Ferrocene and Transition Metal Bis(Dicarbollides) as Platform for Design of Rotatory Molecular Switches
Abstract
:1. Introduction
2. Ferrocene Based Molecular Switches
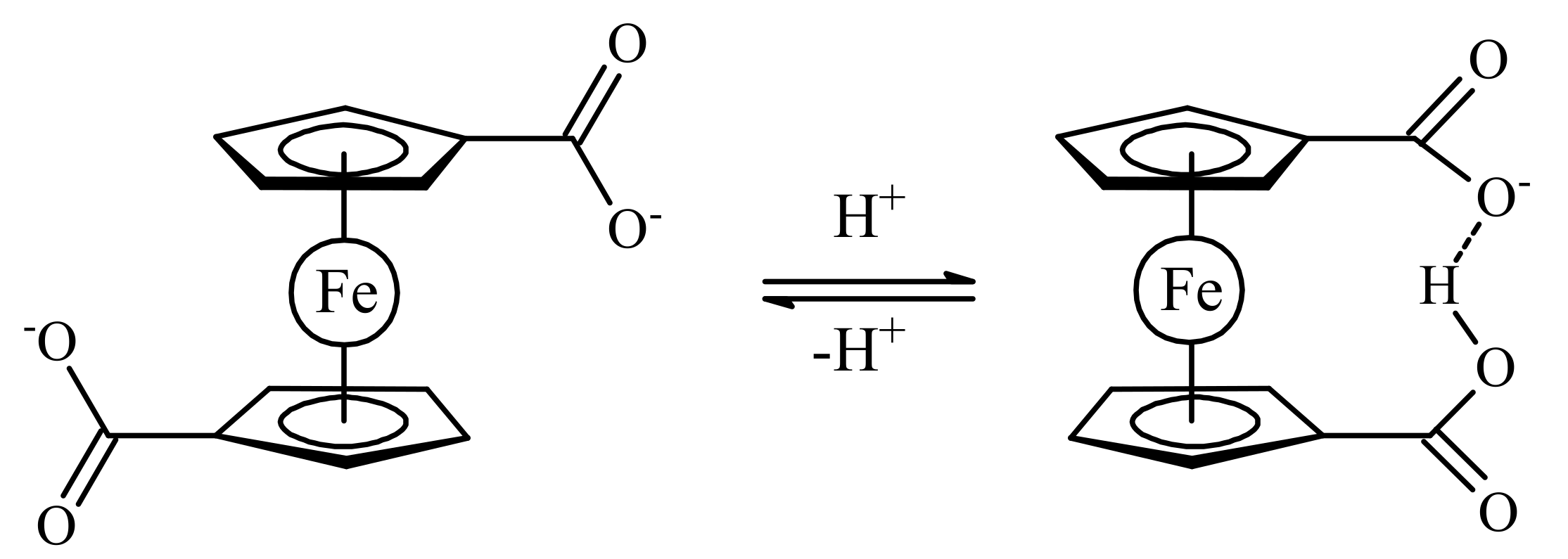
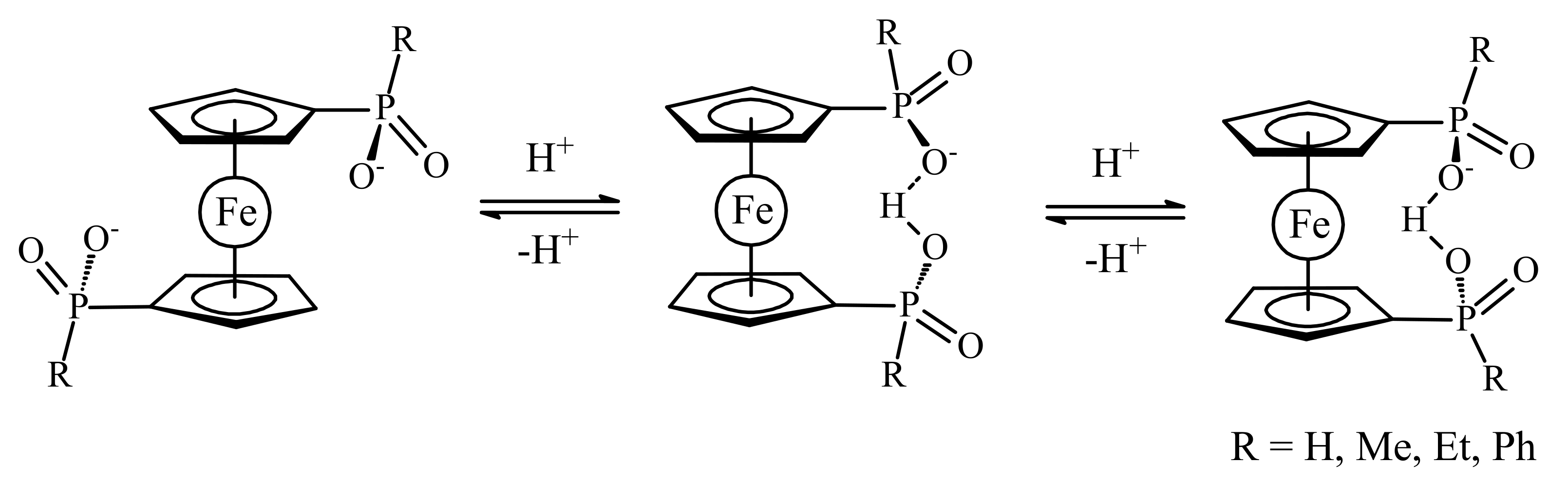
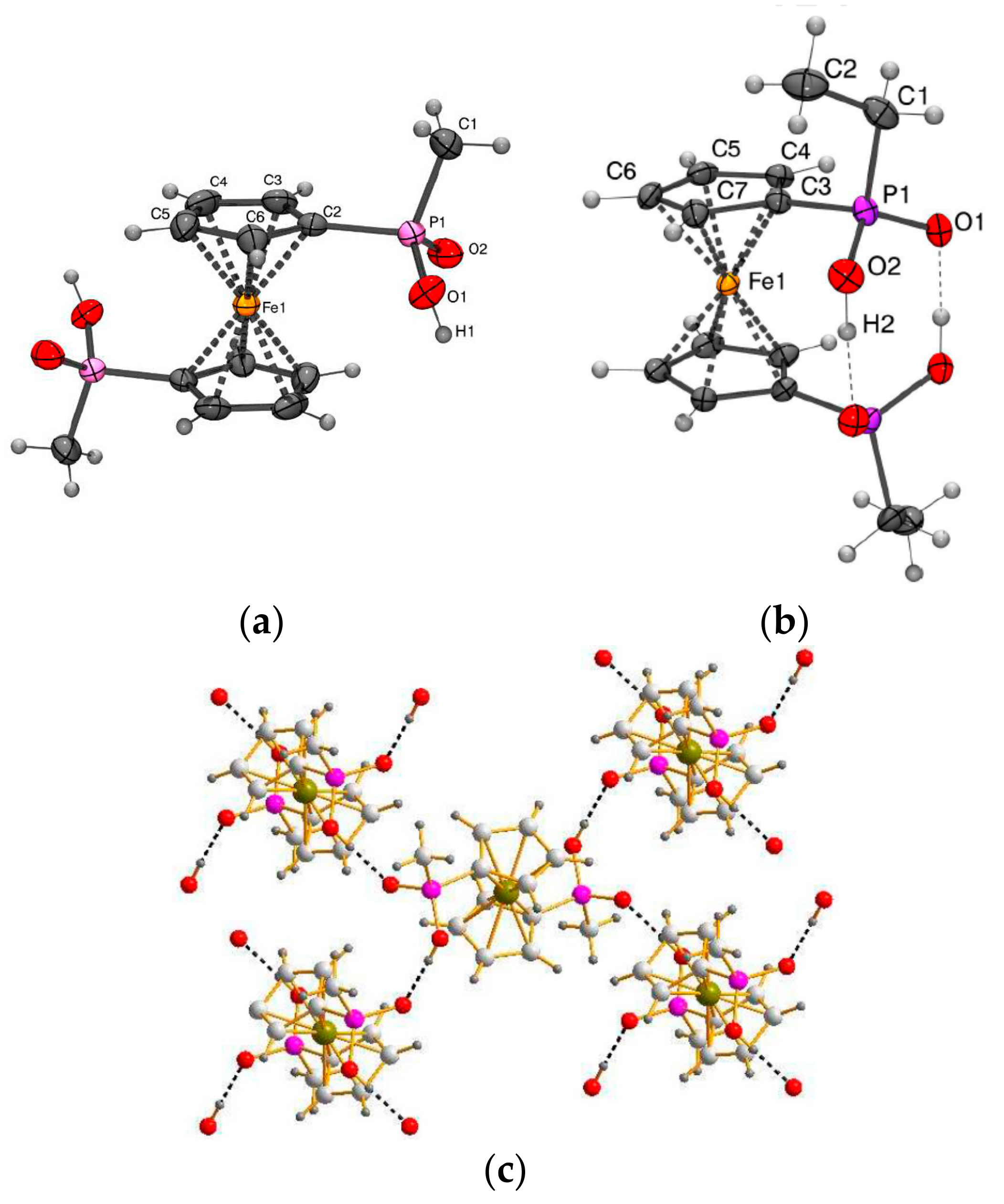
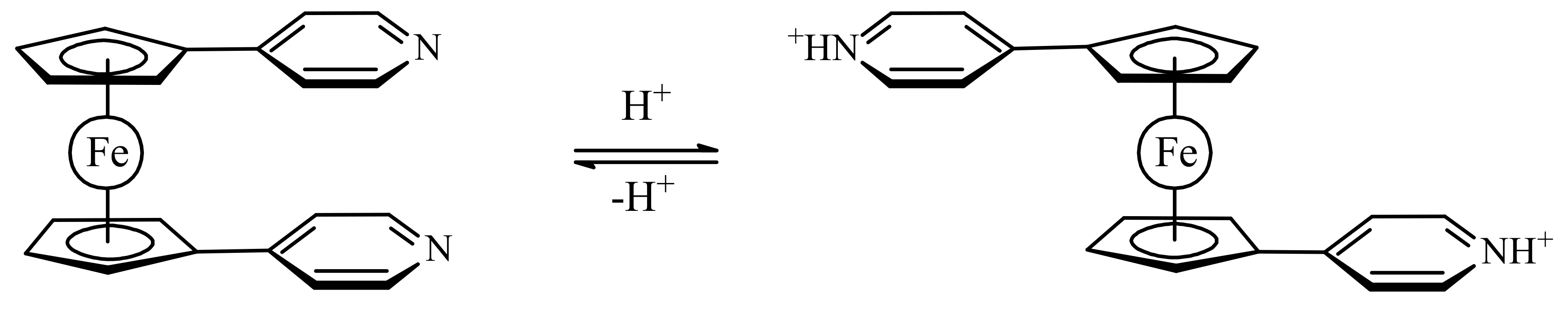
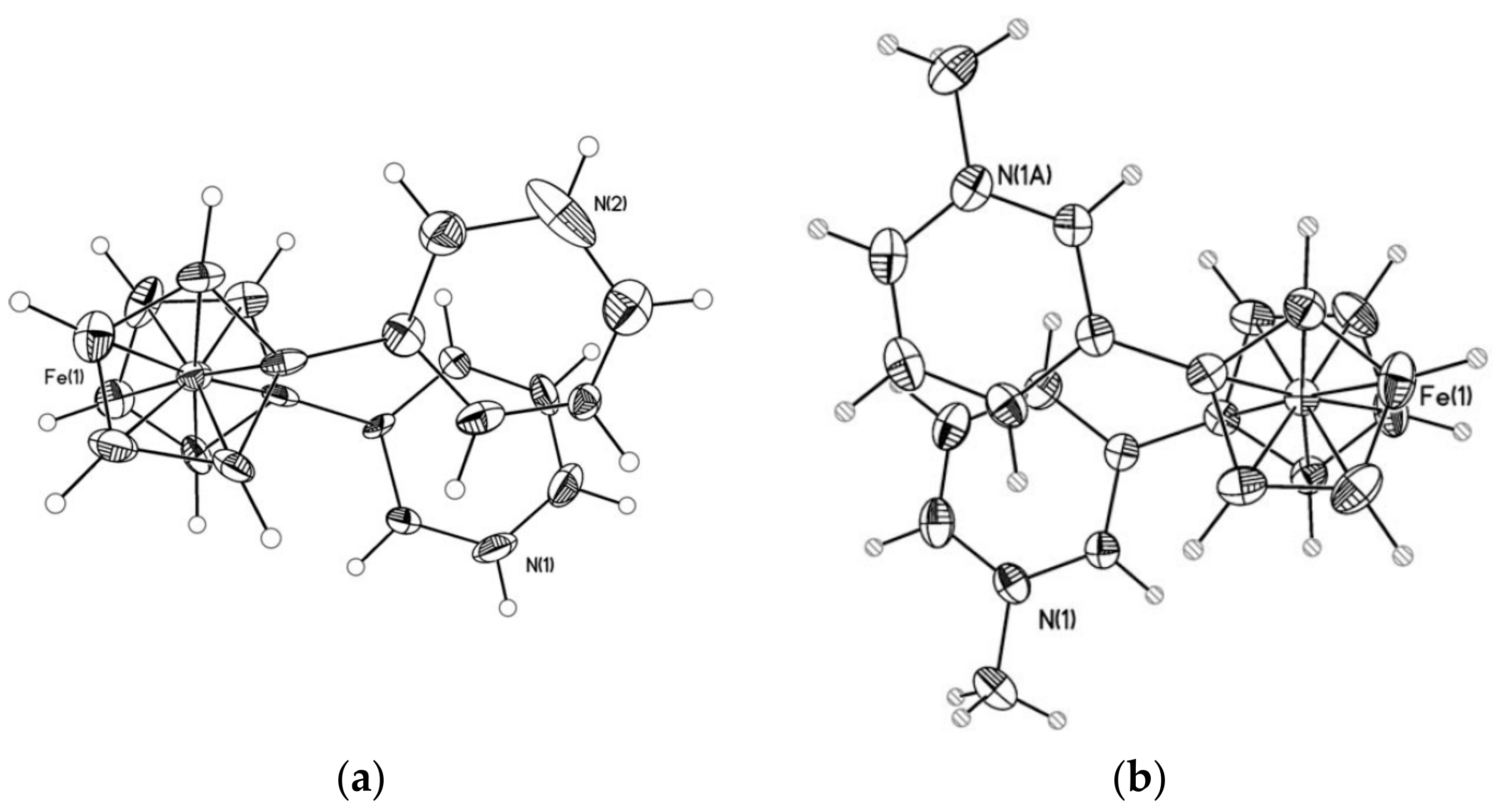
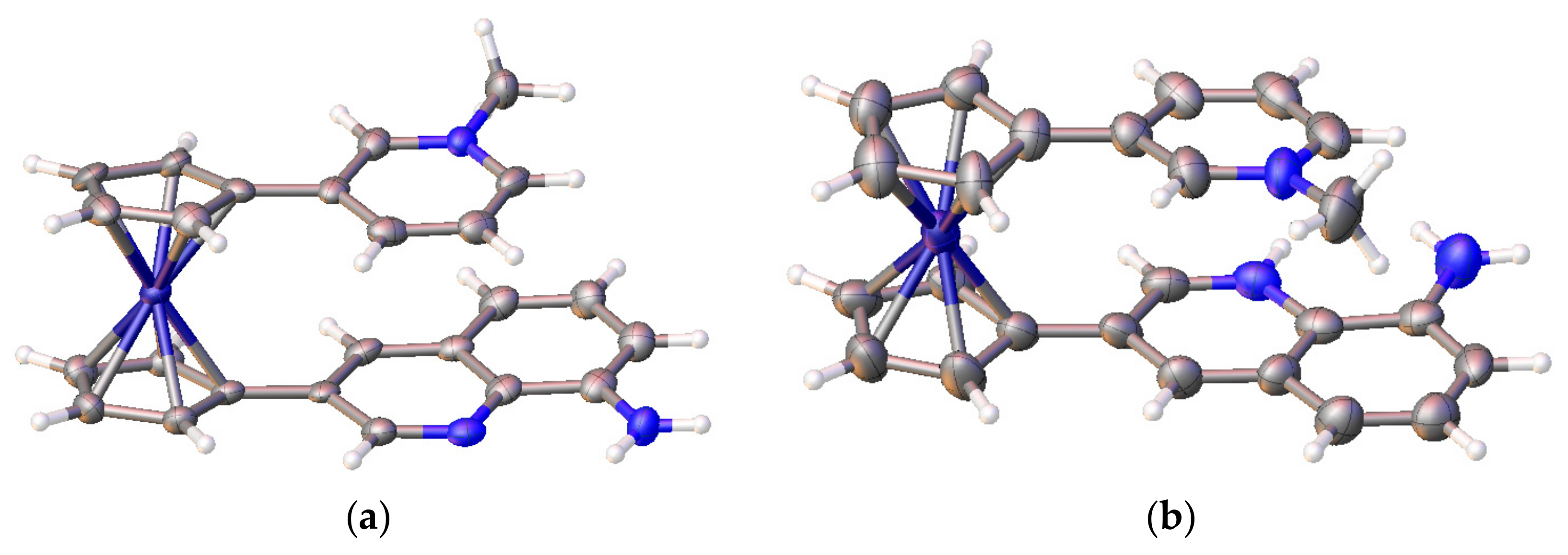
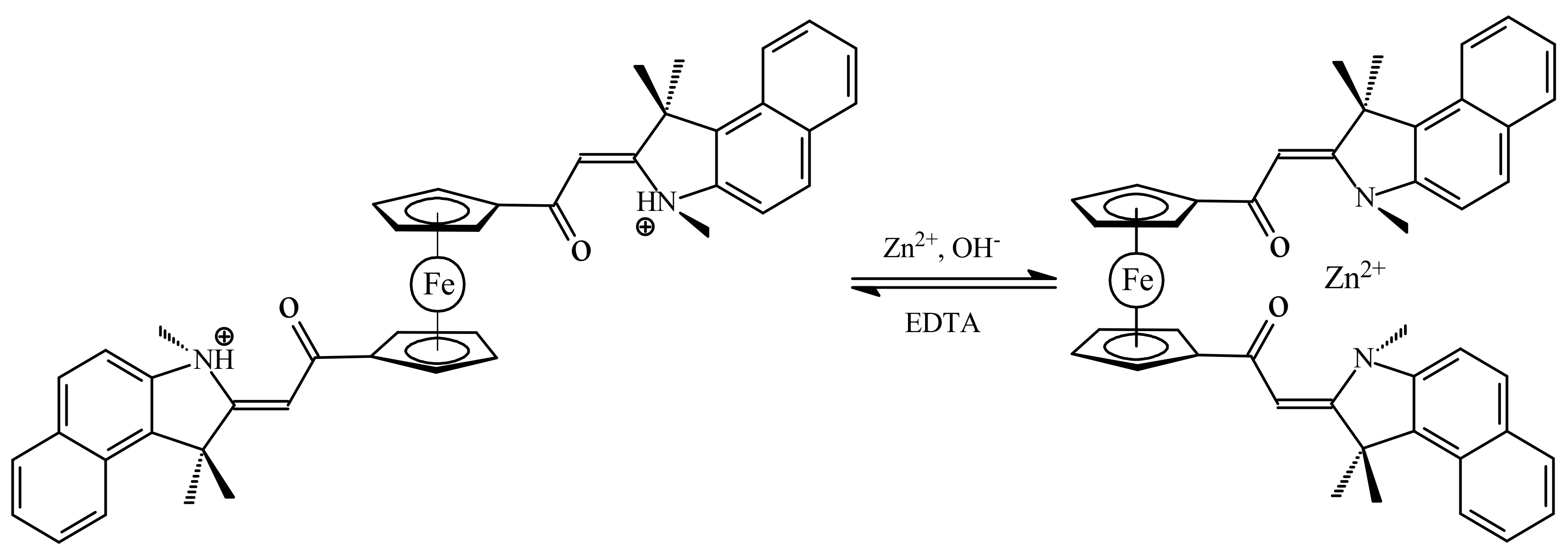
2.1. Protonation-Driven Molecular Switches
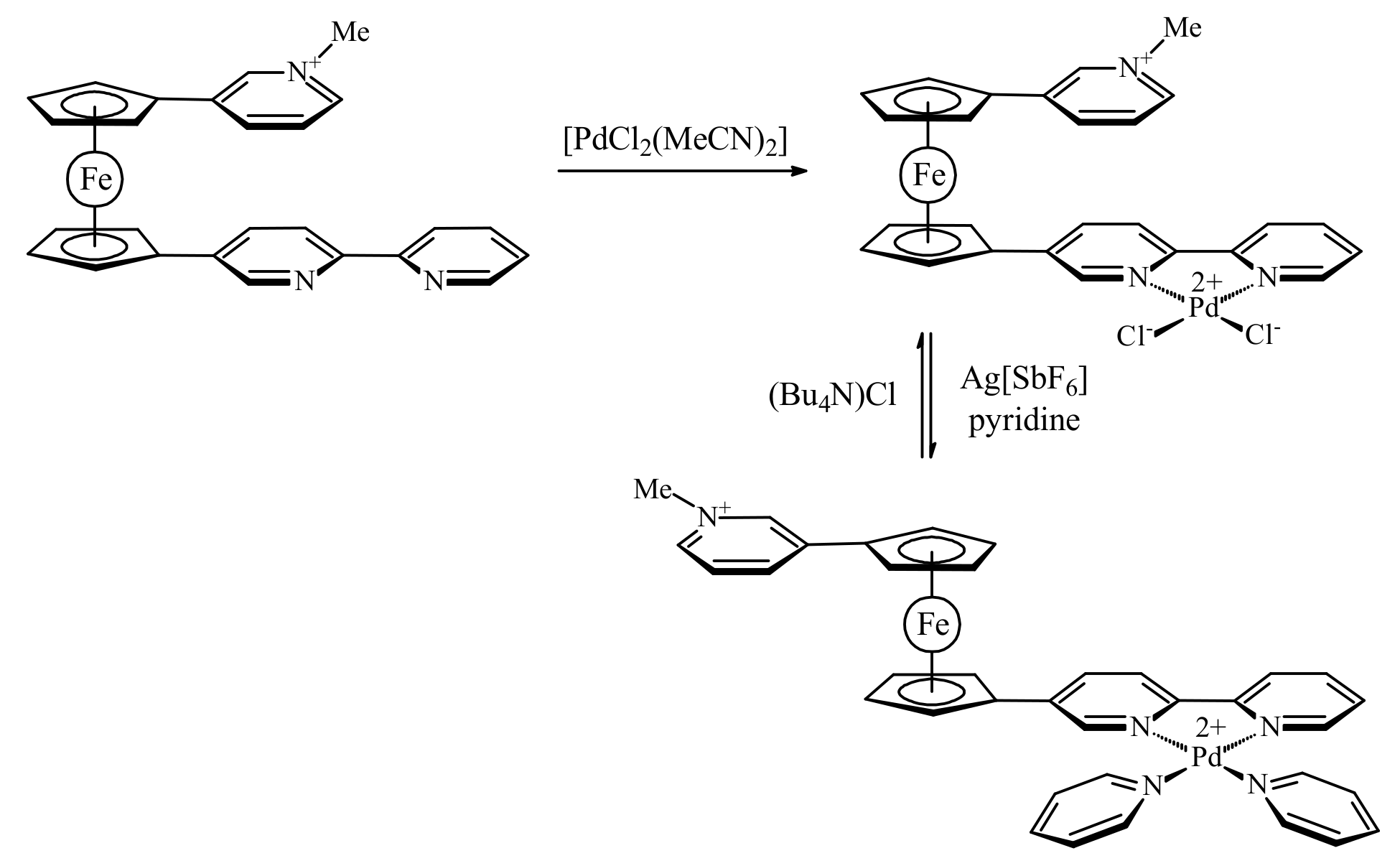
2.2. Complexation-Driven Molecular Switches
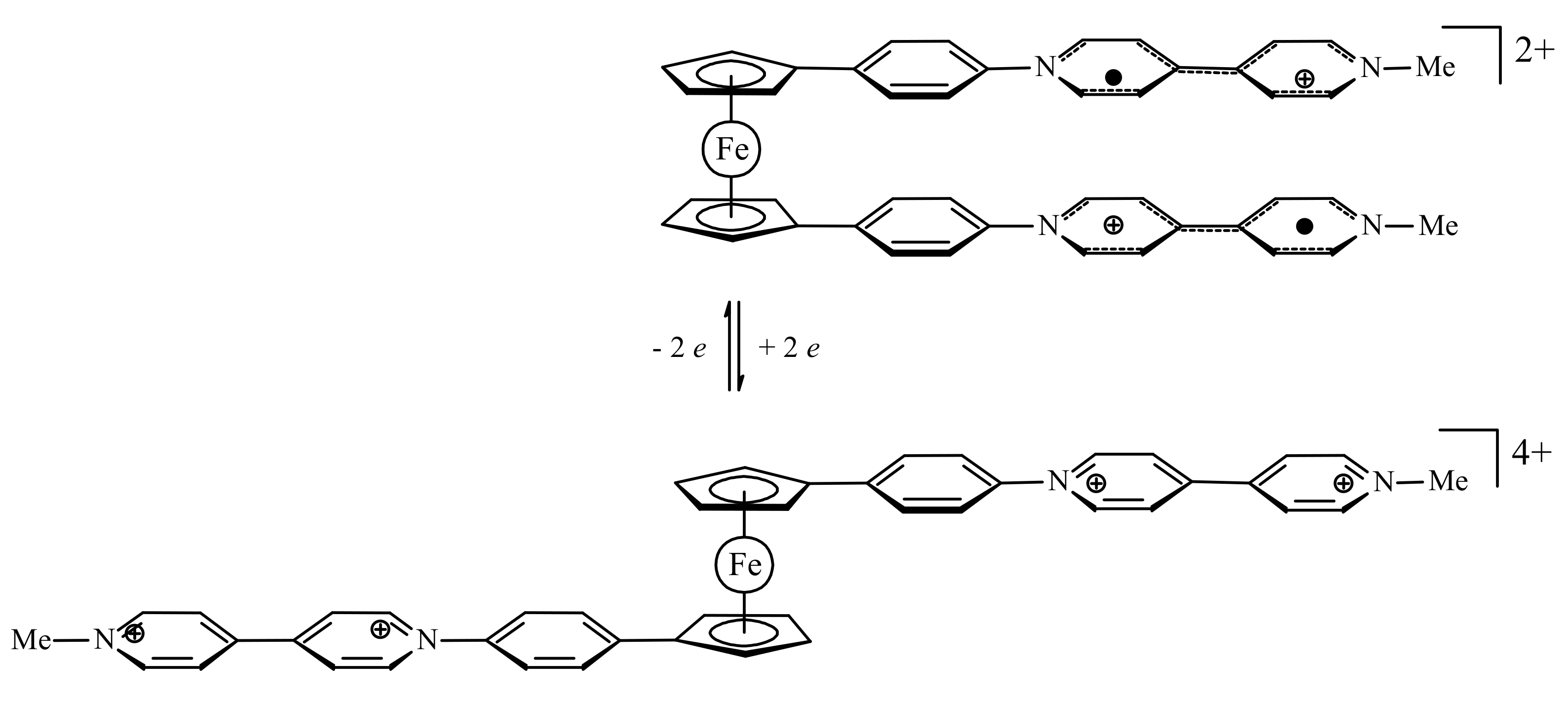
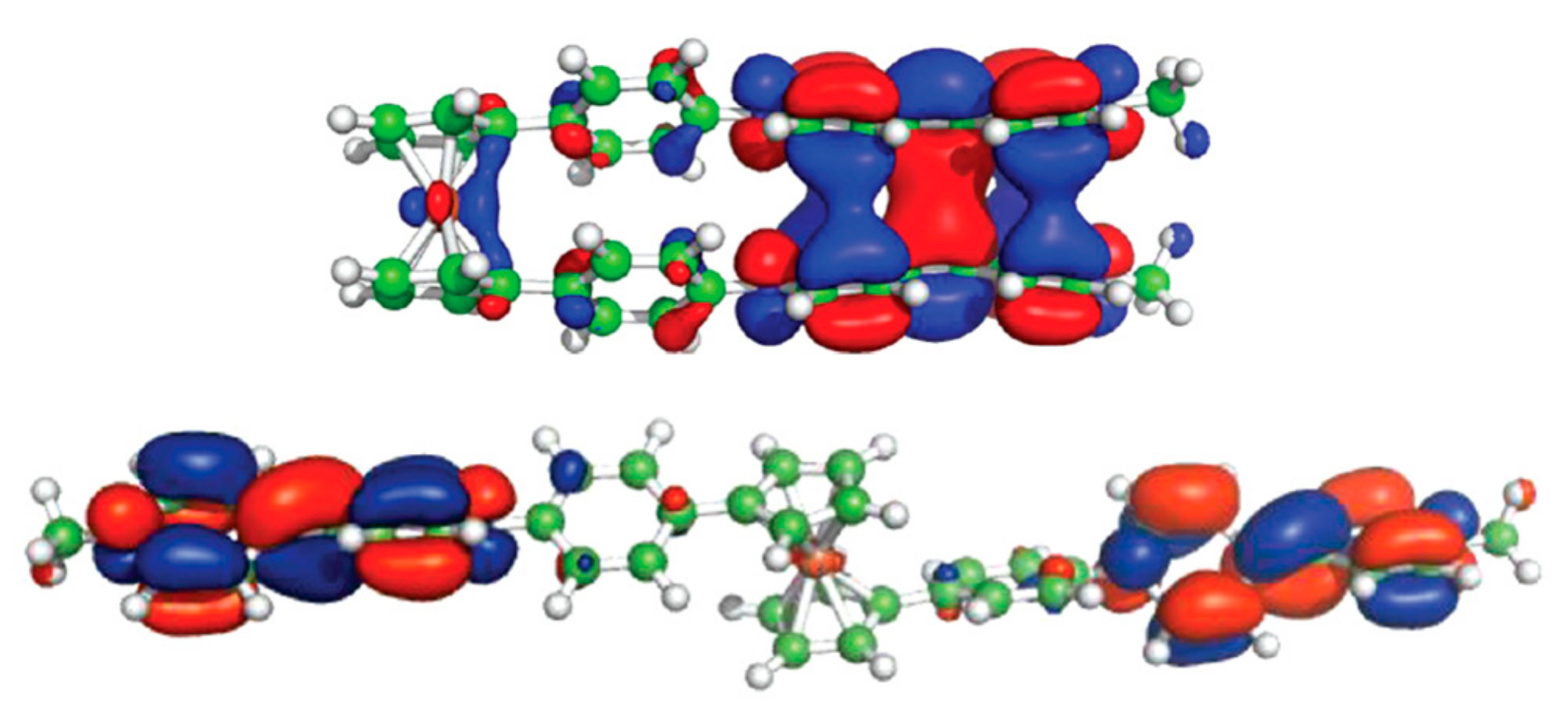
2.3. Electrochemically-Driven Molecular Switches
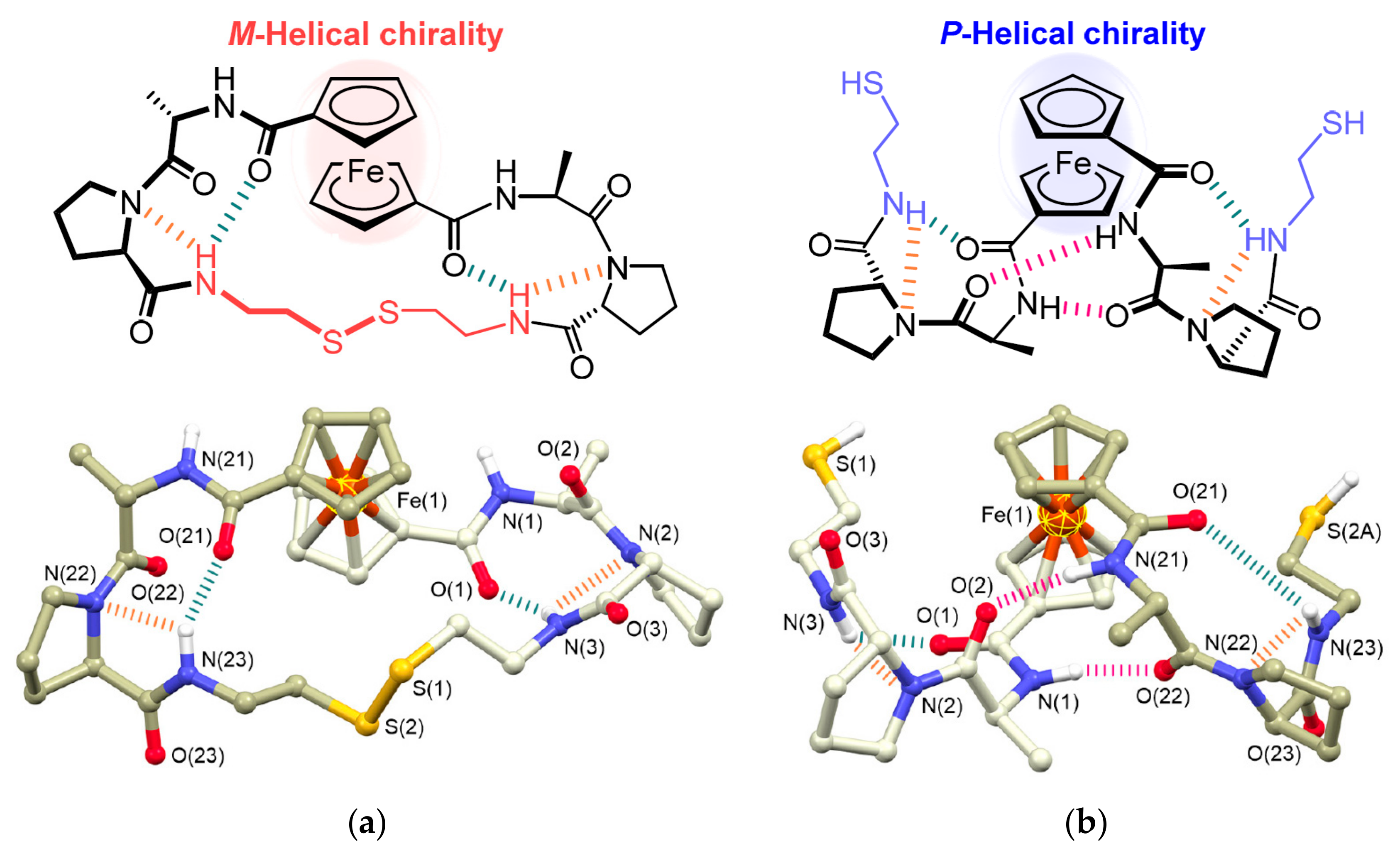
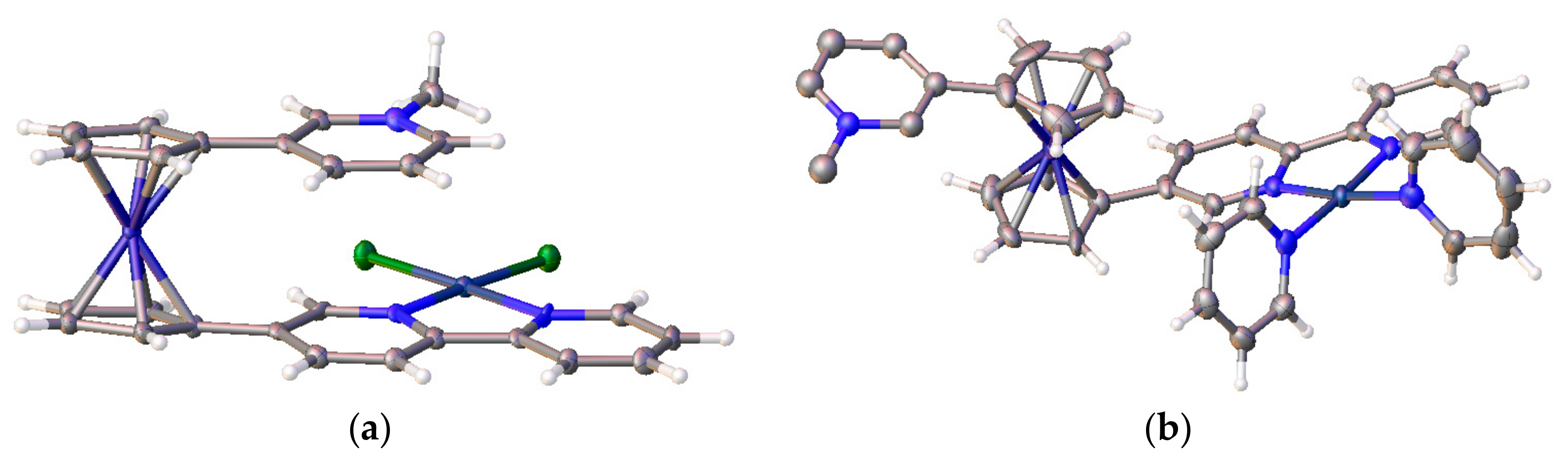
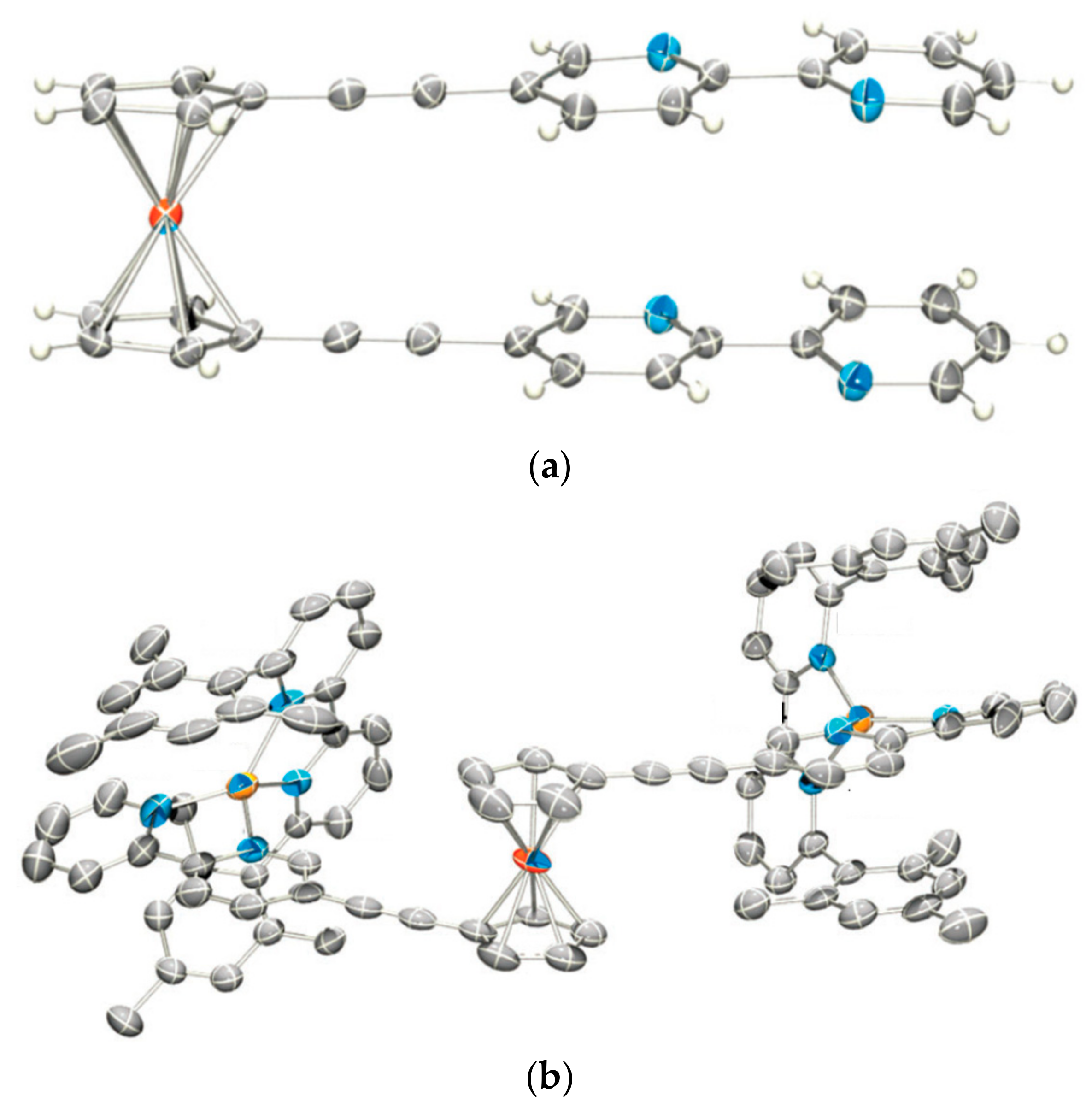
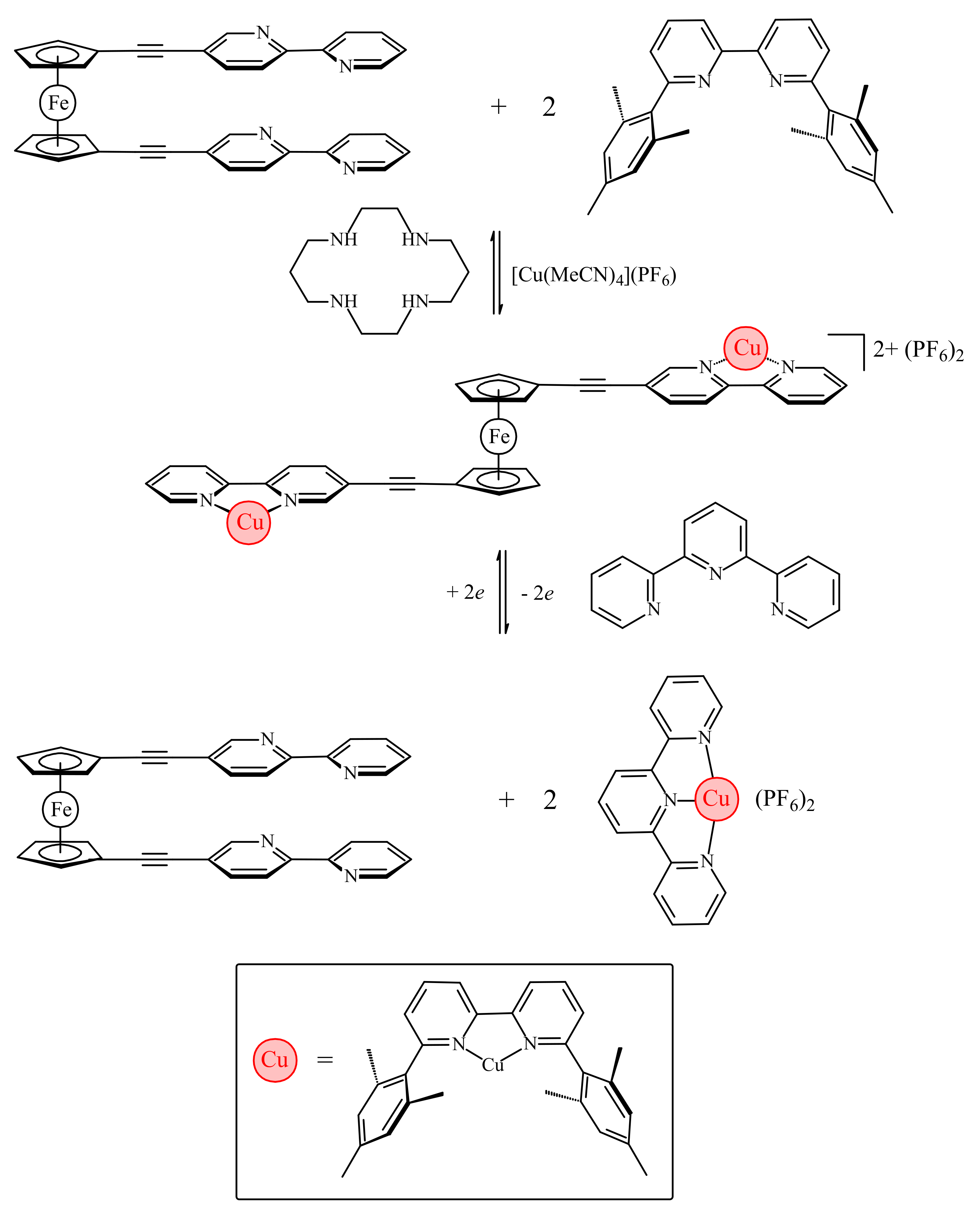
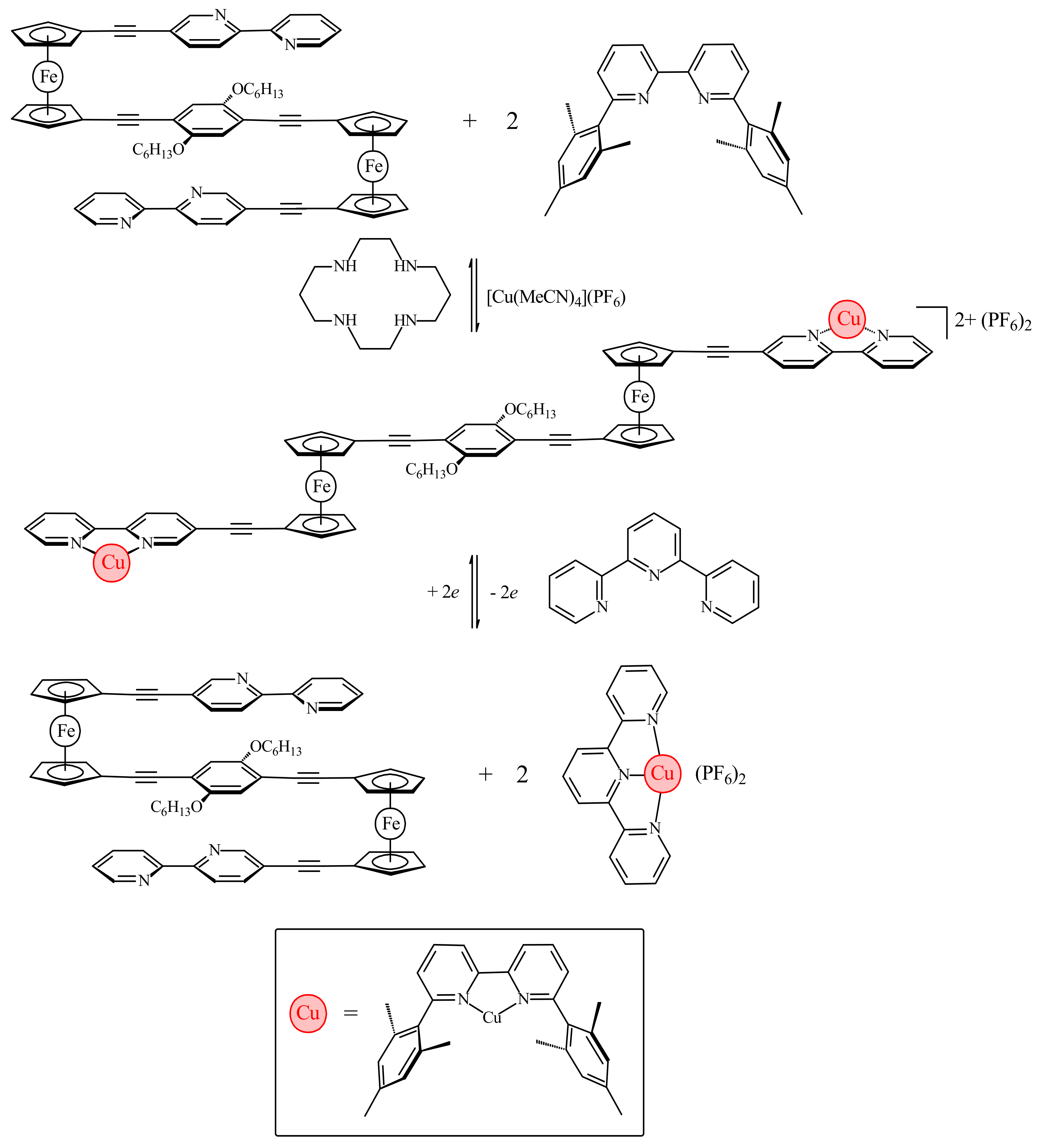
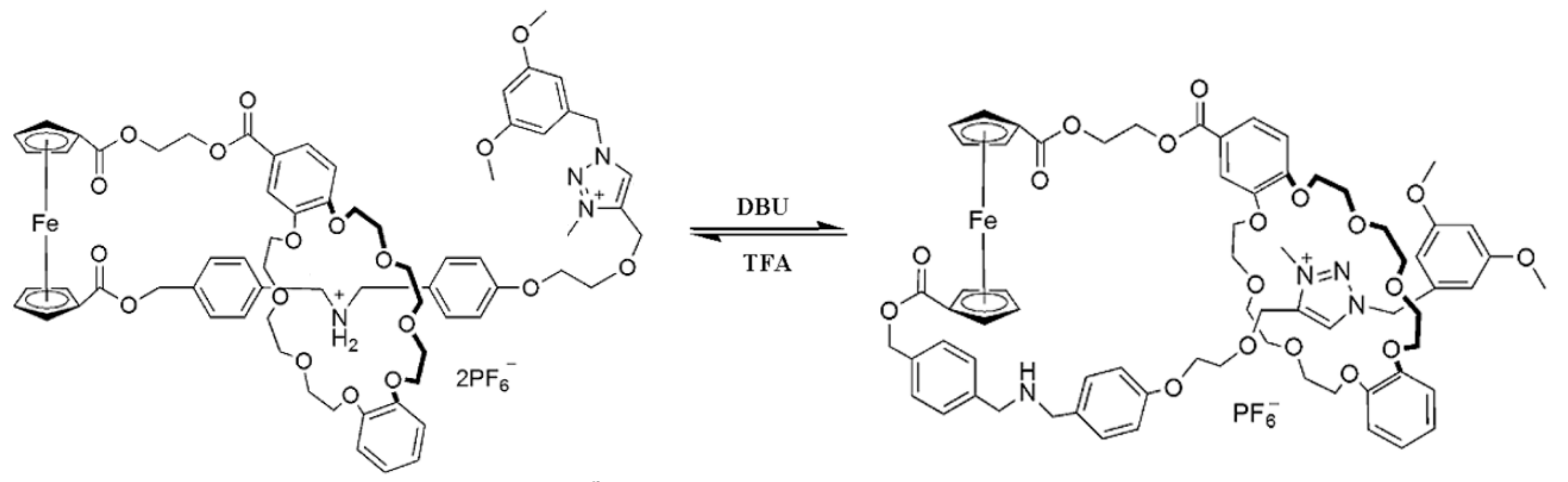
2.4. Ferrocene-Based Molecular Scissors and Pliers
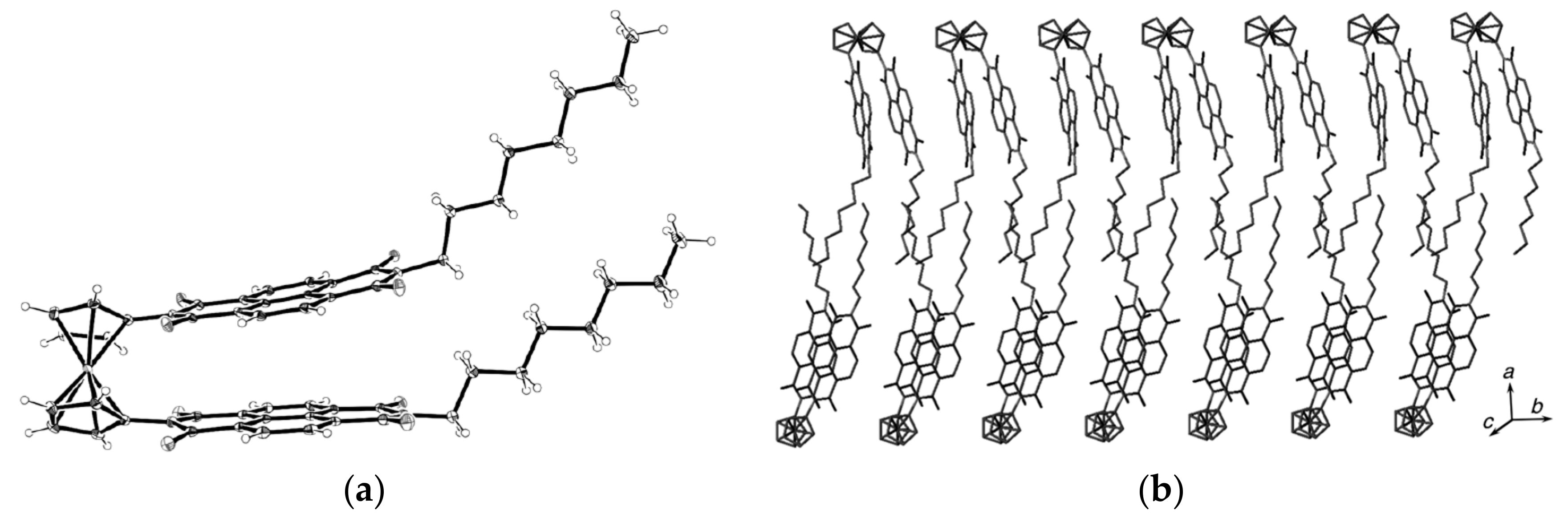
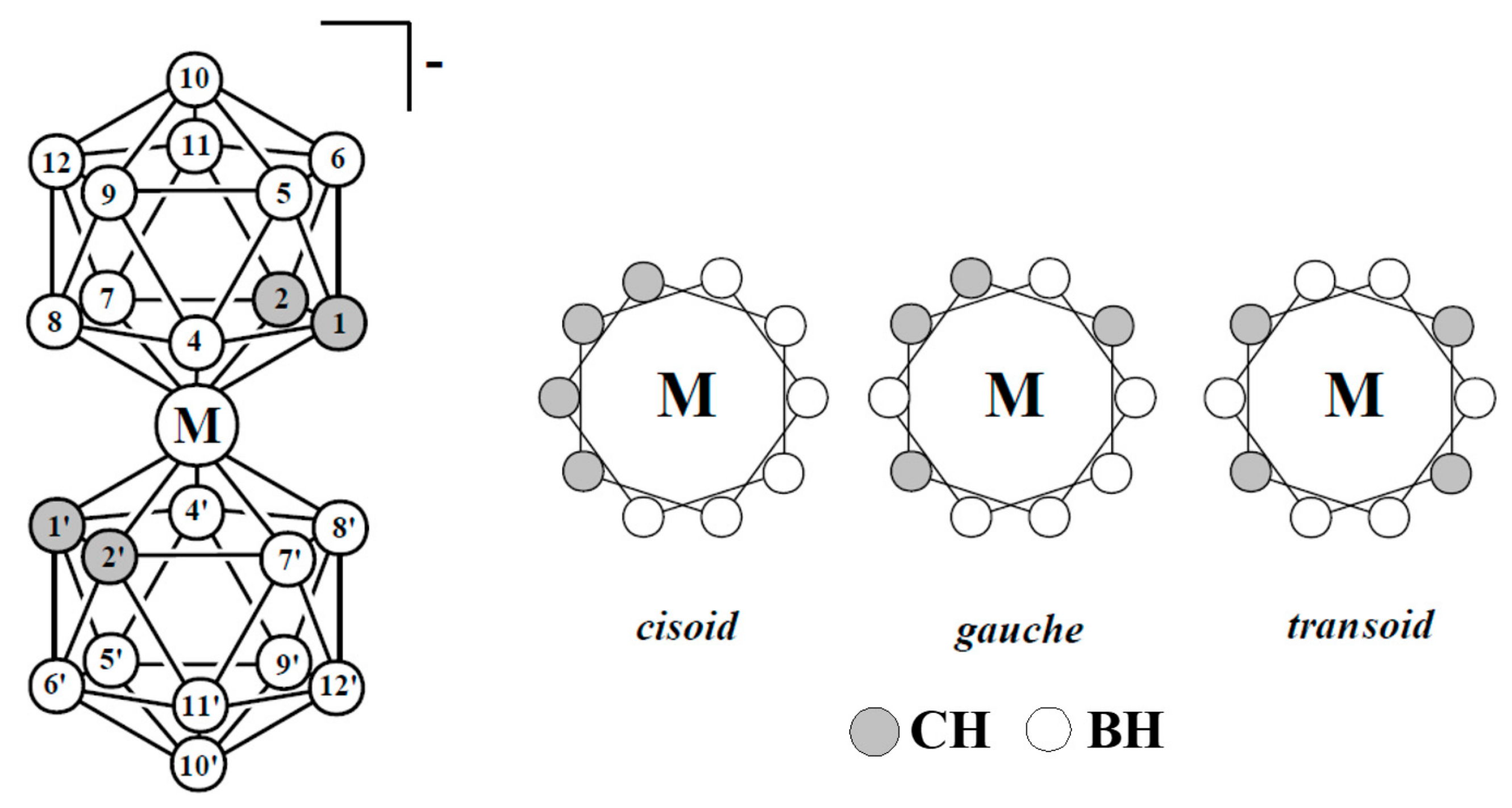
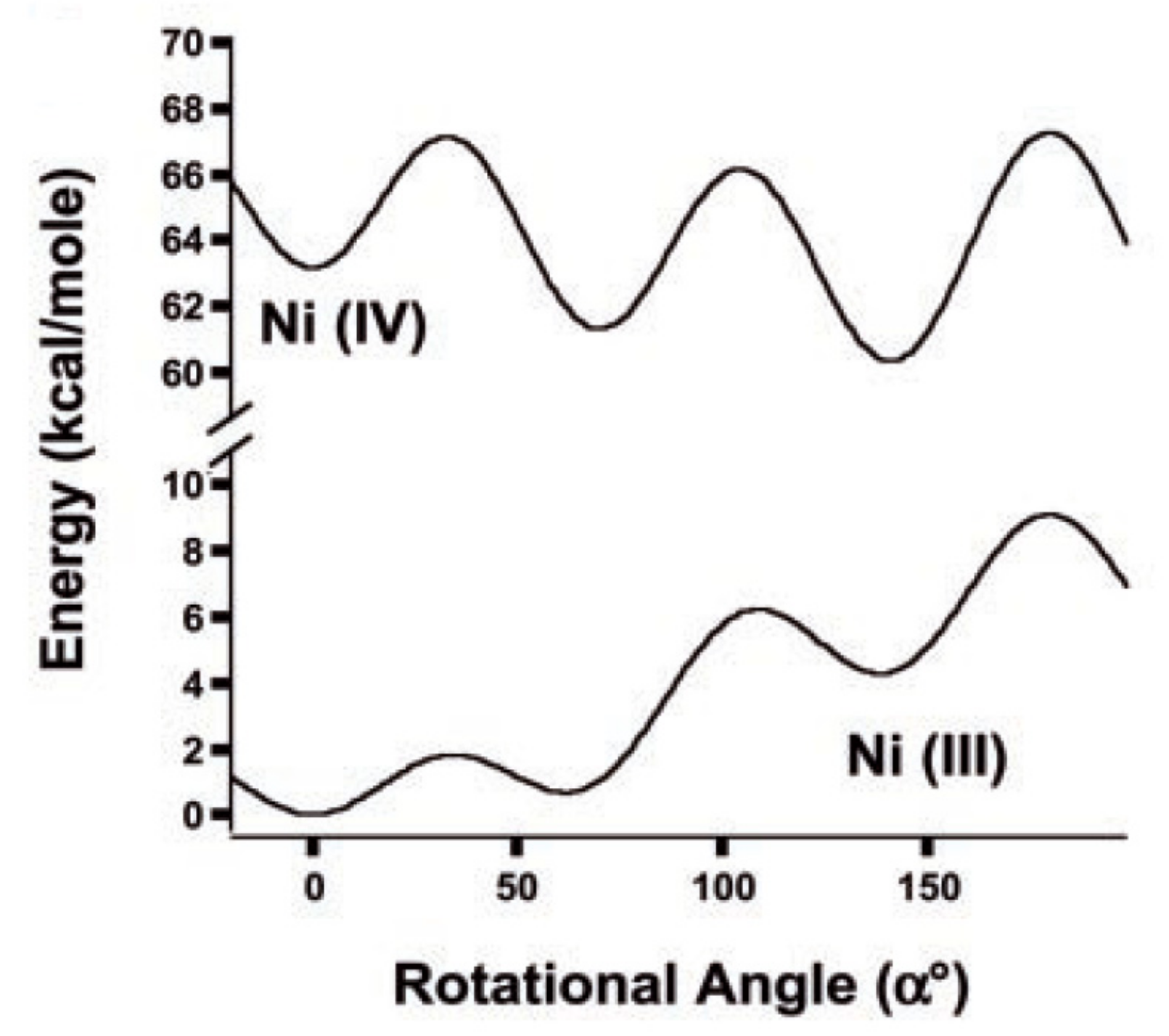
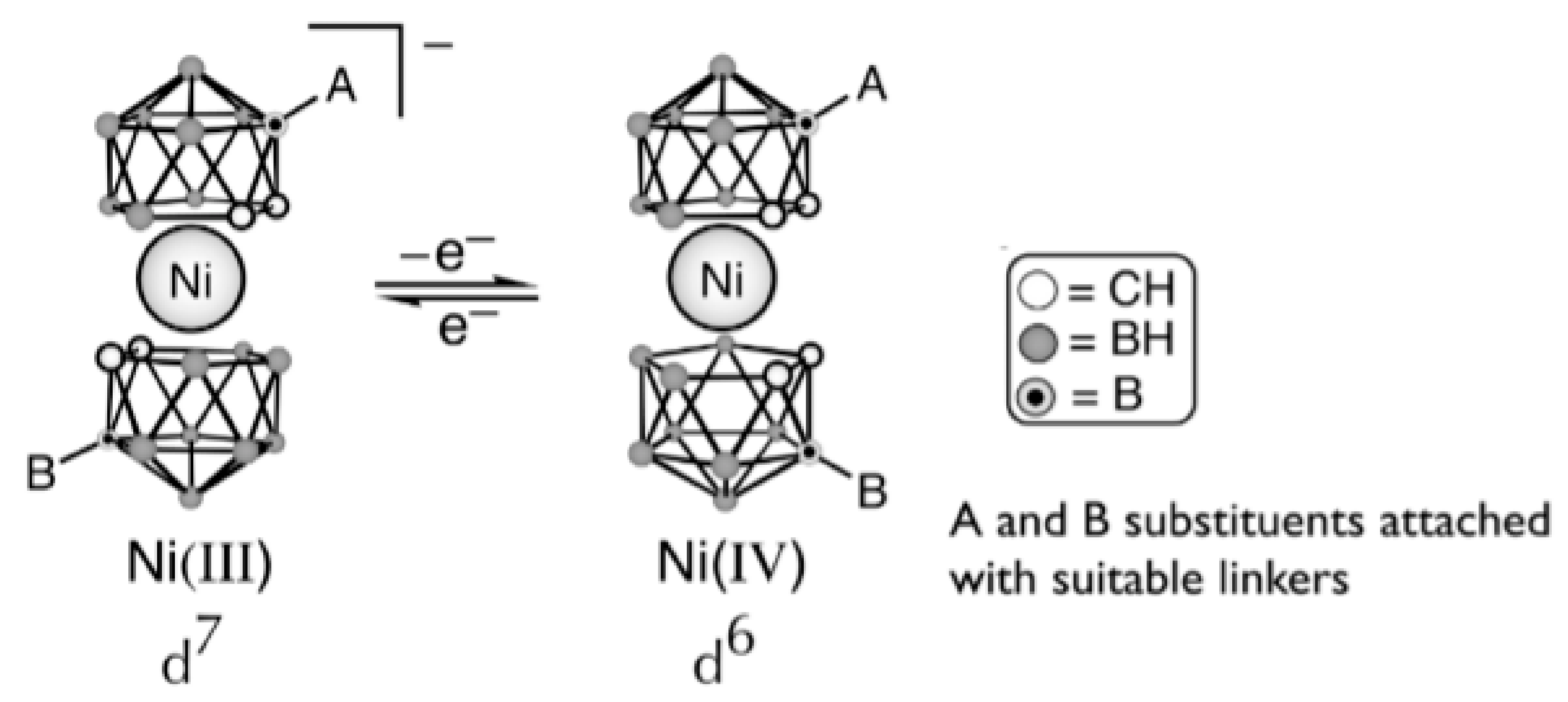
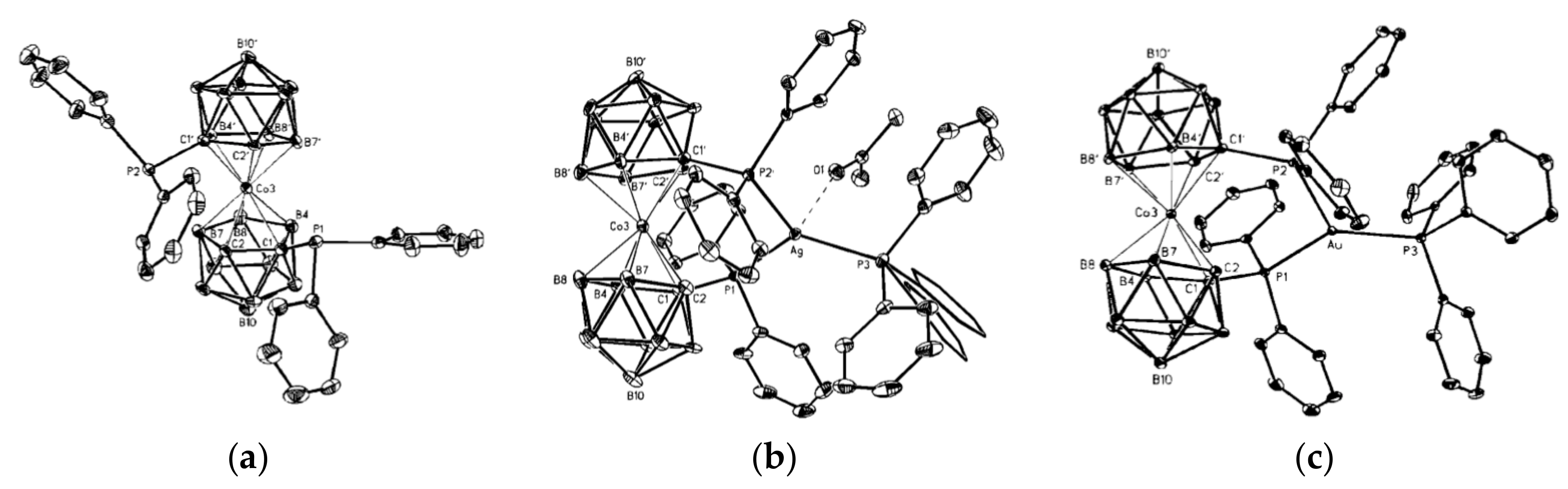
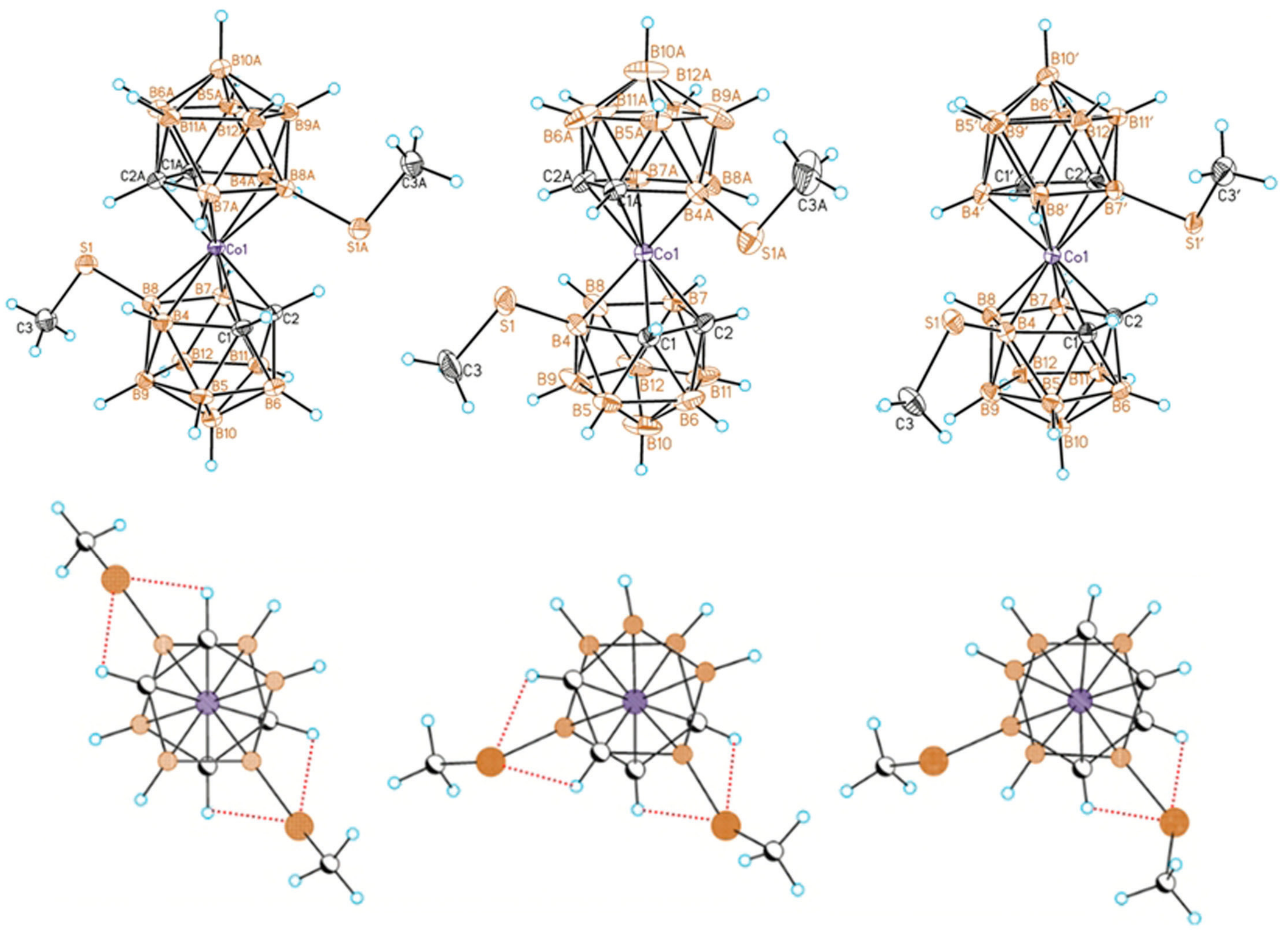
3. Transition Metal Bid(Dicarbollide) Based Molecular Switches
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Feringa, B.L. In control of motion: From molecular switches to molecular motors. Acc. Chem. Res. 2001, 34, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, J.-P. Molecular Machines and Motors (Structure and Bonding, Volume 99); Springer: Berlin, German, 2001; 302p, ISBN 978-3-540-41382-0. [Google Scholar]
- Credi, A.; Silvi, S.; Venturi, M. Molecular Machines and Motors: Recent Advances and Perspectives (Topics in Current Chemistry, Volume 354); Springer: Heidelberg, German, 2014; 342p, ISBN 978-3-319-08677-4. [Google Scholar]
- Erbas-Cakmak, S.; Leigh, D.A.; McTernan, C.T.; Nussbaumer, A.L. Artificial molecular machines. Chem. Rev. 2015, 115, 10081–10206. [Google Scholar] [CrossRef] [PubMed]
- The Nobel Prize in Chemistry 2016. Available online: http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2016/ (accessed on 7 December 2017).
- Budyka, M.F. Molecular switches and logic gates for information processing, the bottom-up strategy: From silicon to carbon, from molecules to supermolecules. Russ. Chem. Rev. 2017, 86, 181–210. [Google Scholar] [CrossRef]
- Feringa, B.L.; Browne, W.R. Molecular Switches, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2011; 792p, ISBN 978-3-52-731365-5. [Google Scholar]
- Canary, J.W.; Mortezaei, S.; Liang, J. Transition metal-based chiroptical switches for nanoscale electronics and sensors. Coord. Chem. Rev. 2010, 254, 2249–2266. [Google Scholar] [CrossRef]
- Werner, H. At least 60 years of ferrocene: The discovery and rediscovery of the sandwich complexes. Angew. Chem. Int. Ed. 2012, 51, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Bohn, R.K.; Haaland, A. On the molecular structure of ferrocene, Fe(C5H5)2. J. Organomet. Chem. 1966, 5, 470–476. [Google Scholar] [CrossRef]
