Extraction Optimization of Flavonoids from Hypericum formosanum and Matrix Metalloproteinase-1 Inhibitory Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Time, Ethanol Concentration, and Temperature on the Extraction of Flavonoids
2.2. Fitting the Model and Response Surface Analysis
2.3. Optimization and Verification
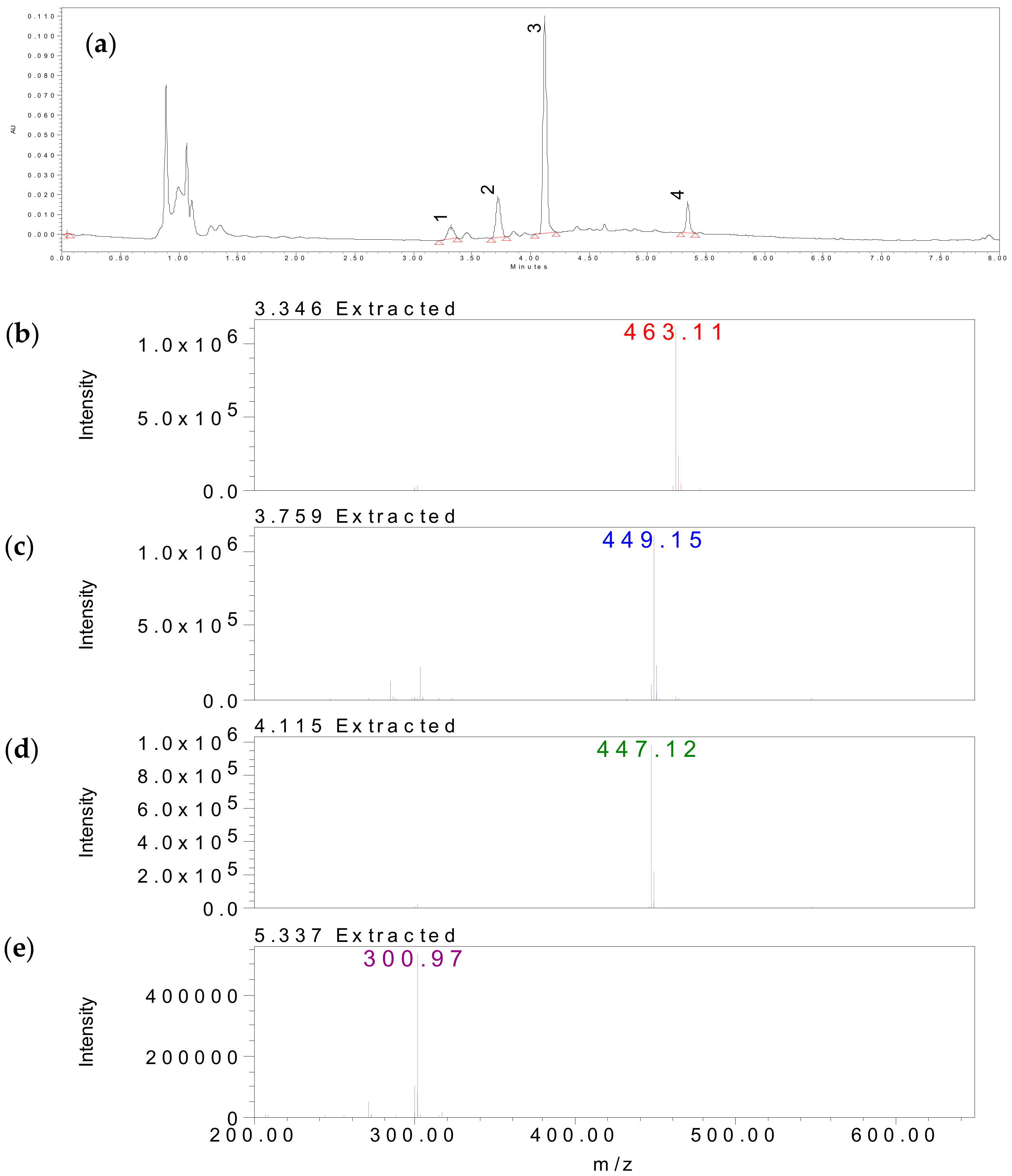
2.4. Identification of Bioactive Compounds by HPLC-MS Analysis
2.5. Matrix Metalloproteinase-1 Inhibitory Activity
3. Materials and Methods
3.1. Plant Materials
3.2. Chemicals
3.3. Plant Materials and Preparation of the Extracts
3.4. Total Flavonoid Content
3.5. Single-Factor Experiments
3.6. Experimental Design
3.7. UPLC–DAD-MS Analysis of the Extract
3.8. Cell Culture
3.9. Cell viability Assay
3.10. AGEsTreatment and Real-Time PCR Analysis
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nahrstedt, A.; Butterweck, V. Lessons learned from herbal medicinal products: The example of St. John’s Wort (perpendicular). J. Nat. Prod. 2010, 73, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Carlo, G.D.; Borrelli, F.; Rrnst, E.; Izzo, A.A. St. John’s wort: Prozac from the plant kingdom. Trends Pharmacol. Sci. 2001, 22, 292–297. [Google Scholar] [CrossRef]
- Greeson, J.M.; Sanford, B.; Monti, D.A. St. John’s wort (Hypericum perforatum): A review of the current pharmacological, toxicological, and clinical literature. Psychopharmacology 2001, 153, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Butterweck, V.; Schmidt, M. St. John’s wort: Role of active compounds for its mechanism of action and efficacy. Wien. Med. Wochenschr. 2007, 157, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Tatsis, E.C.; Boeren, S.; Exarchou, V.; Troganis, A.N.; Vervoort, J.; Gerothanassis, I.P. Identification of the major constituents of Hypericum perforatum by LC/SPE/NMR and/or LC/MS. Phytochemistry 2007, 68, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Skalkos, D.; Stavropoulos, N.E.; Tsimaris, I.; Gioti, E.; Stalikas, C.D.; Nseyo, U.O. The lipophilic extract of Hypericum perforatum exerts significant cytotoxic activity against T24 and NBT-II urinary bladder tumor cells. Planta Med. 2005, 71, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Boveris, A.D.; Puntarulo, S. Free radical scavenging actions of natural antioxidants. Nutr. Res. 1998, 18, 1545–1547. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Katan, M.B. Absorption, metabolism, and health effects of dietary flavonoids in man. Biomed. Pharmacother. 1997, 51, 305–310. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.; Cai, X. Optimization of the extraction of total flavonoids from Scutellaria baicalensis Georgi using the response surface methodology. J. Food Sci. Technol. 2015, 52, 2336–2343. [Google Scholar] [CrossRef] [PubMed]
