Cellulose Nanofibers Prepared via Pretreatment Based on Oxone® Oxidation
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Oxone® Oxidation
3.3. Mechanical Disintegration
3.4. Optical Transmittance
3.5. Preparation of CNF Films
3.6. Scanning Electron Microscopy (SEM)
3.7. Atomic Force Microscopy (AFM)
3.8. Intrinsic Viscosity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Li, H.L.; Li, B.Y.; Liu, M.R.; Zhan, H.Y. Characteristics and properties of cellulose nanofibers prepared by tempo oxidation of corn husk. Bioresources 2016, 11, 5276–5284. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Wata, T.; Kumamoto, Y.; Isogai, A. Transparent and high gas barrier films of cellulose nanofibers prepared by tempo-mediated oxidation. Biomacromolecules 2009, 10, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Tsuzuki, T.; Wang, X.G. Preparation of cellulose nanofiber from softwood pulp by ball milling. Cellulose 2015, 22, 1729–1741. [Google Scholar] [CrossRef]
- Nasri-Nasrabadi, B.; Mehrasa, M.; Rafienia, M.; Bonakdar, S.; Behzad, T.; Gavanji, S. Porous starch/cellulose nanofibers composite prepared by salt leaching technique for tissue engineering. Carbohydr. Polym. 2014, 108, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Tummala, G.K.; Joffre, T.; Lopes, V.R.; Liszka, A.; Buznyk, O.; Ferraz, N.; Persson, C.; Griffith, M.; Mihranyan, A. Hyperelastic nanocellulose-reinforced hydrogel of high water content for ophthalmic applications. ACS Biomater. Sci. Eng. 2016, 2, 2072–2079. [Google Scholar] [CrossRef]
- Gustafsson, S.; Lordat, P.; Hanrieder, T.; Asper, M.; Schaefer, O.; Mihranyan, A. Mille-feuille paper: A novel type of filter architecture for advanced virus separation applications. Mater. Horiz. 2016, 3, 320–327. [Google Scholar] [CrossRef]