- Scottwell, S.Ø.; Crowley, J.D. Ferrocene-containing non-interlocked molecular machines. Chem. Commun. 2016, 52, 2451–2464. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-B.; Dai, B.; Woo, H.-K.; Wang, L.-S. Intramolecular rotation through proton transfer: [Fe(η5-C5H4CO2−)2] versus [(η5-C5H4CO2−)Fe(η5-C5H4CO2H)]. Angew. Chem. Int. Ed. 2005, 44, 6022–6024. [Google Scholar] [CrossRef] [PubMed]
- Palekik, G.J. Crystal and molecular structure of ferrocenedicarboxylic acid. Inorg. Chem. 1969, 8, 2744–2749. [Google Scholar] [CrossRef]
- Takusagawa, F.; Koetzle, T.F. The crystal and molecular structure of 1,1′-ferrocene dicarboxylic acid (triclinic modification): Neutron and X-ray diffraction studies at 78 K and 298 K. Acta Cryst. 1979, B35, 2888–2896. [Google Scholar] [CrossRef]
- Zakaria, C.M.; Ferguson, G.; Lough, A.J.; Glidewell, C. Ferrocene-1,1′-dicarboxylic acid as a building block in supramolecular chemistry: Supramolecular structures in one, two and three dimensions. Acta Cryst. 2002, B58, 786–802. [Google Scholar] [CrossRef]
- Horikoshi, R.; Mochida, T. Ferrocene-containing coordination polymers: Ligand design and assembled structures. Eur. J. Inorg. Chem. 2010, 5355–5371. [Google Scholar] [CrossRef]
- Chandrasekhar, V.; Thirumoorthia, R. Coordination polymers containing ferrocene backbone. Synthesis, structure and electrochemistry. Dalton Trans. 2010, 39, 2684–2691. [Google Scholar] [CrossRef] [PubMed]
- Masello, A.; Sanakis, Y.; Boudalis, A.K.; Abboud, K.A.; Christou, G. Iron(III) chemistry with ferrocene-1,1′-dicarboxylic acid (fdcH2): An Fe7 cluster with an oxidized fdc− ligand. Inorg. Chem. 2011, 50, 5646–5654. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Uehara, H.; Kitagawa, S.; Furukawa, S. Redox reaction in two-dimensional porous coordination polymers based on ferrocenedicarboxylates. Dalton Trans. 2012, 41, 3924–3927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masello, A.; Abboud, K.A.; Wernsdorfer, W.; Christou, G. Mn8 cluster with ferrocene-1,1′-dicarboxylate ligation: Single-molecule magnetism with multiple external redox centers. Inorg. Chem. 2013, 52, 10414–10423. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, V.; Chakraborty, A.; Sañudoc, E.C. Ferrocene-based compartmental ligand for the assembly of neutral ZnII/LnIII heterometallic complexes. Dalton Trans. 2013, 42, 13436–13443. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Bag, P.; Rivière, E.; Mallah, T.; Chandrasekhar, V. Assembly of heterobimetallic NiII–LnIII (LnIII = DyIII, TbIII, GdIII, HoIII, ErIII, YIII) complexes using a ferrocene ligand: Slow relaxation of the magnetization in DyIII, TbIII and HoIII analogues. Dalton Trans. 2014, 43, 8921–8932. [Google Scholar] [CrossRef] [PubMed]
- Ospina Castro, M.L.; Briceño, A.; González, T.; Reiber, A.; Jorge, G.; Duran, M.H. Metallic macrocycles built-up from ferrocenedicarboxylate and ancillary nitrogen heterocycles: Hydrothermal synthesis, crystal structures and electrochemical properties. Inorg. Chim. Acta 2015, 432, 275–282. [Google Scholar] [CrossRef]
