3H), 0.99 (s, 3H). 13C-NMR (75 MHz, CDCl3) δ 160.43, 157.42, 155.70, 138.38, 137.04, 136.46, 135.70, 131.33, 129.21, 128.68, 127.80, 127.31, 126.80, 125.06, 119.37, 78.31, 65.87, 57.89, 43.64, 39.48, 24.88, 22.22, 20.34.
(1E,3Z)-(4′,7′-Dihydroxy-6′,6′-dimethyl-tetrahyrobenzofuran-yl)-3-methyl-pentyl-2-ene-4-yn-1-ol 23 (yield 39%) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 7.35 (d, J = 1.9 Hz, 1H), 6.47 (d, J = 1.9 Hz, 1H), 6.30 (d, J = 15.8 Hz, 1H), 5.91 (d, J = 15.7 Hz, 1H), 5.57 (t, J = 7.2 Hz, 1H), 4.74 (dd, J = 8.3, 5.8 Hz, 1H), 4.12 (qd, J = 12.6, 6.9 Hz, 2H), 2.53 (s, 1H), 1.95 (dd, J = 13.5, 5.8 Hz, 1H), 1.85 (s, 3H), 1.76–1.55 (m, 2H), 1.03 (d, J = 5.1 Hz, 6H). 13C-NMR (75 MHz, CDCl3) δ 151.59, 143.03, 134.79, 132.52, 128.70, 127.27, 121.86, 108.80, 76.08, 63.34, 57.96, 45.40, 41.37, 24.29, 22.29, 20.46.
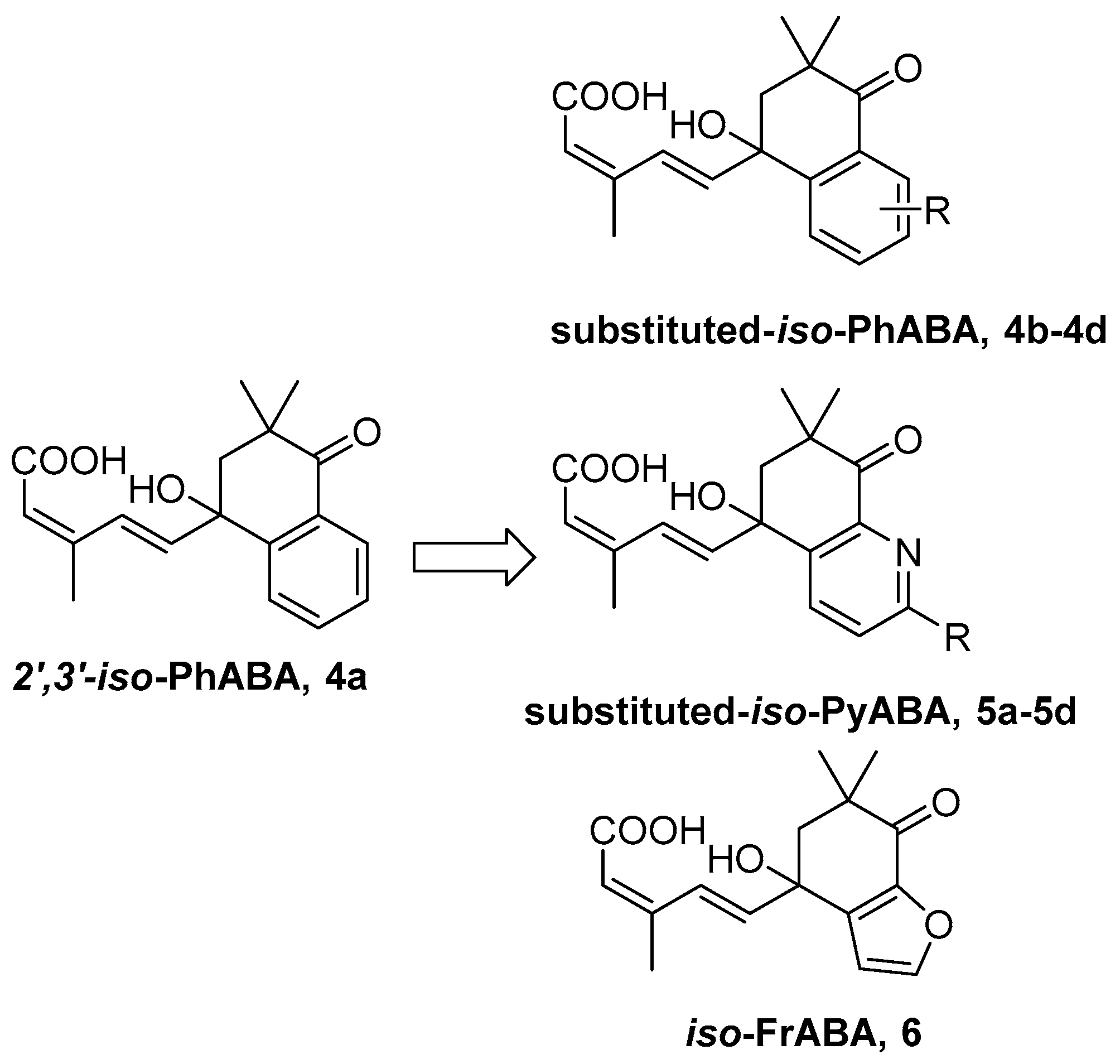
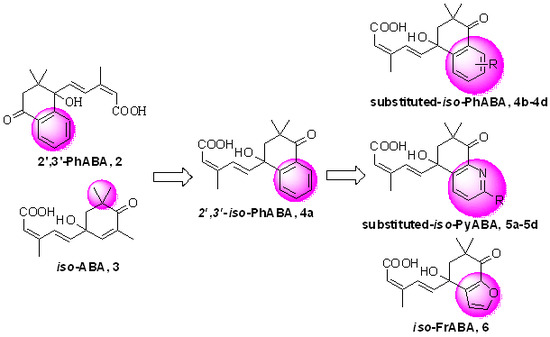
3.2.7. General Procedure for the Preparation of Title Compounds 4b–4d, 5a–5d, 6, 24a–24d, 25a, 25b, 25d and 26 (Use 4b as Example)
To a stirred solution of (1E,3Z)-(1′,4′-dihydroxy-3′,3′-dimethyl-7′-methoxy-tetraloneyl)-3-methyl-pentyl-2-ene-4-yn-1-ol 21b (0.5 g, 1.57 mmol), Dess-Martin periodinane (DMP, 1.32 g, 3.14 mmol) in 30 mL CH2Cl2 at r.t. for 2 h. After added 5 mL aqueous Na2S2O3 solution and 10 mL aqueous NaHCO3 solution, the