Sulfonic Acid Functionalization of Different Zeolites and Their Use as Catalysts in the Microwave-Assisted Etherification of Glycerol with tert-Butyl Alcohol
Abstract
:1. Introduction
2. Results and Discussion
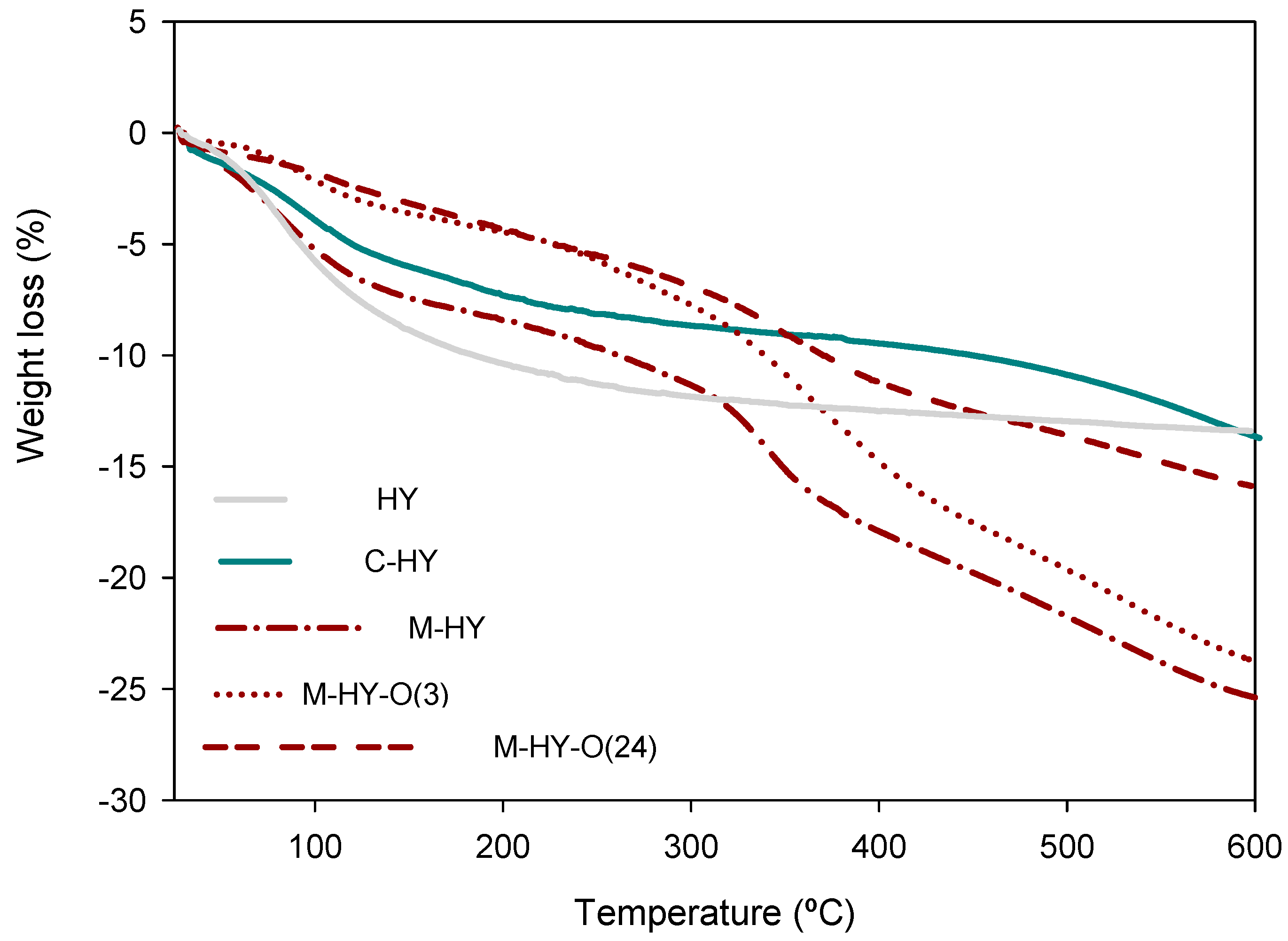
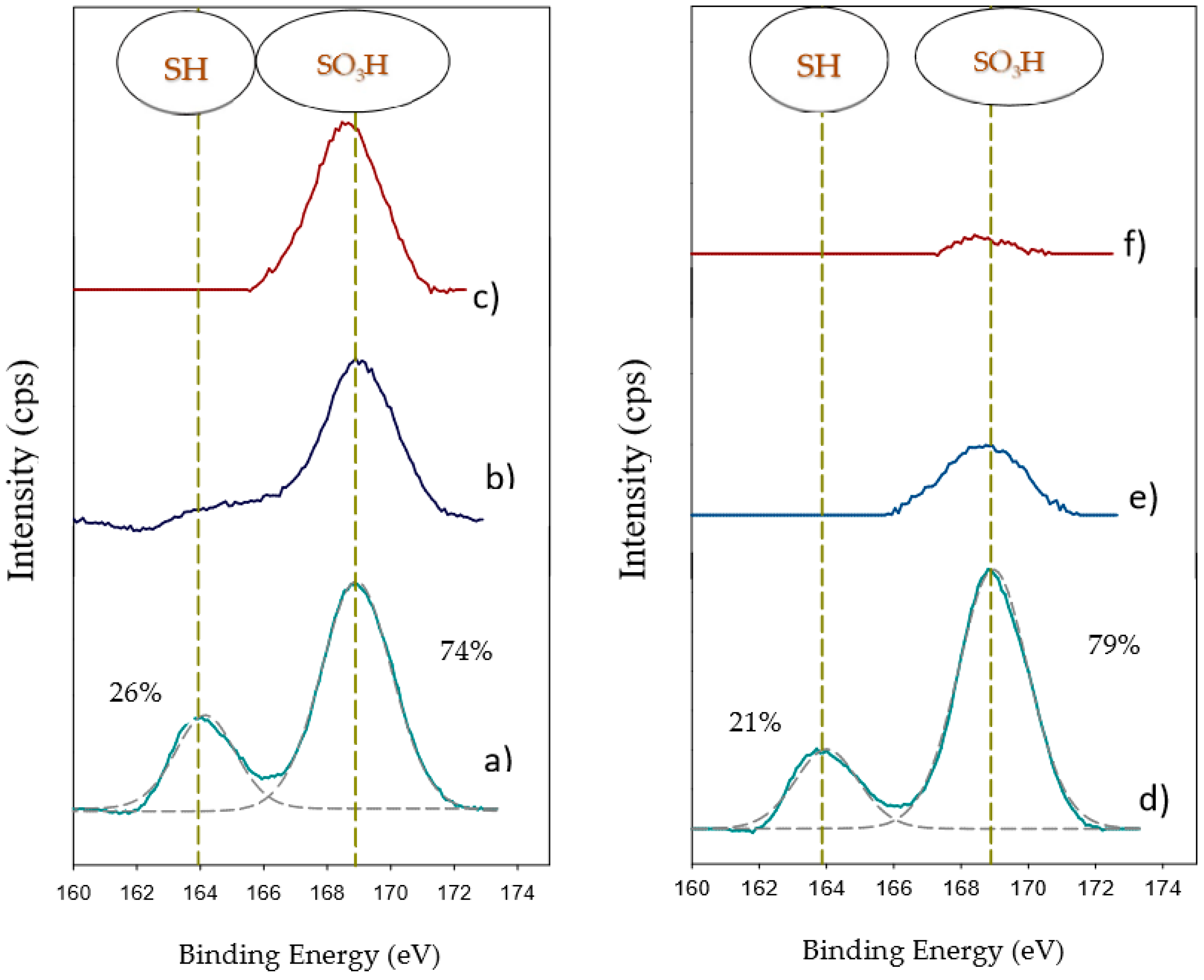
2.1. Characterization of Catalysts
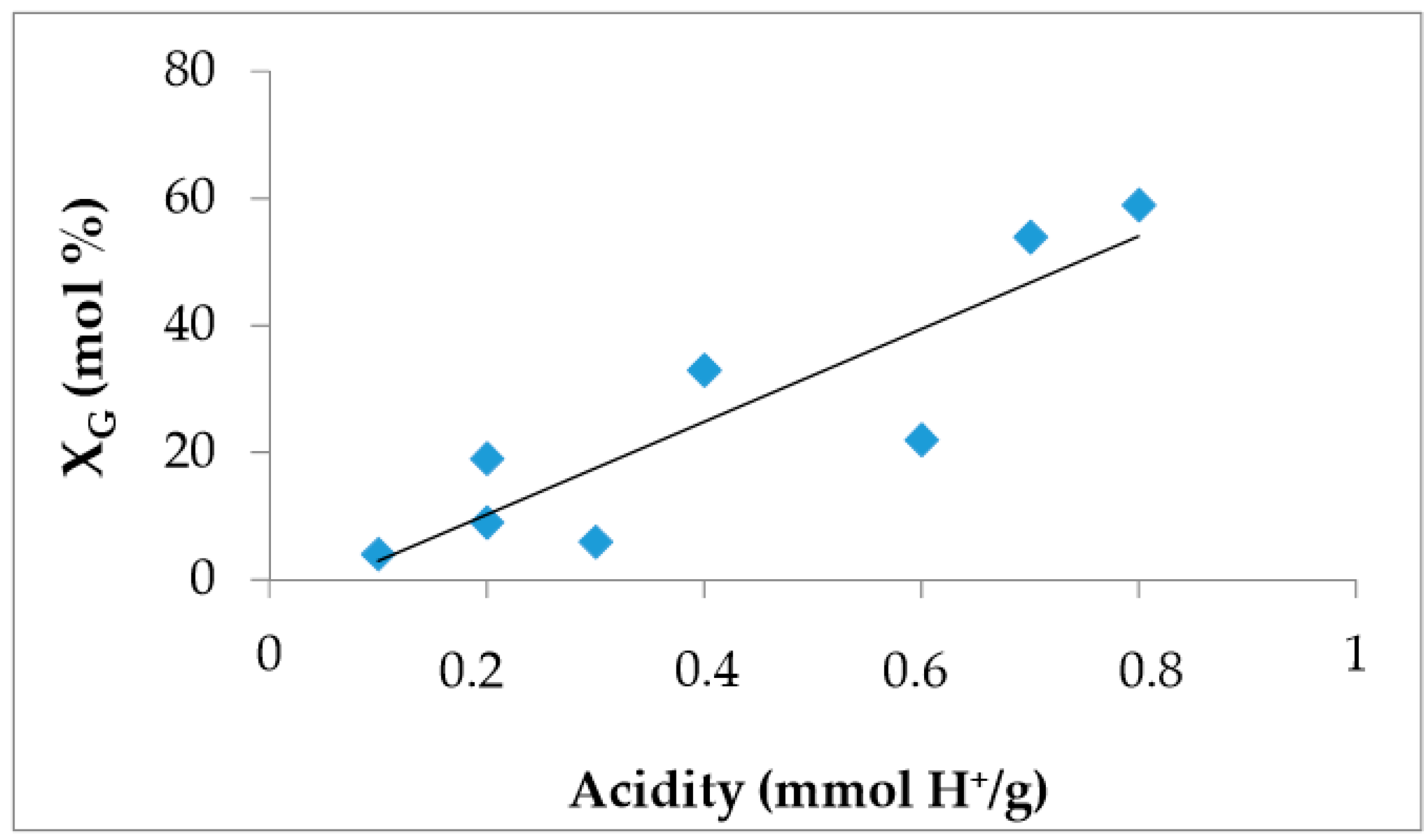
2.2. Determination of the Solid Acidity
2.3. Microwave-Assisted Etherification of Glycerol
3. Materials and Methods
3.1. Synthesis of Catalysts
3.2. Characterization of Catalysts
3.3. Microwave-Assisted Etherification of Glycerol
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Quispe, C.A.; Coronado, C.J.; Carvalho, J.A., Jr. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved utilisation of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; della Pina, C. From Glycerol to Value-Added Products. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Kesling, H.S., Jr.; Karas, L.J.; Liotta, F.J., Jr. Diesel Fuel. U.S. Patent 5,308,365, 3 May 1994. [Google Scholar]