- Ospina-Castro, M.L.; Reiber, A.; Jorge, G.; Ávila, E.E.; Briceño, A. Novel 3-D interpenetrated metal-organometallic networks based on self-assembled Zn(II)/Cu(II) from 1,1′-ferrocenedicarboxylic acid and 4,4′-bipyridine. CrystEngComm 2017, 19, 758–761. [Google Scholar] [CrossRef]
- Yanilkin, V.V.; Nasybullina, G.R.; Nastapova, N.V.; Madzhidov, T.I.; Latypova, L.Z.; Morozov, V.I.; Shekurov, R.P.; Milyukov, V.A. Electrochemically driven molecular rotors based on ferrocene-1,1′-diyl-bisphosphinic acids. Russ. J. Electrochem. 2015, 51, 645–664. [Google Scholar] [CrossRef]
- Shekurov, R.P.; Milyukov, V.A.; Islamov, D.P.; Krivolapov, D.B.; Kataeva, O.N.; Gerasimova, T.P.; Katsyuba, S.A.; Nasybullina, G.R.; Yanilkin, V.V.; Sinyashin, O.G. Synthesis and structure of ferrocenylphosphonic acids. J. Organomet. Chem. 2014, 766, 40–48. [Google Scholar] [CrossRef]
- Shekurov, R.P.; Tufatullin, A.I.; Milyukov, V.A.; Kataeva, O.N.; Sinyashin, O.G. Supramolecular architecture of diammonium ferrocene-1,1′-diyldiphosphinates. Russ. Chem. Bull. 2014, 63, 178–181. [Google Scholar] [CrossRef]
- Toyama, T.; Komori, S.; Yoshino, J.; Hayashi, N.; Higuchi, H. Synthesis and properties of 1,1′-bis[p-(N,N-dimethylaminophenyl)butadiynyl]ferrocene: A methodology for proton-mediated reversible conformation control of two function sites. Tetrahedron Lett. 2013, 54, 66–71. [Google Scholar] [CrossRef]
- Moriuchi, T.; Nomoto, A.; Yoshida, K.; Ogawa, A.; Hirao, T. Chirality Organization of ferrocenes bearing podand dipeptide chains: Synthesis and structural characterization. J. Am. Chem. Soc. 2001, 123, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Moriuchi, T.; Hirao, T. Design of ferrocene-dipeptide bioorganometallic conjugates to induce chirality-organized structures. Acc. Chem. Res. 2010, 43, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Moriuchi, T.; Nishiyama, T.; Nobu, M.; Hirao, T. Control of helical chirality of ferrocene-dipeptide conjugates by the secondary structure of dipeptide chains. Chem. Eur. J. 2017, 23, 12704–12708. [Google Scholar] [CrossRef] [PubMed]
- Deck, P.A.; Lane, M.J.; Montgomery, J.L.; Slebodnick, C.; Fronczek, F.R. Synthesis and structural trends in pentafluorophenyl-substituted ferrocenes, 1,4-tetrafluorophenylene-linked diferrocenes, and 1,1′-ferrocenylene-1,4-tetrafluorophenylene co-oligomers. Organometallics 2000, 19, 1013–1024. [Google Scholar] [CrossRef]
- Baumgardt, I.; Butenschön, H. 1,1′-Diaryl-substituted ferrocenes: Up to three hinges in oligophenylene-ethynylene-type molecular wires. Eur. J. Org. Chem. 2010, 1076–1087. [Google Scholar] [CrossRef]
- Enders, M.; Kohl, G.; Pritzkow, H. Synthesis and coordination behaviour of the new (8-quinolyl)-cyclopentadienyl ligand. J. Organomet. Chem. 2001, 622, 66–73. [Google Scholar] [CrossRef]