resulting mixture, which was stirred for 20 min, was extracted repeatedly with CH2Cl2 (3 × 30 mL). The collected organic extracts were washed with saturate aqueous brine solution, dried, and then concentrated. The crude aldehyde was dissolved in 20 mL solvent (t-BuOH:H2O = 3:1 in volume), stirred with 2-methyl-2-butene (2.2 g, 31.4 mmol, 90%), NaClO2 (1.67 g, 15.7 mmol, 85%) and NaH2PO4·2H2O (0.97 g, 6.28 mmol) at r.t. for 30 min. Extracted repeatedly with EtOAc (3 × 30 mL). The collected organic extracts were washed with saturate aqueous brine solution, dried, and concentrated under reduced pressure afford crude product. The residue was subjected to silica gel chromatography using CH2Cl2 and methanol (20:1) as eluent to afford 7′-OMe-iso-PhABA 4b (0.42 g, yield 81% over two steps) as a white solid. 1H-NMR (300 MHz, MeOD) δ 7.65 (d, J = 16.4 Hz, 1H), 7.50 (d, J = 8.7 Hz, 1H), 7.45 (d, J = 2.8 Hz, 1H), 7.20 (dd, J = 8.7, 2.9 Hz, 1H), 6.48 (d, J = 16.0 Hz, 1H), 5.71 (s, 1H), 3.85 (s, 3H), 2.72 (d, J = 16.8 Hz, 1H), 2.59 (d, J = 17.5 Hz, 1H), 2.04 (d, J = 1.2 Hz, 3H), 1.08 (s, 3H), 1.04 (s, 3H). 13C-NMR (75 MHz, MeOD) δ 199.70, 169.46, 160.69, 151.21, 140.82, 140.37, 133.46, 130.84, 129.57, 122.49, 119.23, 109.90, 78.80, 55.92, 50.91, 42.38, 24.68, 23.95, 21.31. HR-MS (ESI) calcd. for C19H22O5 (M + NH4)+ 348.18055, measured 348.18057.