- Klepáčová, K.; Mravec, D.; Bajus, M. Tert-Butylation of glycerol catalysed by ion-exchange resins. Appl. Catal. A-Gen. 2005, 294, 141–147. [Google Scholar] [CrossRef]
- Klepáčová, K.; Mravec, D.; Kaszonyi, A.; Bajus, M. Etherification of glycerol and ethylene glycol by isobutylene. Appl. Catal. A-Gen. 2007, 328, 1–13. [Google Scholar] [CrossRef]
- Pico, M.P.; Rosas, J.M.; Rodríguez, S.; Santos, A.; Romero, A. Glycerol etherification over acid ion exchange resins: Effect of catalyst concentration and reusability. J. Chem. Technol. Biotechnol. 2013, 88, 2027–2038. [Google Scholar] [CrossRef]
- Celdeira, P.A.; Goncalves, M.; Figueiredo, F.C.; Bosco, S.M.D.; Mandelli, D.; Carvalho, W.A. Sulfonated niobia and pillared clay as catalysts in etherification reaction of glycerol. Appl. Catal. A-Gen. 2014, 478, 98–106. [Google Scholar] [CrossRef]
- Gonçalves, M.; Soler, F.C.; Isoda, N.; Carvalho, W.A.; Mandelli, D.; Sepúlveda, J. Glycerol conversion into value-added products in presence of a green recyclable catalyst: Acid black carbon obtained from coffee ground wastes. J. Taiwan Inst. Chem. Eng. 2016, 60, 294–301. [Google Scholar] [CrossRef]