- Pan, R.J.; Cheung, O.; Wang, Z.H.; Tammela, P.; Huo, J.X.; Lindh, J.; Edström, K.; Strømme, M.; Nyholm, L. Mesoporous cladophora cellulose separators for lithium-ion batteries. J. Power Sources 2016, 321, 185–192. [Google Scholar] [CrossRef]
- Pan, R.J.; Wang, Z.H.; Sun, R.; Lindh, J.; Edström, K.; Strømme, M.; Nyholm, L. Thickness difference induced pore structure variations in cellulosic separators for lithium-ion batteries. Cellulose 2017, 24, 2903–2911. [Google Scholar] [CrossRef]
- Razaq, A.; Nyholm, L.; Sjödin, M.; Strømme, M.; Mihranyan, A. Paper-based energy-storage devices comprising carbon fiber-reinforced polypyrrole-cladophora nanocellulose composite electrodes. Adv. Energy Mater. 2012, 2, 445–454. [Google Scholar] [CrossRef]
- Wang, Z.H.; Carlsson, D.O.; Tammela, P.; Hua, K.; Zhang, P.; Nyholm, L.; Strømme, M. Surface modified nanocellulose fibers yield conducting polymer-based flexible supercapacitors with enhanced capacitances. ACS Nano 2015, 9, 7563–7571. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z.H.; Zhu, J.F. Binder-free nitrogen-doped carbon paper electrodes derived from polypyrrole/cellulose composite for Li–O2 batteries. J. Power Sources 2016, 306, 559–566. [Google Scholar] [CrossRef]
- Galkina, O.L.; Ivanov, V.K.; Agafonov, A.V.; Seisenbaeva, G.A.; Kessler, V.G. Cellulose nanofiber-titania nanocomposites as potential drug delivery systems for dermal applications. J. Mater. Chem. B 2015, 3, 1688–1698. [Google Scholar] [CrossRef]
- Basu, A.; Lindh, J.; Ålander, E.; Strømme, M.; Ferraz, N. On the use of ion-crosslinked nanocellulose hydrogels for wound healing solutions: Physicochemical properties and application-oriented biocompatibility studies. Carbohydr. Polym. 2017, 174, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.Z.; Shao, Z.Q.; Wu, X.; Wang, X.; Li, J.; Zhang, Y.H.; Wang, W.J.; Wang, F.J. Cellulose nanofibers/reduced graphene oxide flexible transparent conductive paper. Carbohydr. Polym. 2013, 97, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef] [PubMed]