6′-OMe-iso-PhABA 4c (yield 83% over two steps) as a white solid. 1H-NMR (300 MHz, CDCl3) δ 8.00 (d, J = 8.7 Hz, 1H), 7.75 (d, J = 15.8 Hz, 1H), 7.06 (d, J = 2.2 Hz, 1H), 6.88 (dd, J = 8.7, 2.5 Hz, 1H), 6.41 (d, J = 16.0 Hz, 1H), 5.72 (s, 1H), 3.83 (s, 3H), 2.70 (d, J = 16.2 Hz, 1H), 2.56 (d, J = 17.3 Hz, 1H), 2.02 (s, 3H), 1.08 (d, J = 11.6 Hz, 6H). 13C-NMR (75 MHz, CDCl3) δ 196.35, 170.87, 164.62, 151.87, 148.60, 139.22, 129.38, 128.33, 124.51, 117.74, 113.90, 111.79, 78.48, 55.51, 49.61, 41.12, 24.25, 23.38, 21.40. HR-MS (ESI) calcd. for C19H22O5 (M + H)+ 331.15400, measured 331.15402.
6′-Br-iso-PhABA 4d (yield 72% over two steps) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 8.05 (d, J = 9.1 Hz, 1H), 7.58 (dt, J = 13.5, 7.2 Hz, 2H), 7.50–7.36 (m, 1H), 6.94 (d, J = 15.3 Hz, 1H), 6.14 (d, J = 15.8 Hz, 1H), 5.98 (s, 1H), 2.83 (d, J = 17.8 Hz, 1H), 2.60 (d, J = 16.9 Hz, 1H), 2.04 (s, 1H), 1.87 (s, 3H), 1.08 (d, J = 4.4 Hz, 6H). 13C-NMR (75 MHz, CDCl3) δ197.21, 169.75, 145.90, 134.47, 134.44, 133.14, 130.98, 128.18, 127.13, 126.72, 126.35, 116.39, 78.21, 49.79, 40.93, 24.33, 23.40, 18.25. HR-MS (ESI) calcd. for C18H19BrO4 (M + H)+ 396.08050, measured 396.08035.
iso-PyABA 5a (yield 78% over two steps) as colorless oil. 1H-NMR (300 MHz, CDCl3) δ 8.73 (dd, J = 4.8, 1.8 Hz, 1H), 8.39 (dd, J = 7.8, 1.8 Hz, 1H), 7.89 (d, J = 15.6 Hz, 1H), 7.48 (dd, J = 7.8, 4.9 Hz, 1H), 6.39 (d, J = 16.0 Hz, 1H), 5.70 (s, 1H), 2.87 (d, J = 18.7 Hz, 1H), 2.66 (d, J = 17.3 Hz, 1H), 2.01 (s, 3H), 1.15 (s, 3H), 1.11 (s, 3H). 13C-NMR (75 MHz, CDCl3) δ 196.24, 169.30, 163.28, 152.65, 150.54, 137.97, 135.20, 128.66, 126.25, 123.29, 117.66, 77.94, 49.44, 39.98, 23.92, 22.94, 20.68. HR-MS (ESI) calcd. for C17H19NO4 (M + H)+ 302.1387, measured 302.1387.