- Galhardo, T.S.; Simone, N.; Gonçalves, M.; Figueiredo, F.C.; Mandelli, D.; Carvalho, W.A. Preparation of sulfonated carbons from rice husk and their application in catalytic conversion of glycerol. ACS Sustain. Chem. Eng. 2013, 1, 1381–1389. [Google Scholar] [CrossRef]
- Frusteri, F.; Arena, F.; Bonura, G.; Cannilla, C.; Spadaro, L.; di Blasi, O. Catalytic etherification of glycerol by tert-butyl alcohol to produce oxygenated additives for diesel fuel. Appl. Catal. A-Gen. 2009, 367, 77–83. [Google Scholar] [CrossRef]
- Cannilla, C.; Bonura, G.; Frusteri, L.; Frusteri, F. Glycerol Etherification with TBA: High Yield to Poly-Ethers Using a Membrane Assisted Batch Reactor. Environ. Sci. Technol. 2014, 48, 6019–6026. [Google Scholar] [CrossRef] [PubMed]
- Estevez, R.; López, M.; Jiménez-Sanchidrián, C.; Luna, D.; Romero-Salguero, F.; Bautista, F. Etherification of glycerol with tert-butyl alcohol over sulfonated hybrid silicas. Appl. Catal. A-Gen. 2016, 526, 155–163. [Google Scholar] [CrossRef]
- Estevez, R.; Lopez-Pedrajas, S.; Luna, D.; Bautista, F. Microwave-assisted etherification of glycerol with tert-butyl alcohol over amorphous organosilica-aluminum phosphates. Appl. Catal. B-Environ. 2017, 213, 42–52. [Google Scholar] [CrossRef]