- Crowley, J.D.; Steele, I.M.; Bosnich, B. Protonmotive force: Development of electrostatic drivers for synthetic molecular motors. Chem. Eur. J. 2006, 12, 8935–8951. [Google Scholar] [CrossRef] [PubMed]
- Siemeling, U.; Vorfeld, U.; Neumann, B.; Stammler, H.-G.; Fontani, M.; Zanello, P. Synthesis and crystal structure of 1,1′-bis[4-(2,2′:6′,2″-terpyridin-4′-yl)phenyl]-octamethylferrocene. J. Organomet. Chem. 2001, 637–639, 733–737. [Google Scholar] [CrossRef]
- Scottwell, S.Ø.; Elliott, A.B.S.; Shaffer, K.J.; Nafady, A.; McAdam, C.J.; Gordon, K.C.; Crowley, J.D. Chemically and electrochemically induced expansion and contraction of a ferrocene rotor. Chem. Commun. 2015, 51, 8161–8164. [Google Scholar] [CrossRef] [PubMed]
- Scottwell, S.Ø.; Barnsley, J.E.; McAdam, C.J.; Gordon, K.C.; Crowley, J.D. A ferrocene based switchable molecular folding ruler. Chem. Commun. 2017, 53, 7628–7631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Q.; Su, J.; Tian, H. A dual-ion-switched molecular brake based on ferrocene. Chem. Commun. 2009, 1700–1702. [Google Scholar] [CrossRef] [PubMed]
- Iordache, A.; Oltean, M.; Milet, A.; Thomas, F.; Baptiste, B.; Sain-Aman, E.; Bucher, C. Redox control of rotary motions in ferrocene-based elemental ball bearing. J. Am. Chem. Soc. 2012, 134, 2653–2671. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Y.; Ma, N.-N.; Sun, S.L.; Qiu, Y.-Q. Redox control of ferrocene-based complexes with systematically extended π-conjugated connectors: Switchable and tailorable second order nonlinear optics. Phys. Chem. Chem. Phys. 2014, 16, 4900–4910. [Google Scholar] [CrossRef] [PubMed]
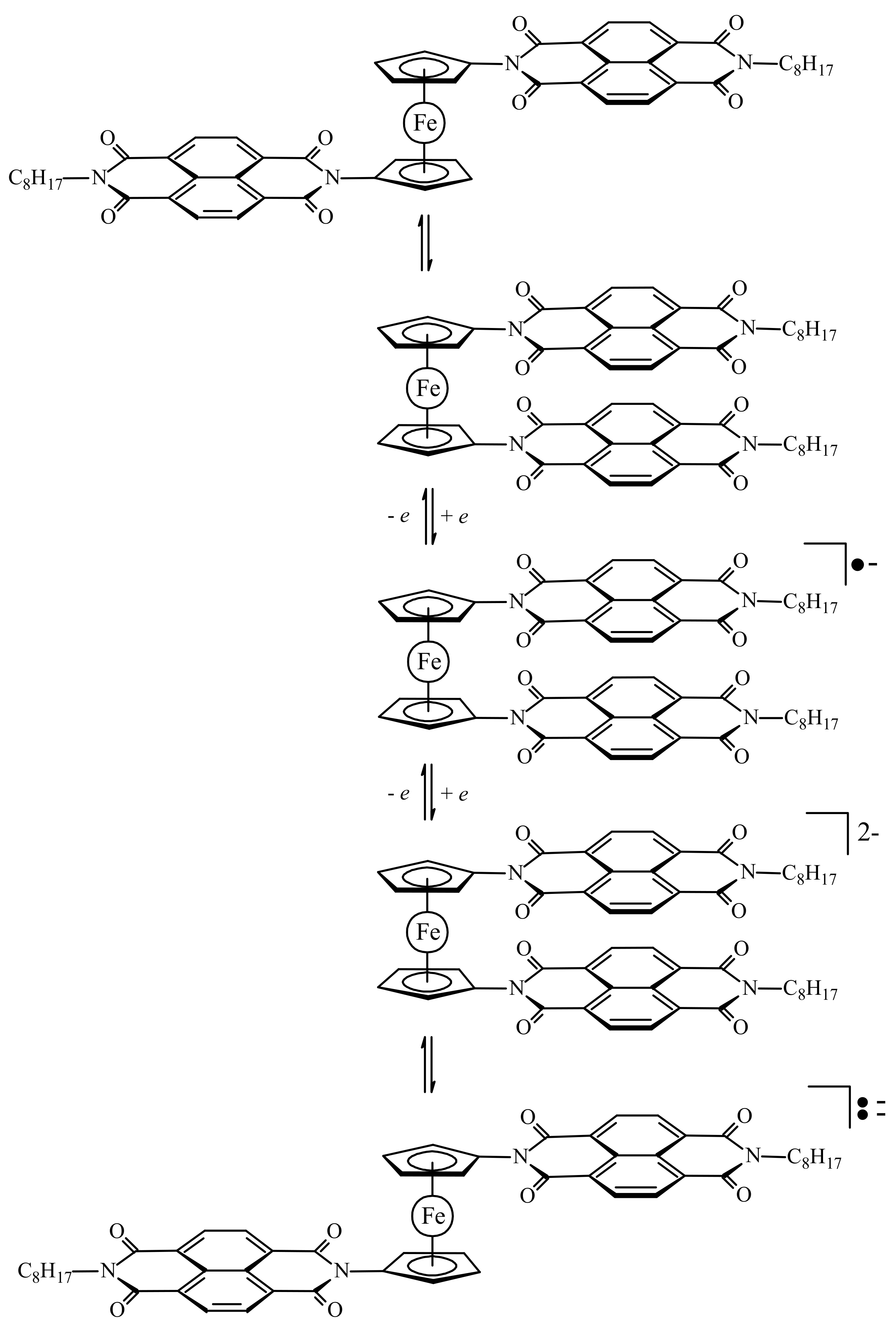
- Takai, A.; Yasuda, T.; Ishizuka, T.; Kojima, T.; Takeuchi, M. A directly linked ferrocene-naphthalenediimide conjugate: Precise control of stacking structures of π-systems by redox stimuli. Angew. Chem. Int. Ed. 2013, 52, 9167–9171. [Google Scholar] [CrossRef] [PubMed]
- Takai, A.; Kajitani, T.; Fukushima, T.; Kishikawa, K.; Yasuda, T.; Takeuchi, M. Supramolecular assemblies of ferrocene-hinged naphthalenediimides: Multiple conformational changes in film states. J. Am. Chem. Soc. 2016, 138, 11245–11253. [Google Scholar] [CrossRef] [PubMed]
- Kinbara, K.; Muraoka, T.; Aida, T. Chiral ferrocenes as novel rotary modules for molecular machines. Org. Biomol. Chem. 2008, 6, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
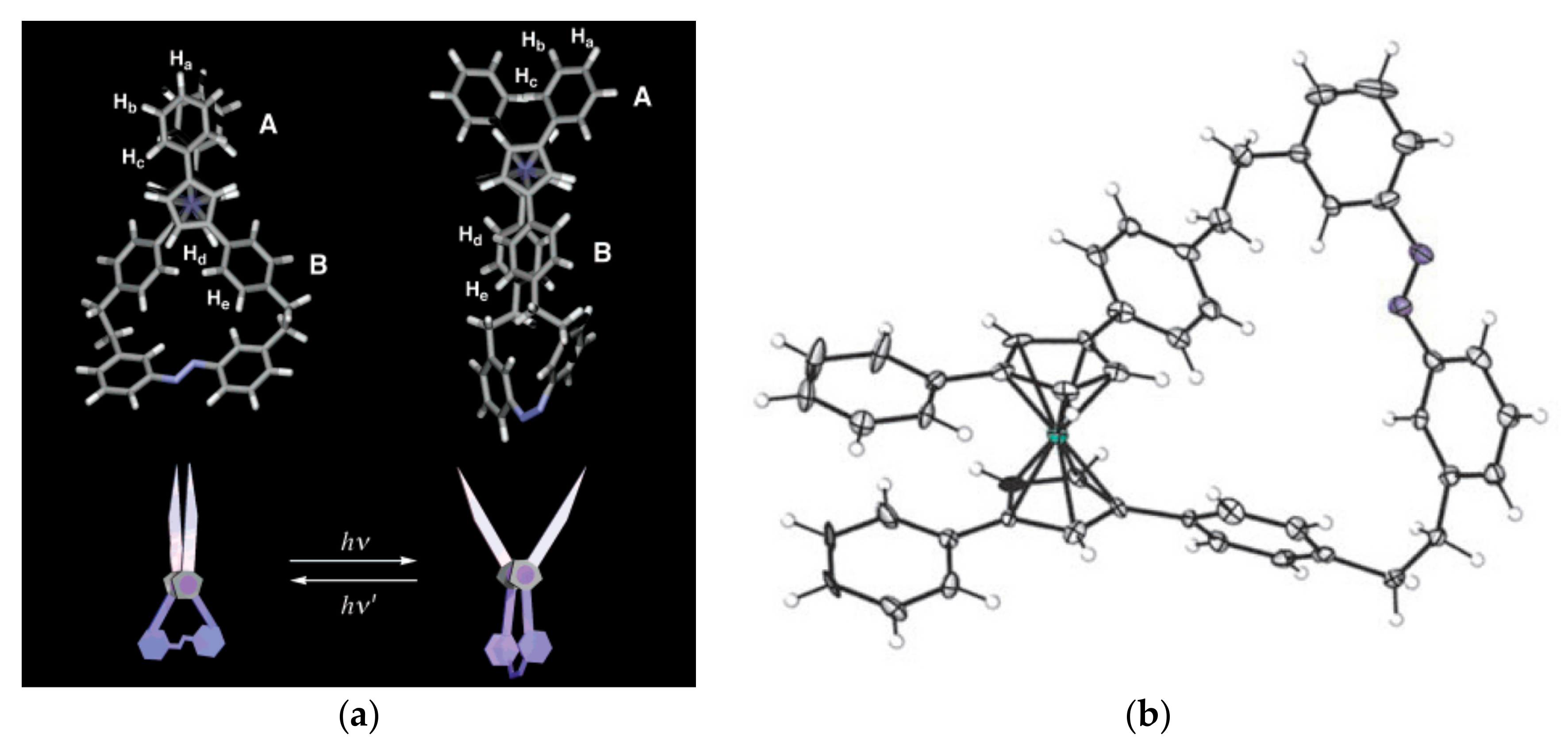
- Muraoka, T.; Kinbara, K.; Kobayashi, Y.; Aida, T. Light-driven open-close motion of chiral molecular scissors. J. Am. Chem. Soc. 2003, 125, 5612–5613. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, T.; Kinbara, K.; Wakamiya, A.; Yamaguchi, S.; Aida, T. Crystallographic and chiroptical studies on tetraarylferrocenes for use as chiral rotary modules for molecular machines. Chem. Eur. J. 2007, 13, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, T.; Kinbara, K.; Aida, T. Reversible operation of chiral molecular scissors by redox and UV light. Chem. Commun. 2007, 1441–1443. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, T.; Kinbara, K.; Aida, T. Mechanical twisting of a guest by a photoresponsive host. Nature 2006, 440, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, T.; Kinbara, K.; Aida, T. A self-locking molecule operative with a photoresponsive key. J. Am. Chem. Soc. 2006, 128, 11600–11605. [Google Scholar] [CrossRef] [PubMed]