2′-Me-iso-PyABA 5b (yield 71% over two steps) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 8.23 (d, J = 8.0 Hz, 1H), 7.64 (d, J = 15.8 Hz, 1H), 7.28 (d, J = 8.0 Hz, 1H), 6.38 (d, J = 16.1 Hz, 1H), 5.68 (s, 1H), 2.68 (s, 2H), 2.63 (s, 3H), 1.99 (s, 3H), 1.16 (s, 3H), 1.05 (s, 3H). 13C-NMR (75 MHz, CDCl3) δ 196.04, 169.66, 163.45, 162.46, 150.81, 138.72, 135.02, 128.48, 123.65, 123.04, 117.58, 77.62, 49.54, 39.99, 24.26, 23.78, 22.85, 20.71. HR-MS (ESI) calcd. for C18H21NO4 (M + H)+ 316.1543, measured 316.1545.
2′-Pr-iso-PyABA 5c (yield 87% over two steps) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 8.24 (d, J = 8.0 Hz, 1H), 7.53 (d, J = 15.6 Hz, 1H), 7.27 (d, J = 6.0 Hz, 1H), 6.39 (d, J = 16.1 Hz, 1H), 5.68 (s, 1H), 2.90–2.80 (m, 2H), 2.77–2.56 (m, 2H), 1.99 (s, 3H), 1.83–1.72 (m, 2H), 1.20 (s, 3H), 1.04 (s, 3H), 0.95 (d, J = 7.4 Hz, 3H). 13C-NMR (75 MHz, CDCl3) δ 196.03, 169.79, 167.22, 162.25, 151.00, 139.08, 134.89, 128.34, 123.72, 122.34, 117.47, 77.59, 49.67, 39.97, 39.86, 23.77, 22.83, 22.15, 20.75, 13.37. HR-MS (ESI) calcd. for C20H25NO4 (M + H)+ 344.1856, measured 316.1854.
2′-Ph-iso-PyABA 5d (yield 80% over two steps) as a yellow solid. 1H-NMR (300 MHz, CDCl3) δ 9.06 (s, 1H), 8.40 (d, J = 8.2 Hz, 1H), 8.08 (dd, J = 7.6, 2.0 Hz, 2H), 7.88 (d, J = 8.2 Hz, 1H), 7.57 (d, J = 15.8 Hz, 1H), 7.53–7.40 (m, 3H), 6.44 (d, J = 16.1 Hz, 1H), 5.67 (s, 1H), 5.13 (s, 1H), 2.82 (d, J = 18.2 Hz, 1H), 2.60 (d, J = 18.0 Hz, 1H), 1.99 (s, 3H), 1.26 (s, 3H), 1.08 (s, 3H). 13C-NMR (75 MHz, CDCl3) δ 196.03, 170.91, 162.86, 160.65, 151.65, 139.57, 137.36, 135.89, 130.39, 128.82, 128.76, 127.50, 124.52, 119.91, 117.83, 78.17, 50.06, 40.28, 24.03, 23.22, 21.09. HR-MS (ESI) calcd. for C23H23NO4 (M + H)+ 378.17000, measured 378.16980.
iso-FrABA 6 (yield 86% over two steps) as a yellow solid. 1H-NMR (300 MHz, MeOD) δ 7.68 (d, J = 16.4 Hz, 1H), 7.58 (s, 1H), 6.67 (s, 1H), 6.32 (d, J = 16.1 Hz, 1H), 5.75 (s, 1H), 3.34 (s, 1H), 2.62 (d, J = 17.3 Hz, 1H), 2.44 (d, J = 15.9 Hz, 1H), 2.04 (s, 3H), 1.07 (d, J = 11.6 Hz, 6H). 13C-NMR (75 MHz, MeOD) δ 196.03, 168.17, 150.56, 145.84, 137.65, 136.47, 130.40, 121.68, 119.99, 106.84, 76.32, 51.84, 45.09, 24.18, 23.28, 21.11. HR-MS (ESI) calcd. for C16H18O5 (M + Na)+ 313.1046, measured 313.1048.