- Ramic, M.; Vidovic, S.; Zekovic, Z.; Vladic, J.; Cvejin, A.; Pavlic, B. Modeling and optimization of ultrasound assisted extraction of polyphenolic compounds from Aronia melanocarpa by products form filter tea factory. Ultrason. Sonochem. 2015, 23, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Ultrasound assisted extraction of soy isoflavones. J. Chromatogr. A 2003, 1012, 119–128. [Google Scholar] [CrossRef]
- Sampaio, P.N.; Calado, C.R.C.; Sousa, L.; Bressler, D.C.; Pais, M.S.; Fonseca, L.P. Optimization of the culture medium composition using response surface methodology for new recombinant cyprosin B production in bioreactor for cheese production. Eur. Food Res. 2010, 231, 339–346. [Google Scholar] [CrossRef]
- Ballarad, T.S.; Mallikarjunan, P.; Zhou, K.; Okkefe, S.F. Optimizing the Extraction of Phenolic Antioxidants from Peanut Skins Using Response Surface Methodology. J. Agric. Food. Chem. 2009, 57, 3064–3072. [Google Scholar] [CrossRef] [PubMed]
- Hayouni, E.A.; Abedrabba, M.; Bouix, M.; Hamdi, M. The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chem. 2007, 105, 1126–1134. [Google Scholar] [CrossRef]
- Huang, W.; Li, Z.; Niu, H.; Li, D.; Zhang, J. Optimization of operating parameters for supercritical carbon dioxide extraction of lycopene by response surface methodology. J. Food Eng. 2008, 89, 298–302. [Google Scholar] [CrossRef]
- Pompeu, D.R.; Silva, E.M.; Rogez, H. Optimization of the solvent extraction of phenolic antioxidants from fruits of Euterpe oleracea using response surface methodology. Bioresour. Technol. 2009, 100, 6076–6082. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.H.; Montgomery, D.C. Response Surface Methodology: Process and Product Optimization Using Design Experiments, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Xi, J.; Wang, B.S. Optimization of ultrahigh-pressure extraction of polyphenolic antioxidants from green tea by response surface methodology. Food Bioprocess Technol. 2013, 6, 2538–2546. [Google Scholar] [CrossRef]
- Dayana, P.S.; Bakthavatsalam, A.K. Optimization of phenol degradation by the microalga Chlorella pyrenoidosa using Plackett-Burman Design and Response Surface Methodology. Bioresour. Technol. 2016, 207, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liang, H.; Yuan, Q. Optimization of ultrasonic-stimulated solvent extraction of sinigrin from Indian mustard seed (Brassica juncea L.) using response surface methodology. Phytochem. Anal. 2011, 22, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Rubilar, M.; Sineiro, J.; Nunez, M.J. Effect of solvent, temperature, and solvent-to-solid ration the total phenolic content and antiradical activity of extracts from different components from grape pomace. J. Agric. Food Chem. 2005, 53, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Giacomoni, P.U.; Rein, G. Factors of skin ageing share common mechanisms. Biogerontology 2001, 2, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Browniee, M.M. Advanced protein glycation in diabetes and aging. Annu. Rev. Med. 1995, 46, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Wondrak, G.T. Let the sun shine in: Mechanisms and potential for therapeutics in skin photodamage. Curr. Opin. Investig. Drugs 2007, 8, 390–400. [Google Scholar] [PubMed]