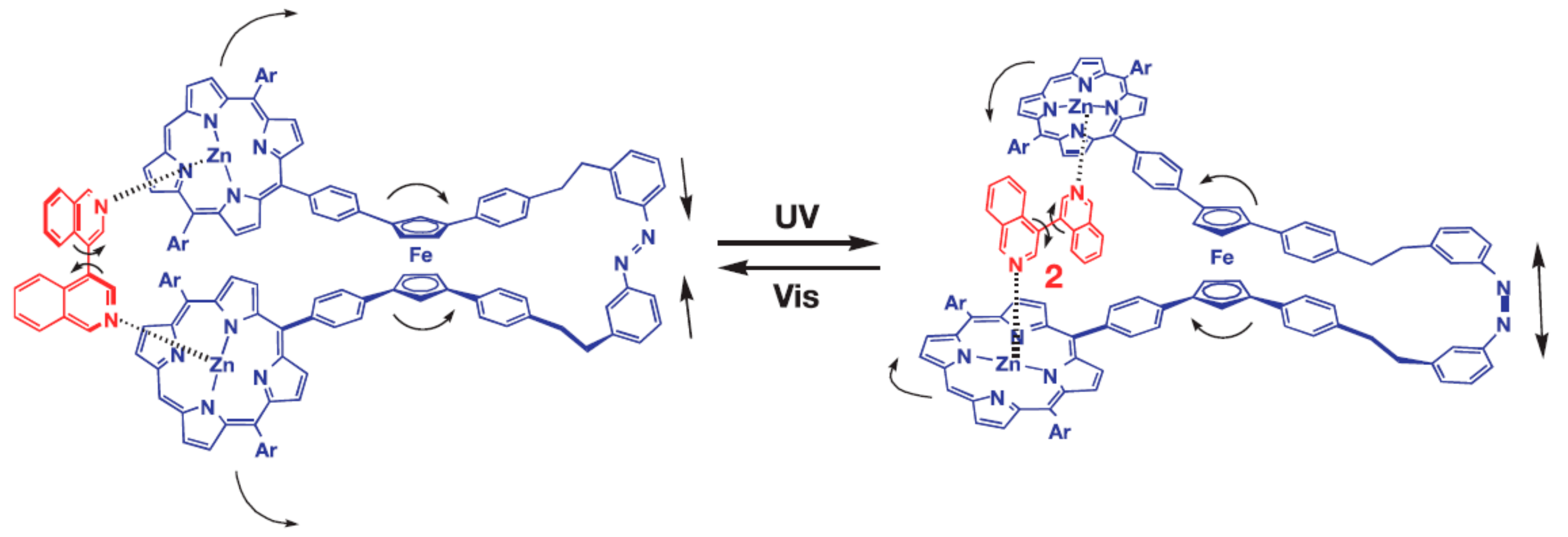
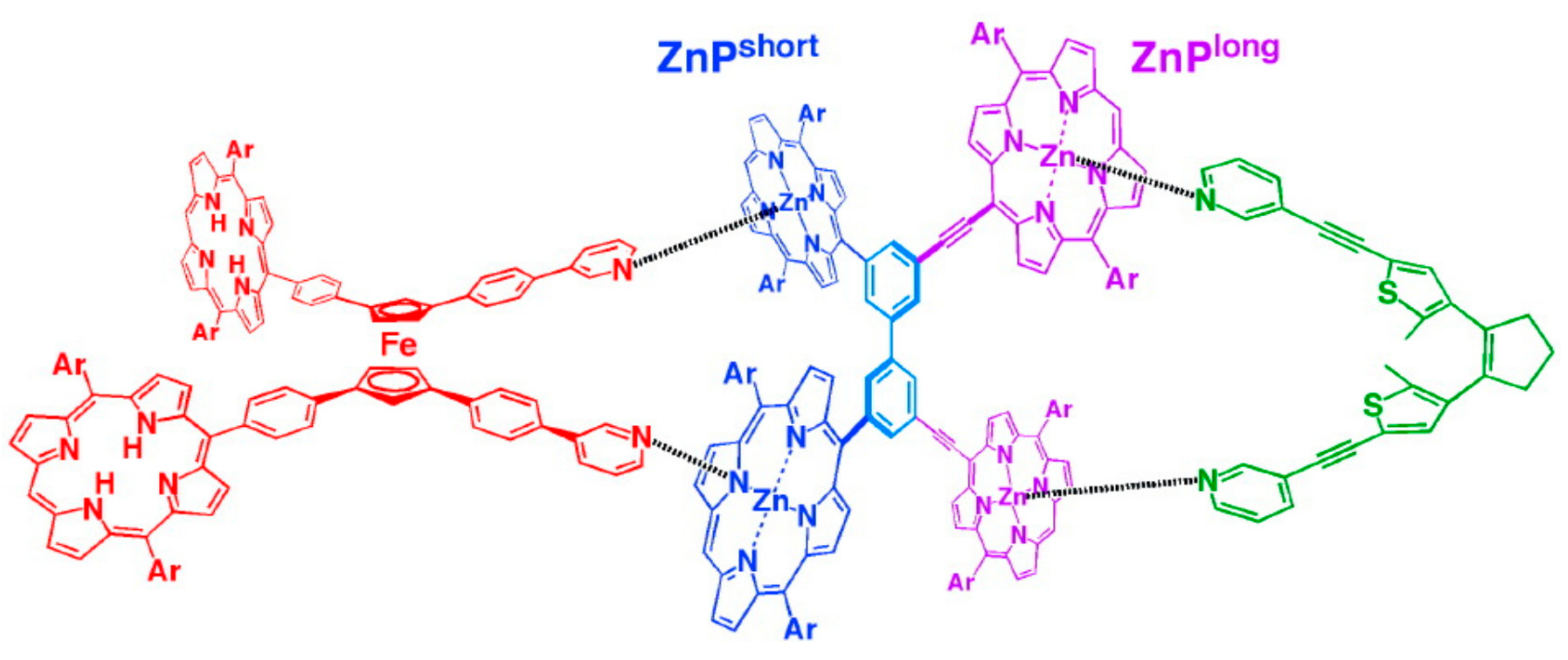
- Kai, H.; Nara, S.; Kinbara, K.; Aida, T. Toward long-distance mechanical communication: Studies on a ternary complex interconnected by a bridging rotary module. J. Am. Chem. Soc. 2008, 130, 6725–6727. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Kinbara, K. Toward autonomously operating molecular machines driven by transition-metal catalyst. Mol. BioSyst. 2008, 4, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Allinger, N.L.; Eliel, E.L.; Schlögl, K. Stereochemistry of Metallocenes. In Topics in Stereochemistry, Volume 1; Allinger, N.L., Eliel, E.L., Eds.; Interscience Publishers: New York, NY, USA, 1967; pp. 39–91. ISBN 978-0-47-002465-2. [Google Scholar]
- Li, H.; Zhang, H.; Zhang, Q.-W.; Qu, D.-H. A switchable ferrocene-based [1]rotaxane with an electrochemical signal output. Org. Lett. 2012, 14, 5900–5903. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, M.F.; Young, D.C.; Wegner, P.A. Carbametallic boron hydride derivatives. I. Apparent analogs of ferrocene and ferricinium ion. J. Am. Chem. Soc. 1965, 87, 1818–1819. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Andrews, T.D. Carborane analogues of cobalticinium ion. Chem. Commun. 1965, 443–444. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Chemistry of cobalt bis(dicarbollides). A review. Collect. Czech. Chem. Commun. 1999, 64, 783–805. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Chemistry of nickel and iron bis(dicarbollides). A review. J. Organomet. Chem. 2000, 614–615, 27–36. [Google Scholar] [CrossRef]
- Dash, B.P.; Satapathy, R.; Swain, B.R.; Mahanta, C.S.; Jena, B.B.; Hosmane, N.S. Cobalt bis(dicarbollide) anion and its derivatives. J. Organomet. Chem. 2017, 849–850, 170–194. [Google Scholar] [CrossRef]
- Bühl, M.; Hnyk, D.; Macháček, J. Computational study of structures and properties of metallaboranes: Cobalt bis(dicarbollide). Chem. Eur. J. 2005, 11, 4109–4120. [Google Scholar] [CrossRef] [PubMed]
- Bühl, M.; Holub, J.; Hnyk, D.; Macháček, J. Computational studies of structures and properties of metallaboranes. 2. Transition-metal dicarbollide complexes. Organometallics 2006, 25, 2173–2181. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Zink, J.I.; Skelton, J.M.; Bayer, M.B.; Liu, C.; Livshits, E.; Baer, R.; Neuhauser, D. Electrical or photocontrol of the rotary motion of a metallacarborane. Science 2004, 303, 1849–1852. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.F.; Hawthorne, M.F. Chemistry of the bis[π-(3)-1,2-dicarbollyl] metalates of nickel and palladium. J. Am. Chem. Soc. 1970, 92, 1157–1173. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Ramachandran, B.M.; Kennedy, R.D.; Knobler, C.B. Approaches to rotary molecular motors. Pure Appl. Chem. 2006, 78, 1299–1304. [Google Scholar] [CrossRef]