- Xiao, L.; Mao, J.; Zhou, J.; Guo, X.; Zhang, S. Enhanced performance of HY zeolites by acid wash for glycerol etherification with isobutene. Appl. Catal. A-Gen. 2011, 393, 88–95. [Google Scholar] [CrossRef]
- González, M.D.; Salagre, P.; Taboada, E.; Llorca, J.; Cesteros, Y. Microwave-assisted synthesis of sulfonic acid-functionalized microporous materials for the catalytic etherification of glycerol with isobutene. Green Chem. 2013, 15, 2230–2239. [Google Scholar] [CrossRef]
- González, M.D.; Cesteros, Y.; Llorca, J.; Salagre, P. Boosted selectivity toward high glycerol tertiary butyl ethers by microwave-assisted sulfonic acid-functionalization of SBA-15 and beta zeolite. J. Catal. 2012, 290, 202–209. [Google Scholar] [CrossRef]
- González, M.D.; Cesteros, Y.; Salagre, P. Establishing the role of Brønsted acidity and porosity for the catalytic etherification of glycerol with tert-butanol by modifying zeolites. Appl. Catal. A-Gen. 2013, 450, 178–188. [Google Scholar] [CrossRef]
- Felice, V.; Ntais, S.; Tavares, A.C. Propyl sulfonic acid functionalization of faujasite-type zeolites: Effect on water and methanol sorption and on proton conductivity. Microporous Mesoporous Mater. 2013, 169, 128–136. [Google Scholar] [CrossRef]
- Yu, N.; Zheng, J.; Tang, Q.; Wu, D.; Sun, Y.; Hu, W.; Liu, W.; Deng, F. Preparation of hydrothermally stable mercapto-functionalized mesoporous silicas via assembly of nanoclustered zeolite Y seeds and 3-mercaptopropyltrimethoxysilane. Stud. Surf. Sci. Catal. 2005, 156, 213–220. [Google Scholar]
- González, M.D.; Cesteros, Y.; Salagre, P. Comparison of dealumination of zeolites beta, mordenite and ZSM-5 by treatment with acid under microwave irradiation. Microporous Mesoporous Mater. 2011, 144, 162–170. [Google Scholar] [CrossRef]
- Müller, M.; Harvey, G.; Prins, R. Comparison of the dealumination of zeolites beta, mordenite, ZSM-5 and ferrierite by thermal treatment, leaching with oxalic acid and treatment with SiCl 4 by 1 H, 29 Si and 27 Al MAS NMR. Microporous Mesoporous Mater. 2000, 34, 135–147. [Google Scholar] [CrossRef]
- Melero, J.A.; Stucky, G.D.; van Grieken, R.; Morales, G. Direct syntheses of ordered SBA-15 mesoporous materials containing arenesulfonic acid groups. J. Mater. Chem. 2002, 12, 1664–1670. [Google Scholar] [CrossRef]