- Farris, P.K. Innovative cosmeceuticals: Sirtuin activators and anti-glycation compounds. Semin. Cutan. Med. Surg. 2011, 30, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Pageon, H. Reaction of glycation and human skin: The effects on the skin and its components, reconstructed skin as a model. Pathol. Biol. 2010, 58, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jin, M.; Yang, F.; Zhu, J.; Xiao, Q.; Zhang, L. Matrix metalloproteinases: Inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediat. Inflamm. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Ham, Y.M.; Yoon, W.J.; Roh, S.W.; Jeon, Y.J.; Oda, T. Quercitrin protects against ultraviolet B-induced cell death in vitro and in an in vivo zebrafish model. Photochem. Photobiol. B 2012, 114, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Morand, C.; Manach, C.; Crespy, V.; Remesy, C. Quercetin 3-O-beta-glucoside is better absorbed than other quercetin forms and is not present in rat plasma. Free Radic. Res. 2000, 33, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Hanamura, T.; Hagiwara, T.; Kawagishi, H. Structural and functional characterization of polyphenols isolated from Acerola (Malpighia emarginata DC.) fruit. Biosci. Biotechnol. Biochem. 2005, 69, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.S.; Kim, J.M.; Kim, J.; Yoo, J.L.; Kim, Y.S.; Kim, J.S. Effects of compounds isolated from the fruits of Rumex japonicus on the protein glycation. Chem. Biodivers. 2008, 5, 2718–2723. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Tornberg, E.; Gekas, V. Clarification of high-added value products from olive mill wastewater. J. Food Eng. 2010, 99, 190–197. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.W.; Cho, S.Y.; Lee, S.R.; Lee, K.S. Onion extract and quercetin induce matrix metalloproteinase-1 in vitro and in vivo. Int. J. Mol. Med. 2010, 25, 347–352. [Google Scholar] [PubMed]
- Jnawali, H.N.; Lee, E.; Shin, A.; Park, Y.G.; Kim, Y. Effect of Quercetin in the UV-Irradiated Human Keratinocyte HaCaT Cells and A Model of Its Binding To p38 MAPK. Bull. Korean Chem. Soc. 2014, 35, 2787–2790. [Google Scholar] [CrossRef]
- Lee, S.Y. Synergistic effect of maclurin on ginsenoside compound K induced inhibition of the transcriptional expression of MMP-1 in HaCaT human keratinocyte cells. J. Ginseng Res. 2017, in press. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, T.; Chen, M.; Jiang, M.; Wang, X.; Liu, Q.; Zhan, Z.; Zhang, X. Research progress on synergistic anti-tumor mechanisms of compounds in Traditional Chinese Medicine. J Tradit. Chin. Med. 2014, 34, 100–105. [Google Scholar] [CrossRef]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.J. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |