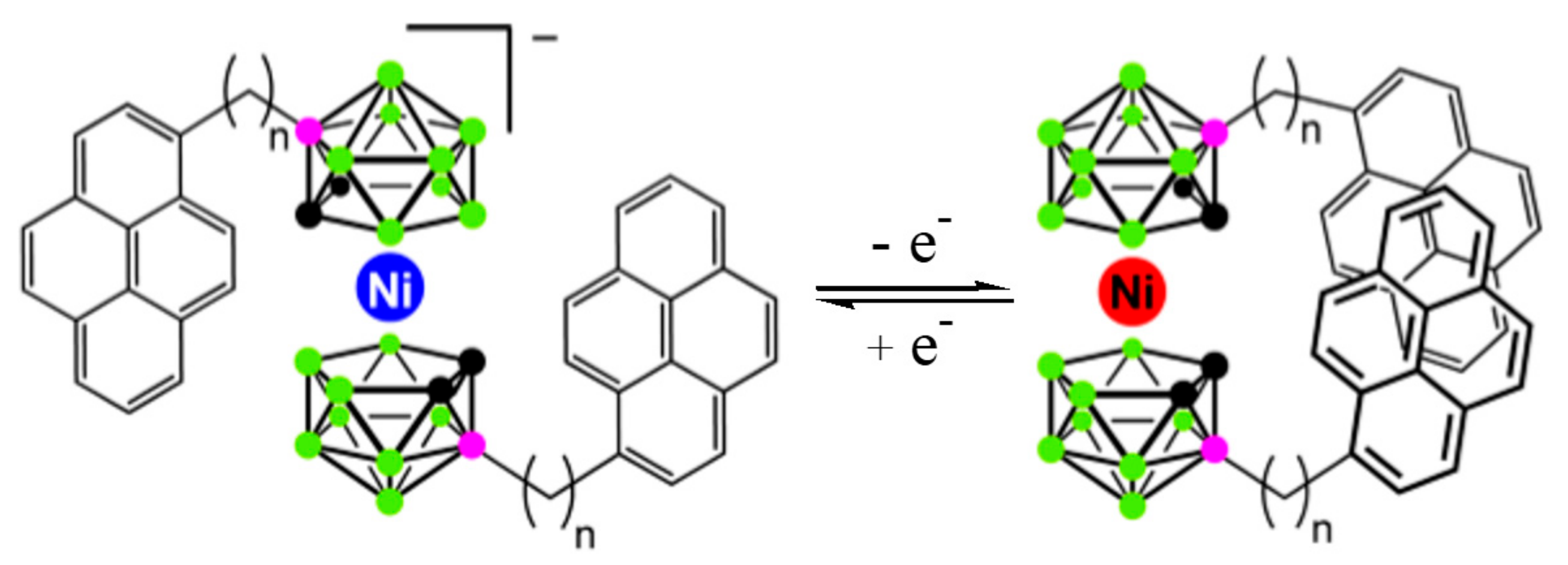
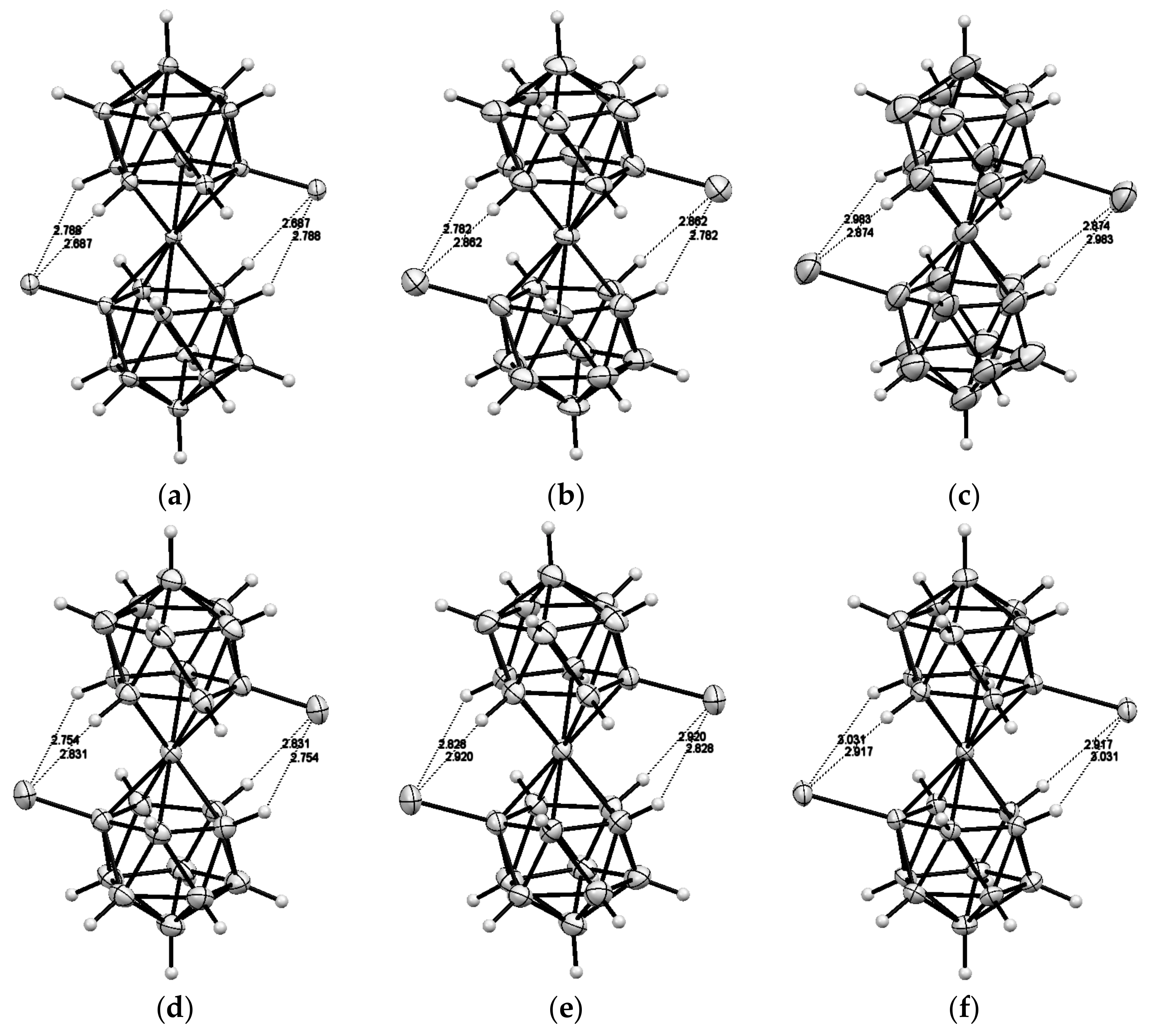
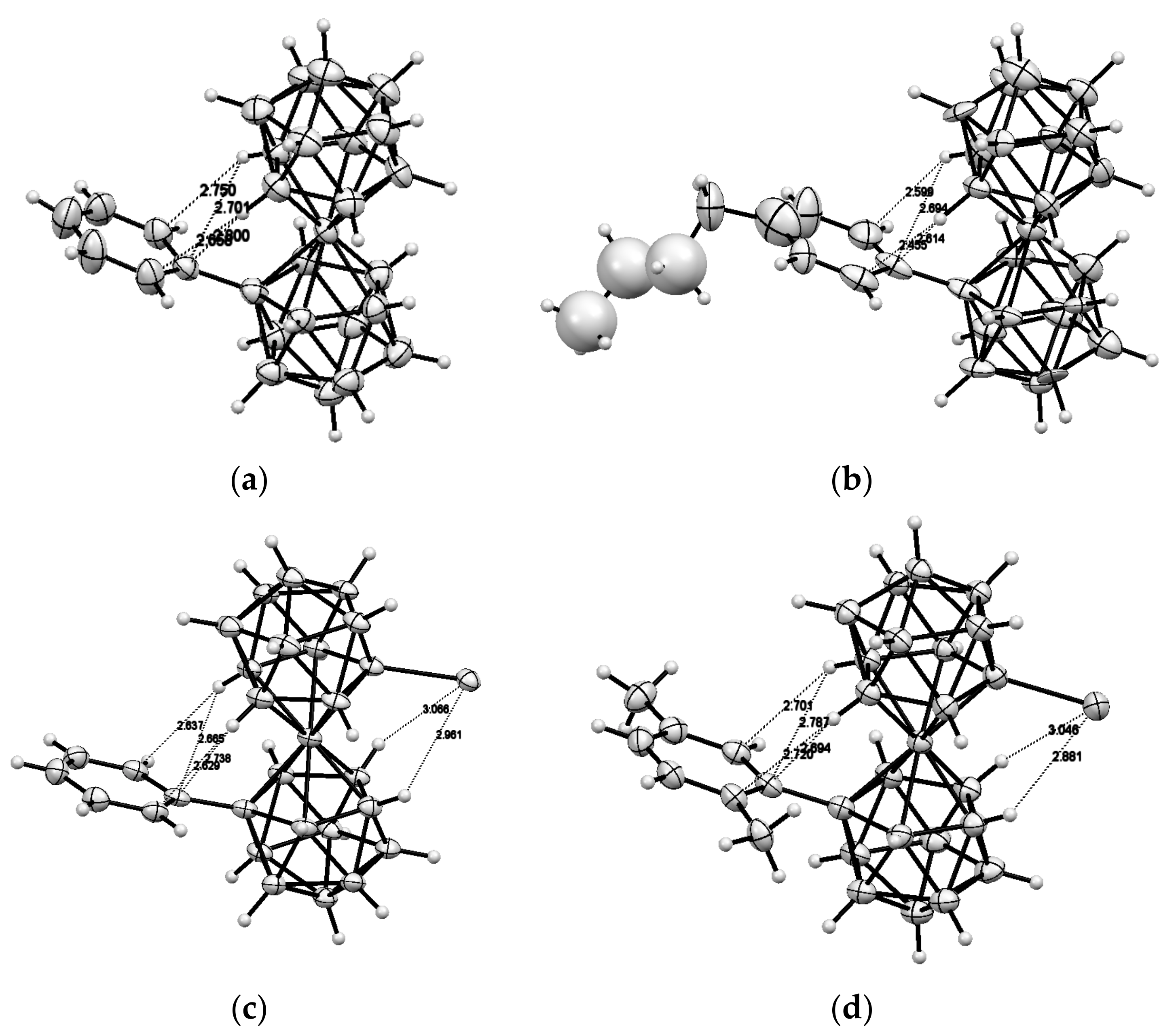
- Safronov, A.V.; Shlyakhtina, N.I.; Everett, T.A.; VanGordon, M.R.; Sevryugina, Y.V.; Jalisatgi, S.S.; Hawthorne, M.F. Direct observation of bis(dicarbollyl)nickel conformers in solution by fluorescence spectroscopy: An approach to redox-controlled metallacarborane molecular motors. Inorg. Chem. 2014, 53, 10045–10053. [Google Scholar] [CrossRef] [PubMed]
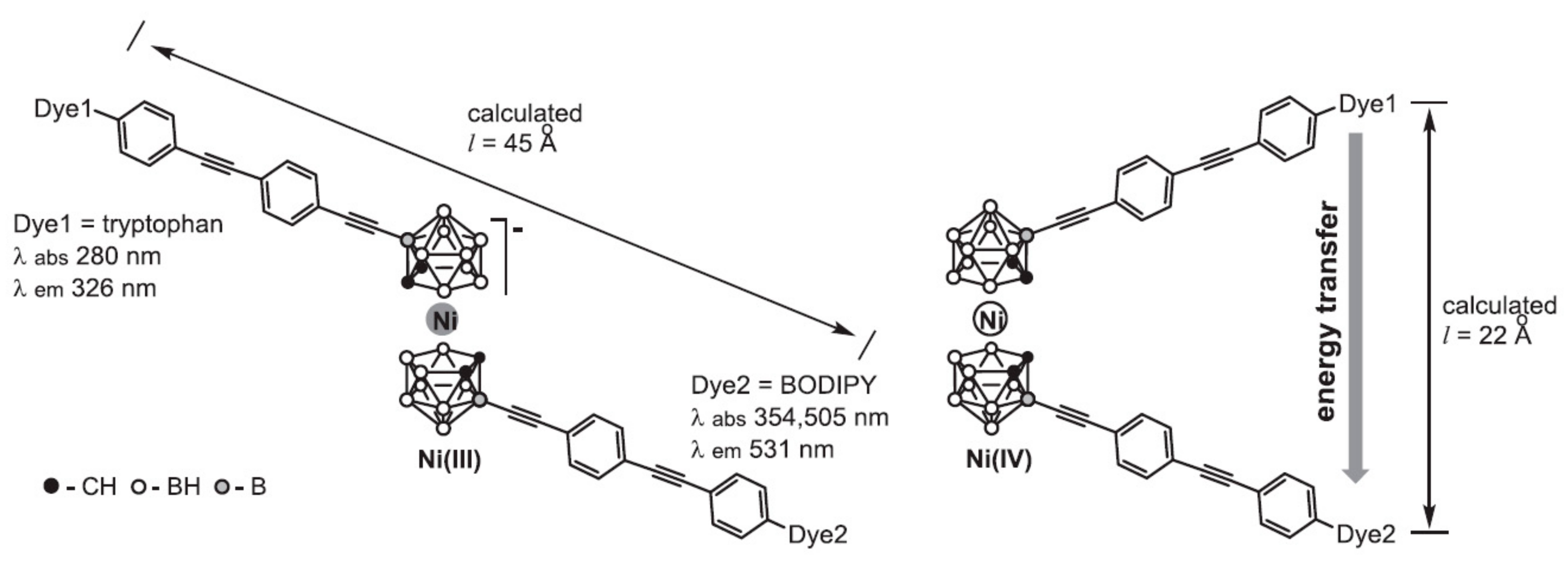
- Shlyakhtina, N.I.; Safronov, A.V.; Sevryugina, Y.V.; Jalisatgi, S.S.; Hawthorne, M.F. Synthesis, characterization, and preliminary fluorescence study of a mixed ligand bis(dicarbollyl)nickel complex bearing a tryptophan-BODIPY FRET couple. J. Organomet. Chem. 2015, 798, 234–244. [Google Scholar] [CrossRef]
- Kirillova, N.I.; Zhdanov, A.S.; Gusev, A.I.; Kirin, V.N.; Knyazev, S.P.; Sokolova, T.V. The molecular structures of the dicarbaboryl derivatives [3,3′-M(8,1,2-ClC2B9H10)2]K. Organomet. Chem. USSR 1989, 2, 448–450. [Google Scholar]
- Hurlburt, P.K.; Miller, R.L.; Abney, K.D.; Foreman, T.M.; Butcher, R.J.; Kinkead, S.A. New synthetic routes to B-halogenated derivatives of cobalt dicarbollide. Inorg. Chem. 1995, 34, 5215–5219. [Google Scholar] [CrossRef]
- Kazheva, O.N.; Kravchenko, A.V.; Aleksandrov, G.G.; Sivaev, I.B.; Bregadze, V.I.; Kosenko, I.D.; Lobanova, I.A.; Buravov, L.I.; Starodub, V.A.; Dyachenko, O.A. Syntheses, structures, and electroconductivity of bis(ethylenedithio)tetrathiafulvalene (BEDT-TTF) and bis(methylenedithio)tetrathiafulvalene (BMDT-TTF) salts with cobalt 8,8′-dichloro-3,3′-bis(1,2-dicarbollide). Russ. Chem. Bull. 2014, 63, 1322–1329. [Google Scholar] [CrossRef]
- Kazheva, O.; Alexandrov, G.; Kravchenko, A.; Starodub, V.; Lobanova, I.; Sivaev, I.; Bregadze, V.; Buravov, L.; Dyachenko, O. First molecular conductors with 8,8′-dibromo cobalt bis(dicarbollide) anion. Solid State Sci. 2008, 10, 1734–1739. [Google Scholar] [CrossRef]
- Sivy, P.; Preisinger, A.; Baumgartner, O.; Valach, F.; Koreñ, B.; Matel, L. Structure of caesium 3,3′-commo-bis(decahydro-8-iodo-1,2-dicarba-3-cobalta-closo-dodecaborate)(1-). Acta Cryst. C 1986, 42, 28–30. [Google Scholar] [CrossRef]
- Kazheva, O.N.; Alexandrov, G.G.; Kravchenko, A.V.; Starodub, V.A.; Lobanova, I.A.; Sivaev, I.B.; Bregadze, V.I.; Titov, L.V.; Buravov, L.I.; Dyachenko, O.A. Molecular conductors with 8,8’-diiodo cobalt bis(dicarbollide) anion. J. Organomet. Chem. 2009, 694, 2336–2342. [Google Scholar] [CrossRef]
- Kazheva, O.N.; Kravchenko, A.V.; Aleksandrov, G.G.; Kosenko, I.D.; Lobanova, I.A.; Bregadze, V.I.; Chudak, D.M.; Buravov, L.I.; Protasova, S.G.; Starodub, V.A.; Dyachenko, O.A. Synthesis, structure, and properties of a new bifunctional radical cation salt with ferracarborane anion: (BEDT-TTF)2[8,8′-Cl2–3,3′-Fe(1,2-C2B9H10)2]. Russ. Chem. Bull. 2016, 65, 2195–2201. [Google Scholar] [CrossRef]