- González, M.D.; Salagre, P.; Taboada, E.; Llorca, J.; Molins, E.; Cesteros, Y. Sulfonic acid-functionalized aerogels as high resistant to deactivation catalysts for the etherification of glycerol with isobutene. Appl. Catal. B-Environ. 2013, 136, 287–293. [Google Scholar] [CrossRef]
- Jamróz, M.E.; Jarosz, M.; Witowska-Jarosz, J.; Bednarek, E.; Tęcza, W.; Jamróz, M.H.; Dobrowolski, J.C.; Kijeński, J. Mono-, di-, and tri-tert-butyl ethers of glycerol: A molecular spectroscopic study. Spectrochim. Acta Part A 2007, 67, 980–988. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
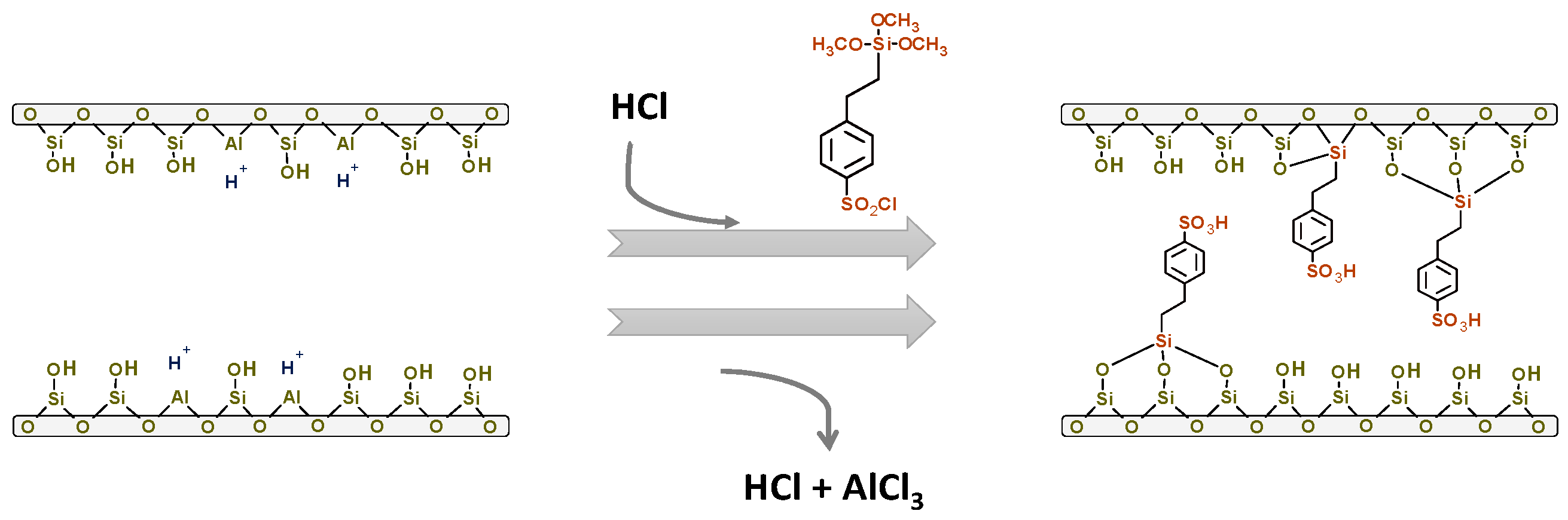
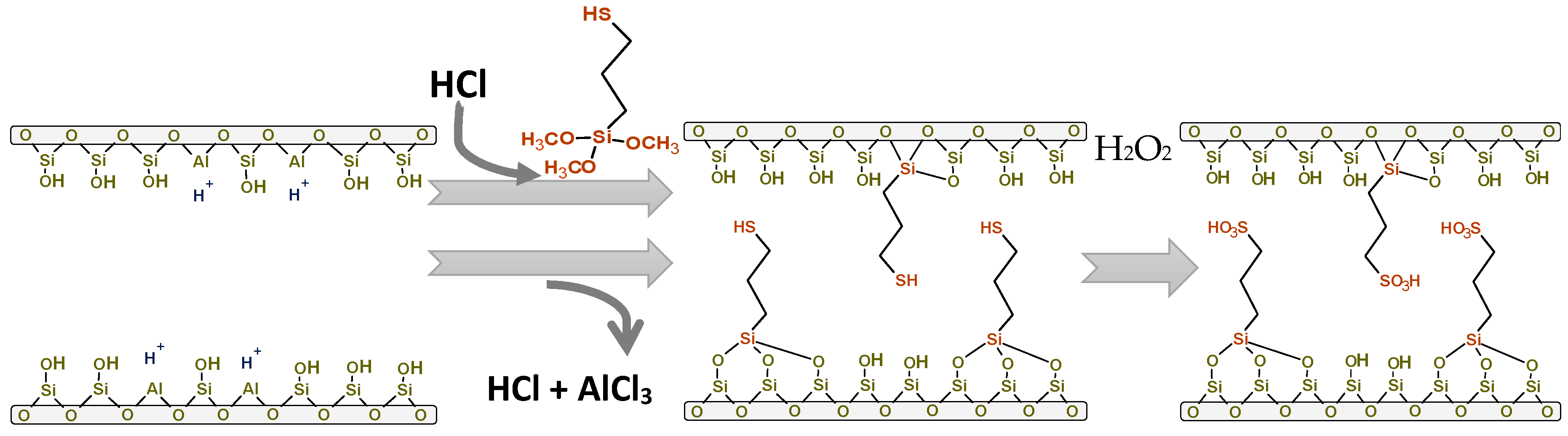
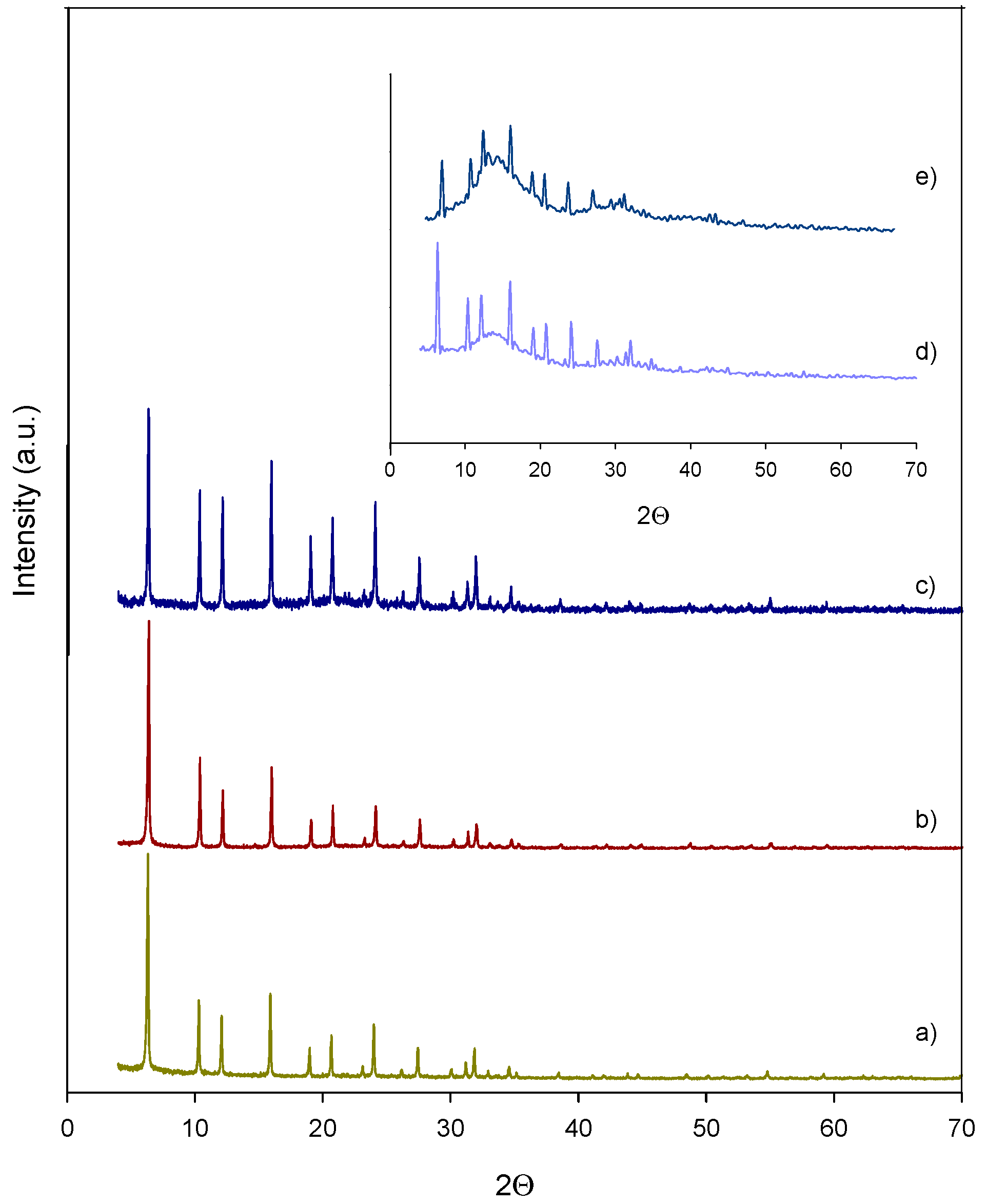

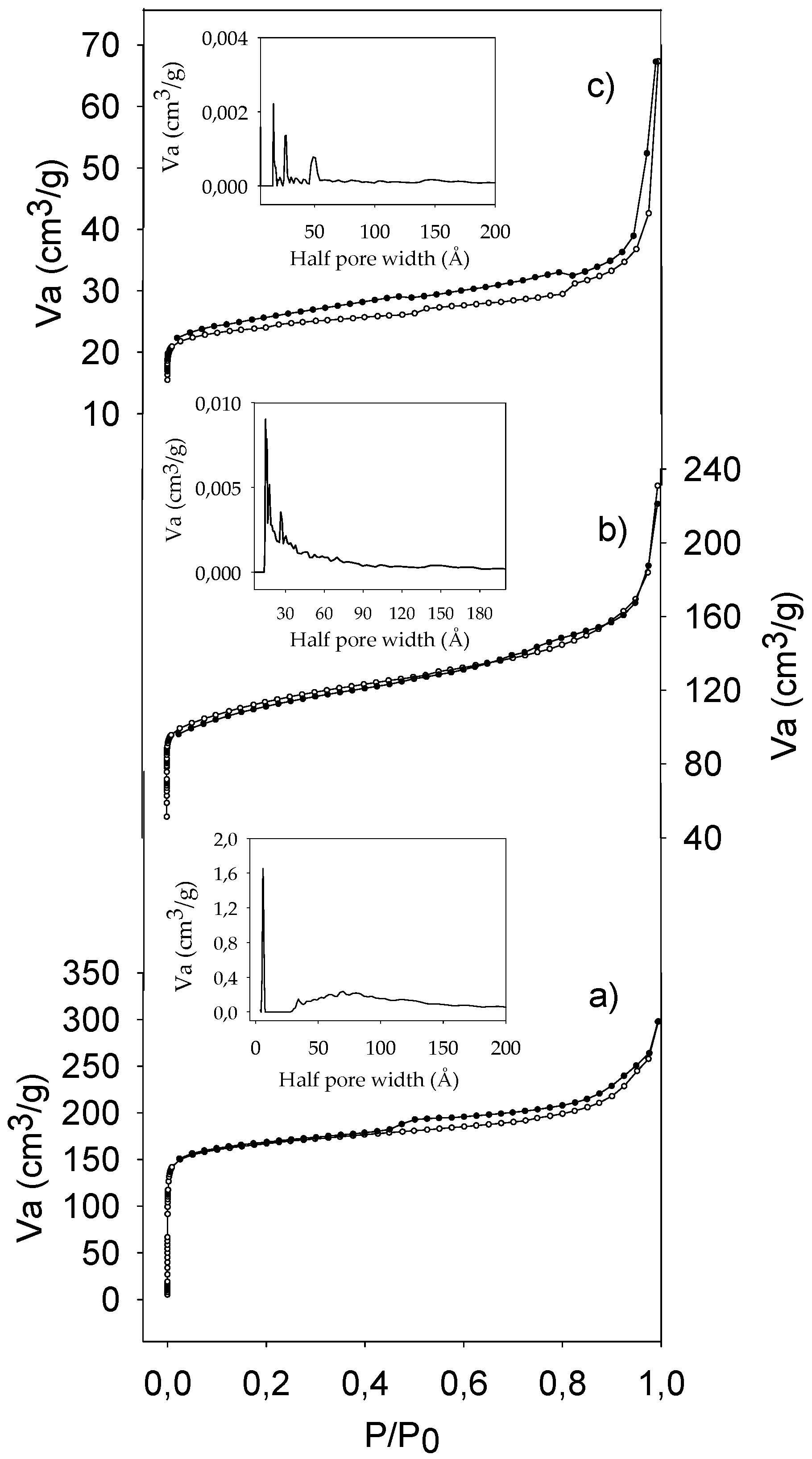
| Catalyst | SBET (m2/g) | Sext (m2/g) | Smicrop (m2/g) | VP (cm3/g) | Meso (%) | Micro (%) | NaOH Consumed (mL) | Acidity (mmol H+/g) | Acidity (mmol H+/g) b |
|---|---|---|---|---|---|---|---|---|---|
| HY a | 545 | 54 | 491 | 0.36 | 44 | 56 | 0.30 | 0.2 | − |