Acetylenic iso-PhABA 24a (yield 84% over two steps) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 7.98 (d, J = 7.74 Hz, 2H), 7.60 (t, J = 7.50 Hz, 1H), 7.41 (t, J = 7.44 Hz, 1H), 6.05 (s, 1H), 2.77 (m, 2H), 2.10 (s, 3H), 1.17 (s, 6H). 13C-NMR (75 MHz, CDCl3) δ 197.5, 169.2, 143.7, 136.5, 134.3, 130.0, 128.6, 127.5, 126.8, 124.4, 101.8, 86.1, 74.7, 48.07, 41.5, 25.1, 24.8. HR-MS (ESI) calcd. for C18H18O4 (M + Na)+ 321.1097, measured 321.1096.
Acetylenic 7′-OMe-iso-PhABA 24b (yield 71% over two steps) as a yellow solid. 1H-NMR (300 MHz, CDCl3) δ 7.65 (d, J = 8.7 Hz, 1H), 7.51 (d, J = 2.9 Hz, 1H), 7.18 (dd, J = 8.7, 2.9 Hz, 1H), 6.09 (d, J = 1.5 Hz, 1H), 4.67 (s, 1H), 3.88 (s, 3H), 2.90 (d, J = 18.1 Hz, 1H), 2.60 (d, J = 17.5 Hz, 1H), 2.44 (s, 3H), 1.23 (s, 3H), 1.10 (s, 3H). 13C-NMR (75 MHz, CDCl3) δ 196.65, 165.16, 159.67, 156.72, 146.97, 136.16, 133.76, 129.19, 125.58, 121.79, 118.97, 108.31, 83.52, 55.54, 49.81, 43.32, 25.19, 24.72, 18.37. HR-MS (ESI) calcd. for C19H20O5 (M + H)+ 329.13835, measured 329.13821.
Acetylenic 6′-OMe-iso-PhABA 24c (yield 85% over two steps) as a yellow solid. 1H-NMR (300 MHz, CDCl3) δ 7.95 (d, J = 8.7 Hz, 1H), 7.47 (s, 1H), 6.87 (dd, J = 8.7, 2.1 Hz, 1H), 6.05 (s, 1H), 3.87 (s, 3H), 2.82 (d, J = 24.2 Hz, 4H), 2.07 (s, 3H), 1.17 (d, J = 8.6 Hz, 6H). 13C-NMR (75 MHz, CDCl3) δ 196.15, 164.44, 146.40, 129.49, 124.76, 124.70, 123.71, 114.57, 111.89, 111.83, 101.62, 86.20, 74.83, 55.61, 48.52, 41.64, 29.68, 25.12, 24.84. HR-MS (ESI) calcd. for C19H20O5 (M + H)+ 329.13835, measured 329.13828.
Acetylenic 6′-Br-iso-PhABA 24d (yield 79% over two steps) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 8.13 (s, 1H), 7.84 (d, J = 8.3 Hz, 1H), 7.54 (dd, J = 8.3, 1.9 Hz, 1H), 6.05 (d, J = 1.8 Hz, 1H), 2.79 (s, 2H), 2.08 (s, 3H), 1.17 (d, J = 17.4 Hz, 6H). 13C-NMR (75 MHz, CDCl3) δ 195.72, 168.33, 144.62, 135.17, 130.93, 129.60, 128.65, 127.87, 127.63, 123.85, 99.99, 85.67, 73.33, 47.61, 40.80, 24.13, 23.75, 21.66. HR-MS (ESI) calcd. for C18H17BrO4 (M + H)+ 394.06485, measured 394.06485.