- Yamane, C.; Abe, K.; Satho, M.; Miyamoto, H. Dissolution of cellulose nanofibers in aqueous sodium hydroxide solution. Nord. Pulp Pap. Res. J. 2015, 30, 92–98. [Google Scholar] [CrossRef]
- Ohkawa, K.; Hayashi, S.; Nishida, A.; Yamamoto, H.; Ducreux, J. Preparation of pure cellulose nanofiber via electrospinning. Text. Res. J. 2009, 79, 1396–1401. [Google Scholar] [CrossRef]
- Saito, T.; Nishiyama, Y.; Putaux, J.L.; Vignon, M.; Isogai, A. Homogeneous suspensions of individualized microfibrils from tempo-catalyzed oxidation of native cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Sugiyama, J.; Nakagaito, A.N.; Nogi, M.; Matsuura, T.; Hikita, M.; Handa, K. Optically transparent composites reinforced with networks of bacterial nanofibers. Adv. Mater. 2005, 17, 153–155. [Google Scholar] [CrossRef]
- Saito, T.; Hirota, M.; Tamura, N.; Kimura, S.; Fukuzumi, H.; Heux, L.; Isogai, A. Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation using tempo catalyst under neutral conditions. Biomacromolecules 2009, 10, 1992–1996. [Google Scholar] [CrossRef] [PubMed]
- Coseri, S.; Biliuta, G.; Simionescu, B.C.; Stana-Kleinschek, K.; Ribitsch, V.; Harabagiu, V. Oxidized cellulose-survey of the most recent achievements. Carbohydr. Polym. 2013, 93, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, D.O.; Lindh, J.; Nyholm, L.; Strømme, M.; Mihranyan, A. Cooxidant-free tempo-mediated oxidation of highly crystalline nanocellulose in water. RSC Adv. 2014, 4, 52289–52298. [Google Scholar] [CrossRef]