- Kazheva, O.N.; Kravchenko, A.V.; Kosenko, I.D.; Alexandrov, G.G.; Chudak, D.M.; Starodub, V.A.; Lobanova, I.A.; Bregadze, V.I.; Buravov, L.I.; Protasova, S.G.; Dyachenko, O.A. First hybrid radical-cation salts with halogen substituted iron bis(dicarbollide) anions—Synthesis, structure, properties. J. Organomet. Chem. 2017, 849–850, 261–267. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond, 3rd ed.; Cornell University Press: Ithaca, NY, USA, 1960; 644p, ISBN 978-0-80-140333-0. [Google Scholar]
- Gashti, A.N.; Huffman, J.C.; Edwards, A.; Szekeley, G.; Siedle, A.R.; Karty, J.A.; Reilly, J.P.; Todd, L.J. Fluorination studies of the [commo-3,3′-Co(3,1,2-CoC2B9H11)2]− ion. J. Organomet. Chem. 2000, 614–615, 120–124. [Google Scholar] [CrossRef]
- Kazheva, O.N.; Alexandrov, G.G.; Kravchenko, A.V.; Sivaev, I.B.; Starodub, V.A.; Kosenko, I.D.; Lobanova, I.A.; Bregadze, V.I.; Buravov, L.V.; Dyachenko, O.A. New organic conductors with halogen and phenyl substituted cobalt bis(dicarbollide) anions. J. Chem. Eng. Chem. Res. 2015, 2, 497–503. [Google Scholar]
- Sivy, P.; Preisinger, A.; Baumgartner, O.; Valach, F.; Koreñ, B.; Matel, L. Structure of caesium 8-iodo-3,3′-commo-bis(decahydro-1,2-dicarba-3-cobalta-closo-dodecaborate)(1-). Acta Cryst. C 1986, 42, 30–33. [Google Scholar] [CrossRef]
- Kazheva, O.N.; Alexandrov, G.G.; Kravchenko, A.V.; Starodub, V.A.; Sivaev, I.B.; Lobanova, I.A.; Bregadze, V.I.; Buravov, L.I.; Dyachenko, O.A. New fulvalenium salts of bis(dicarbollide) cobalt and iron: Synthesis, crystal structure and electrical conductivity. J. Organomet. Chem. 2007, 692, 5033–5043. [Google Scholar] [CrossRef]
- Kazheva, O.N.; Alexandrov, G.G.; Kravchenko, A.V.; Kosenko, I.D.; Lobanova, I.A.; Sivaev, I.B.; Filippov, O.A.; Shubina, E.S.; Bregadze, V.I.; Starodub, V.A.; et al. Molecular conductors with a 8-hydroxy cobalt bis(dicarbollide) anion. Inorg. Chem. 2011, 50, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Rokitskaya, T.I.; Kosenko, I.D.; Sivaev, I.B.; Antonenko, Y.N.; Bregadze, V.I. Fast flip-flop of halogenated cobalt bis(dicarbollide) anion in a lipid bilayer membrane. Phys. Chem. Chem. Phys. 2017, 19, 25122–25126. [Google Scholar] [CrossRef] [PubMed]
- Plešek, J.; Heřmánek, S.; Franken, A.; Císařová, I.; Nachtigal, C. Dimethyl sulfate induced nucleophilic substitution of the [bis(1,2-dicarbollido)-3-cobalt(1-)]ate ion. Syntheses, properties and structures of its 8,8′-μ-sulfato,8-phenyl and 8-dioxane derivatives. Collect. Czech. Chem. Commun. 1997, 62, 47–56. [Google Scholar] [CrossRef]
- Rojo, I.; Teixidor, F.; Viñas, C.; Kivekäs, R.; Sillanpää, R. Relevance of the electronegativity of boron in η5-coordinating ligands: Regioselective monoalkylation and monoarylation in cobaltabisdicarbollide [3,3′-Co(1,2-C2B9H11)2]−clusters. Chem. Eur. J. 2003, 9, 4311–4323. [Google Scholar] [CrossRef] [PubMed]