| C-HY | 597 | 125 | 472 | 0.27 | 35 | 65 | 0.80 | 0.4 | 0.3 |
| M-HY | 241 | 56 | 185 | 0.16 | 51 | 49 | 0.35 | 0.2 | − |
| M-HY-O(3) | 246 | 76 | 170 | 0.17 | 69 | 31 | 1.60 | 0.8 | 0.6 |
| M-HY-O(24) | 374 | 77 | 297 | 0.21 | 34 | 66 | 1.15 | 0.6 | 0.4 |
| HZSM-5 a | 349 | 81 | 268 | 0.27 | 44 | 56 | 0.20 | 0.1 | − |
| C-HZSM-5 | 337 | 131 | 206 | 0.29 | 35 | 65 | 0.35 | 0.2 | 0.1 |
| M-HZSM-5 | 60 | 24 | 36 | 0.15 | 60 | 40 | 0.15 | 0.1 | − |
| M-HZSM-5-O(3) | 127 | 50 | 77 | 0.10 | 65 | 35 | 1.26 | 0.7 | 0.5 |
| M-HZSM-5-O(24) | 193 | 68 | 125 | 0.20 | 55 | 45 | 0.50 | 0.3 | 0.1 |
| Catalizador | XG (%) | SMTBG (%) | Sh-GTBE (%) |
|---|---|---|---|
| HY | 9 | 87 | 13 |
| C-HY | 33 | 75 | 25 |
| M-HY | − | − | − |
| M-HY-O(3) | 59 | 78 | 22 (2.1) a |
| M-HY-O(3) b | 30 | 80 | 20 (1.5) |
| M-HY-O(3) c | 53 | 81 | 19 (1.7) |
| M-HY-O(24) | 22 | 81 | 19 |
| HZSM-5 | 4 | 99 | 1 |
| C-HZSM-5 | 19 | 93 | 7 |
| M-HZSM-5 | − | − | − |
| M-HZSM-5-O(3) | 54 | 78 | 22 (1.5) |
| M-HZSM-5-O(24) | 6 | 95 |
| Catalyst | Temperature (°C) | Yh-GTBE (%) | STY (h−1) | References |
|---|---|---|---|---|
| C(10)AlPO(1.5)-250 | 85 | 21 | 152 | [15] |