Acetylenic iso-PyABA 25a (yield 77% over two steps) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 8.74 (dd, J = 4.8, 1.8 Hz, 1H), 8.32 (dd, J = 7.8, 1.8 Hz, 1H), 7.45 (dd, J = 7.8, 4.8 Hz, 1H), 5.84 (s, 1H), 5.60 (s, 1H), 5.07 (s, 1H), 2.73 (d, J = 17.4 Hz, 1H), 2.63 (d, J = 17.1 Hz, 1H), 2.15 (s, 3H), 1.28 (s, 3H), 1.05 (s, 3H). 13C-NMR (75 MHz, CDCl3) δ 195.96, 167.91, 162.11, 155.35, 153.02, 149.76, 134.68, 126.88, 123.78, 116.39, 113.15, 77.20, 49.64, 41.50, 24.31, 22.68, 11.95. HR-MS (ESI) calcd. for C17H17NO4 (M + H)+ 300.1230, measured 300.1230.
Acetylenic 2′-Me-iso-PyABA 25b (yield 90% over two steps) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 8.20 (d, J = 8.0 Hz, 1H), 7.27 (d, J = 7.7 Hz, 3H), 5.84 (s, 1H), 5.57 (s, 1H), 5.24 (s, 1H), 2.71 (d, J = 16.9 Hz, 1H), 2.62 (s, 3H), 2.56 (d, J = 17.5 Hz, 1H), 2.13 (s, 3H), 1.28 (s, 3H), 1.03 (s, 3H). 13C-NMR (75 MHz, CDCl3) δ 195.83, 168.11, 163.25, 161.47, 155.39, 149.63, 134.89, 124.46, 123.47, 116.33, 113.37, 76.91, 49.68, 41.47, 24.81, 24.31, 22.58, 11.93. HR-MS (ESI) calcd. for C18H19NO4 (M + H)+ 314.13868, measured 314.13858.
Acetylenic 2′-Ph-iso-PyABA 25d (yield 75% over two steps) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 8.35 (d, J = 8.2 Hz, 1H), 8.11 (dd, J = 7.4, 2.0 Hz, 2H), 7.85 (d, J = 8.2 Hz, 1H), 7.63–7.40 (m, 3H), 5.97 (s, 1H), 5.59 (s, 1H), 3.38 (d, J = 17.6 Hz, 1H), 2.59 (d, J = 17.7 Hz, 1H), 2.01 (s, 3H), 1.47 (s, 3H), 0.99 (s, 3H). 13C-NMR (75 MHz, CDCl3) δ 195.97, 168.91, 160.65, 160.24, 137.27, 136.37, 136.16, 130.41, 128.83, 127.44, 124.34, 123.67, 120.23, 101.71, 85.06, 74.04, 50.09, 41.24, 25.03, 24.50, 20.99. HR-MS (ESI) calcd. for C23H21NO4 (M + H)+ 376.15433, measured 376.15414.
Acetylenic iso-FrABA 26 (yield 89% over two steps) as a yellow oil. 1H-NMR (300 MHz, CDCl3) δ 7.46 (d, J = 1.7 Hz, 1H), 6.67 (d, J = 1.7 Hz, 1H), 6.10 (s, 1H), 5.78 (s, 2H), 2.75 (d, J = 16.9 Hz, 1H), 2.58 (d, J = 16.5 Hz, 1H), 2.08 (s, 3H), 1.23 (d, J = 6.1 Hz, 6H). 13C-NMR (75 MHz, CDCl3) δ 193.42, 163.13, 144.19, 141.58, 132.98, 127.89, 119.68, 106.58, 97.64, 86.13, 70.69, 49.85, 44.28, 24.91, 24.62, 22.42. HR-MS (ESI) calcd. for C16H16O5 (M + H)+ 289.1071, measured 289.1073.