| Run | X1 | X2 | X3 | Response Value |
|---|---|---|---|---|
| Concentration of Ethanol (%) | Extraction Time (min) | Extraction Temperature (°C) | TFC (mg/g) | |
| 1 | 75 | 20 | 80 | 90.1 |
| 2 | 52.5 | 40 | 60 | 89.9 |
| 3 | 30 | 20 | 40 | 65.5 |
| 4 | 30 | 40 | 60 | 80.2 |
| 5 | 30 | 60 | 80 | 70.5 |
| 6 | 75 | 20 | 40 | 81.9 |
| 7 | 52.5 | 40 | 60 | 94.7 |
| 8 | 75 | 40 | 60 | 101.5 |
| 9 | 52.5 | 40 | 60 | 94.5 |
| 10 | 52.5 | 40 | 80 | 90.2 |
| 11 | 52.5 | 40 | 40 | 75.3 |
| 12 | 30 | 20 | 80 | 70.2 |
| 13 | 52.5 | 60 | 60 | 90.2 |
| 14 | 30 | 60 | 40 | 67.7 |
| 15 | 75 | 60 | 80 | 92.1 |
| 16 | 52.5 | 20 | 60 | 85.2 |
| 17 | 52.5 | 40 | 60 | 95.2 |
| 18 | 75 | 60 | 40 | 90.2 |
| 19 | 52.5 | 40 | 60 | 94.9 |
| 20 | 52.5 | 40 | 60 | 95.1 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Model | 2084.65 | 9 | 231.63 | 28.33 | <0.0001 | Significant |
| X1 | 1034.29 | 1 | 1034.29 | 126.5 | <0.0001 | |
| X2 | 31.68 | 1 | 31.68 | 3.88 | 0.1234 | |
| X3 | 105.62 | 1 | 105.62 | 12.92 | 0.0773 | |
| X1X2 | 7.61 | 1 | 7.61 | 0.93 | 0.0049 | |
| X1X3 | 0.84 | 1 | 0.84 | 0.1 | 0.3576 | |
| X2X3 | 8.4 | 1 | 8.4 | 1.03 | 0.7545 | |
| X12 | 4.39 | 1 | 4.39 | 0.54 | 0.3345 | |
| X22 | 53.57 | 1 | 53.57 | 6.55 | 0.0284 | |
| X32 | 241.11 | 1 | 241.11 | 29.49 | 0.0003 | |
| Residual | 81.76 | 10 | 8.18 | |||
| Lack of Fit | 60.77 | 5 | 12.15 | 2.89 | 0.1342 | Not significant |
| Pure Error | 20.99 | 5 | 4.20 | |||
| Core total | 2166.41 | 19 | ||||
| R2 | 0.9587 |
| Optimal Conditions | Total Flavonoid Content (mg/g) | |||
|---|---|---|---|---|
| Concentration of Ethanol (%) | Time (min) | Temperature (°C) | Experimental Result | Predicted Value |
| 73.5 | 38.3 | 62.5 | 101.7 ± 1.7 | 101.1 |
| Independent Variable | Symbol | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Concentration of ethanol (%) | X1 | 30 | 60 | 75 |
| Time (min) | X2 | 20 | 40 | 60 |
| Temperature (°C) | X3 | 40 | 60 | 80 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-S.; Liaw, E.-T. Extraction Optimization of Flavonoids from Hypericum formosanum and Matrix Metalloproteinase-1 Inhibitory Activity. Molecules 2017, 22, 2172. https://doi.org/10.3390/molecules22122172
Huang H-S, Liaw E-T. Extraction Optimization of Flavonoids from Hypericum formosanum and Matrix Metalloproteinase-1 Inhibitory Activity. Molecules. 2017; 22(12):2172. https://doi.org/10.3390/molecules22122172
Chicago/Turabian StyleHuang, Ho-Shin, and Ean-Tun Liaw. 2017. "Extraction Optimization of Flavonoids from Hypericum formosanum and Matrix Metalloproteinase-1 Inhibitory Activity" Molecules 22, no. 12: 2172. https://doi.org/10.3390/molecules22122172