| A-15 | 75 | 7 | 13 | [15] |
| C-HY | 75 | 8 | 174 | This work |
| C-HZSM-5 | 75 | 1 | 58 | This work |
| M-HY-O(3) | 75 | 12 | 149 | This work |
| M-HZSM5-O(3) | 75 | 12 | 130 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estevez, R.; Iglesias, I.; Luna, D.; Bautista, F.M. Sulfonic Acid Functionalization of Different Zeolites and Their Use as Catalysts in the Microwave-Assisted Etherification of Glycerol with tert-Butyl Alcohol. Molecules 2017, 22, 2206. https://doi.org/10.3390/molecules22122206
Estevez R, Iglesias I, Luna D, Bautista FM. Sulfonic Acid Functionalization of Different Zeolites and Their Use as Catalysts in the Microwave-Assisted Etherification of Glycerol with tert-Butyl Alcohol. Molecules. 2017; 22(12):2206. https://doi.org/10.3390/molecules22122206
Chicago/Turabian StyleEstevez, Rafael, Ivan Iglesias, Diego Luna, and Felipa M. Bautista. 2017. "Sulfonic Acid Functionalization of Different Zeolites and Their Use as Catalysts in the Microwave-Assisted Etherification of Glycerol with tert-Butyl Alcohol" Molecules 22, no. 12: 2206. https://doi.org/10.3390/molecules22122206