- Bregadze, V.I.; Kosenko, I.D.; Lobanova, I.A.; Starikova, Z.A.; Godovikov, I.A.; Sivaev, I.B. C-H Bond activation of arenes by [8,8′-μ-I-3,3′-Co(1,2-C2B9H10)2] in the presence of sterically hindered Lewis bases. Organometallics 2010, 29, 5366–5372. [Google Scholar] [CrossRef]
- Kosenko, I.D.; Lobanova, I.A.; Godovikov, I.A.; Starikova, Z.A.; Sivaev, I.B.; Bregadze, V.I. Mild C-H activation of activated aromatics with [8,8′-μ-I-3,3′-Co(1,2-C2B9H10)2]: Just mix them. J. Organomet. Chem. 2012, 721–722, 70–77. [Google Scholar] [CrossRef]
- Rojo, I.; Teixidor, F.; Viñas, C.; Kivekäs, R.; Sillanpää, R. Synthesis and coordinating ability of an anionic cobaltabisdicarbollide ligand geometrically analogous to BINAP. Chem. Eur. J. 2004, 10, 5376–5385. [Google Scholar] [CrossRef] [PubMed]
- Teixidor, F.; Pedrajas, J.; Rojo, I.; Viñas, C.; Kivekäs, R.; Sillanpää, R.; Sivaev, I.; Bregadze, V.; Sjöberg, S. Chameleonic capacity of [3,3’-Co(1,2-C2B9H11)2]− in coordination. Generation of the highly uncommon S(thioether)-Na bond. Organometallics 2003, 22, 3414–3423. [Google Scholar] [CrossRef]
- Kazakov, G.S.; Stogniy, M. Y.; Sivaev, I.B.; Suponitsky, K.Y.; Godovikov, I.A.; Kirilin, A.D.; Bregadze, V.I. Synthesis of crown ethers with the incorporated cobalt bis(dicarbollide) fragment. J. Organomet. Chem. 2015, 798, 196–203. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Sivaev, I.B.; Suponitsky, K.Y.; Godovikov, I.A.; Bregadze, V.I. Synthesis of 10-methylsulfide and 10-alkylmethylsulfonium nido-carborane derivatives: B-H…π interactions between the B-H-B hydrogen atom and alkyne group in 10-RC≡CCH2S(Me)-7,8-C2B9H11. Eur. J. Inorg. Chem. 2017, 4436–4443. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Sivaev, I.B.; Suponitsky, K.Y.; Bregadze, V.I. Practical synthesis of 9-methylthio-7,8-nido-carborane [9-MeS-7,8-C2B9H11]−. Some evidences of BH … X hydride-halogen bonds in 9-XCH2(Me)S-7,8-C2B9H11 (X = Cl, Br, I). J. Organomet. Chem. 2017, 849–850, 315–323. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Erokhina, S.A.; Suponitsky, K.Y.; Godovikov, I.A.; Filippov, O.A.; Fabrizi de Biani, F.; Corsini, M.; Chizhov, A.O.; Sivaev, I.B. Methylsulfanyl-stabilized rotamers of cobalt bis(dicarbollide). Eur. J. Inorg. Chem. 2017, 4444–4451. [Google Scholar] [CrossRef]
- Timofeev, S.V.; Anufriev, S.A.; Sivaev, I.B.; Bregadze, V.I. Synthesis of pentacarbonyl tungsten complexes of 8,8′-dimethylsulfanyl cobalt bis(dicarbollide). Russ. Chem. Bull. Submitted.










© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivaev, I.B. Ferrocene and Transition Metal Bis(Dicarbollides) as Platform for Design of Rotatory Molecular Switches. Molecules 2017, 22, 2201. https://doi.org/10.3390/molecules22122201
Sivaev IB. Ferrocene and Transition Metal Bis(Dicarbollides) as Platform for Design of Rotatory Molecular Switches. Molecules. 2017; 22(12):2201. https://doi.org/10.3390/molecules22122201
Chicago/Turabian StyleSivaev, Igor B. 2017. "Ferrocene and Transition Metal Bis(Dicarbollides) as Platform for Design of Rotatory Molecular Switches" Molecules 22, no. 12: 2201. https://doi.org/10.3390/molecules22122201