- Kachkarova-Sorokina, S.L.; Gallezot, P.; Sorokin, A.B. A novel clean catalytic method for waste-free modification of polysaccharides by oxidation. Chem. Commun. 2004, 2844–2845. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.Q.; Strømme, M.; Mihranyan, A.; Lindh, J. Favored surface-limited oxidation of cellulose with oxone® in water. RSC Adv. 2017, 7, 40600–40607. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. Tempo-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Perez, D.D.; Montanari, S.; Vignon, M.R. Tempo-mediated oxidation of cellulose III. Biomacromolecules 2003, 4, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- ASTM Standard D1795-96(2007)e1: Standard Test Method For Intrinsic Viscosity of Cellulose; ASTM International: West Conshohocken, PA, USA, 1996.
Sample Availability: Not available. |
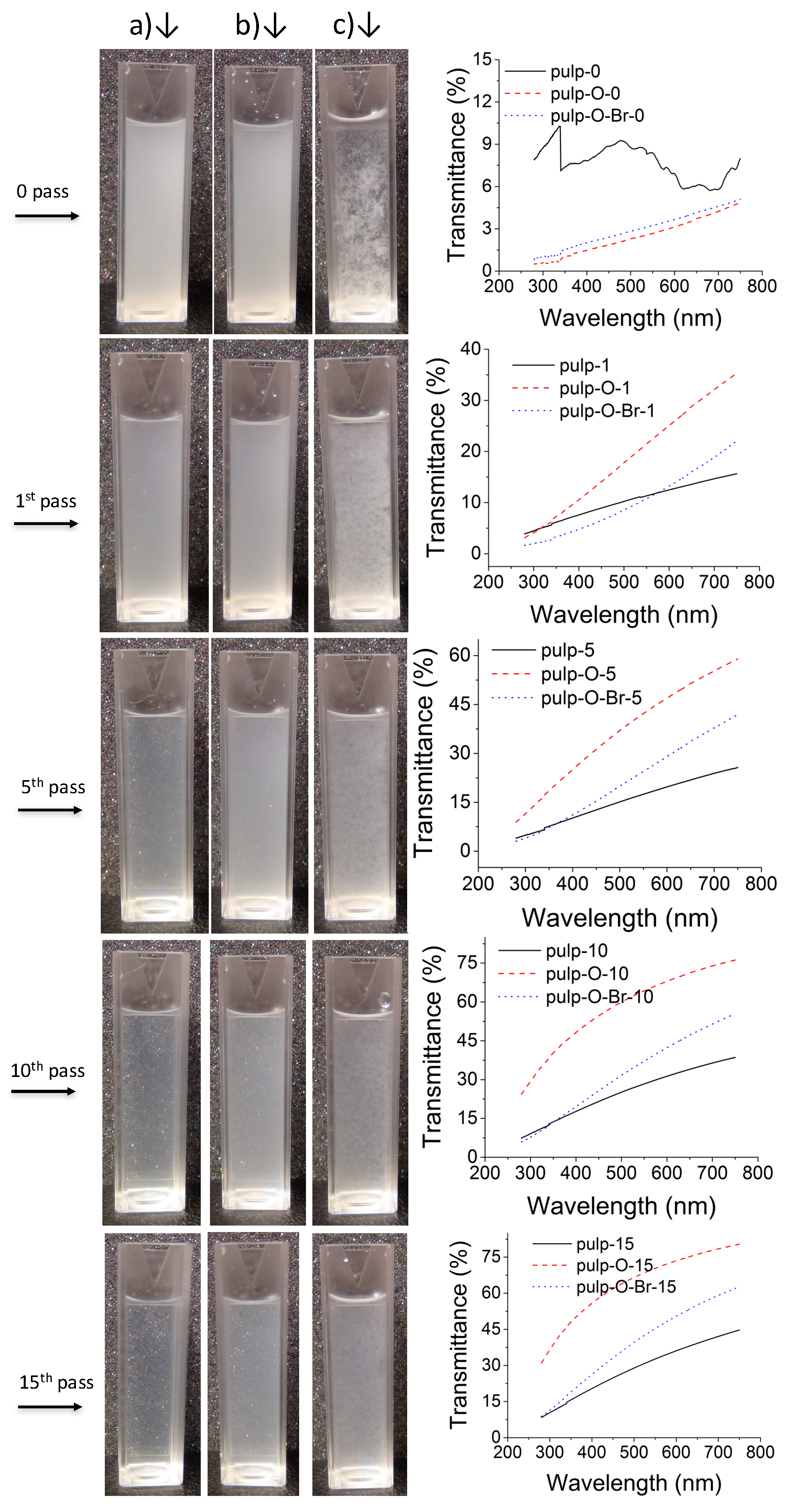
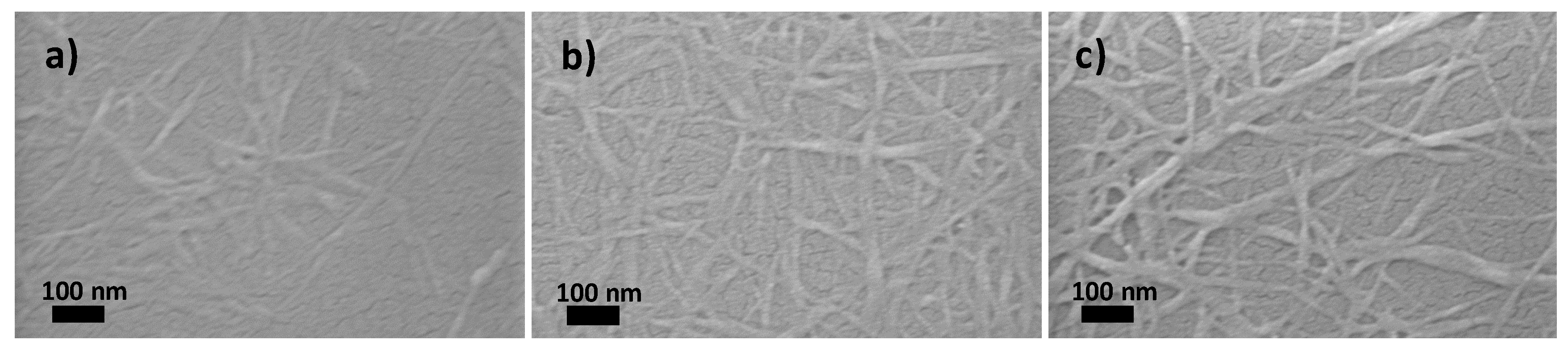
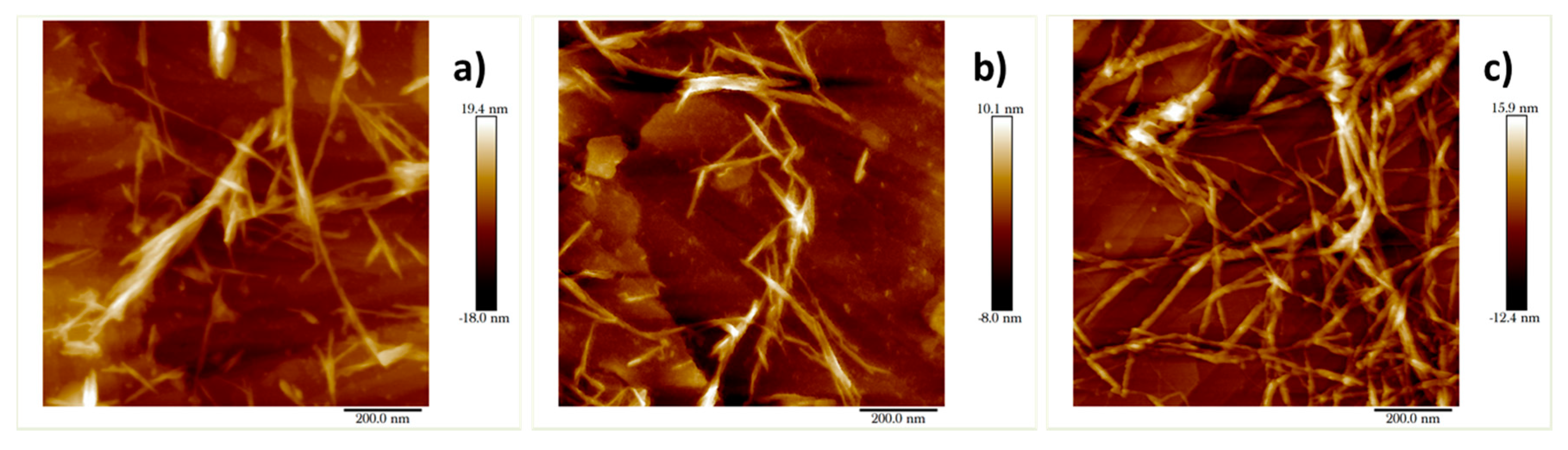

| Sample | pulp | pulp-15 | pulp-O | pulp-O-15 | pulp-O-Br | pulp-O-Br-15 |
|---|---|---|---|---|---|---|
| Intrinsic viscosity (η) (dL/g) | 2.01 | 1.149 | 0.375 | 0.294 | 0.510 | 0.456 |
| Transmittance at 550 nm (%) | 8.37 | 32.69 | 2.67 | 70.34 | 3.23 | 45.31 |
| Carboxylic acid content (mmol/g) | 0.077 | / | 1.073 | / | 0.585 | / |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, C.-Q.; Gustafsson, S.; Strømme, M.; Mihranyan, A.; Lindh, J. Cellulose Nanofibers Prepared via Pretreatment Based on Oxone® Oxidation. Molecules 2017, 22, 2177. https://doi.org/10.3390/molecules22122177
Ruan C-Q, Gustafsson S, Strømme M, Mihranyan A, Lindh J. Cellulose Nanofibers Prepared via Pretreatment Based on Oxone® Oxidation. Molecules. 2017; 22(12):2177. https://doi.org/10.3390/molecules22122177
Chicago/Turabian StyleRuan, Chang-Qing, Simon Gustafsson, Maria Strømme, Albert Mihranyan, and Jonas Lindh. 2017. "Cellulose Nanofibers Prepared via Pretreatment Based on Oxone® Oxidation" Molecules 22, no. 12: 2177. https://doi.org/10.3390/molecules22122177





